Abstract
Objectives
Coke oven emissions (COE) containing polycyclic aromatic hydrocarbons (PAHs) can induce both benzo[a]pyrene‐r‐7, t‐8, t‐9,c‐10‐tetrahydotetrol‐albumin (BPDE‐Alb) adducts and DNA damage. However, the relation between these biomarkers for early biological effects is not well documented in coke oven workers.
Methods
In this study, the authors recruited 207 male workers exposed to COE and 102 controls not exposed to COE in the same steel plant in northern China. They measured BPDE‐Alb adduct concentrations in plasma with reverse‐phase high performance liquid chromatography and DNA damage in peripheral blood lymphocytes with alkaline comet assay.
Results
The results showed that the median concentration of BPDE‐Alb adducts in the exposed group (34.36 fmol/mg albumin) was significantly higher than that in the control group (21.90 fmol/mg albumin, p = 0.012). The mean Olive tail moment (Olive TM) of DNA damage in the exposed and control groups were 1.20 and 0.63, respectively (p = 0.000). Multivariate logistic regression analysis revealed that the odds ratio (OR) for BPDE‐Alb adduct and Olive TM associated with the exposure were 1.72 (95% CI 1.06 to 2.81) and 1.96 (95% CI 1.20 to 3.19), respectively. These results show significant correlations between the concentrations of BPDE‐Alb adduct and Olive TM levels in exposed group (r = 0.235, p = 0.001) but not in control group (r = 0.093, p = 0.353).
Conclusion
The results suggest that occupational exposure to COE may induce both BPDE–Alb adducts and DNA damage in the lymphocytes of coke oven workers and that these two markers are useful for monitoring exposure to COE in the workplace.
Coke oven workers are exposed to coke oven emissions (COE) that contain a wide variety of volatile organic solvents and particulates, especially polycyclic aromatic hydrocarbons (PAHs).1 Epidemiological studies suggest an aetiological link between carcinogenic PAHs exposure and lung cancer risk in coke oven workers exposed to COE, and coke oven workers were found to have a three‐ to sevenfold increased risk for developing lung cancer.1,2
Benzo[a]pyrene (B[a]P), the most potent and well‐studied carcinogen in PAHs mixtures, has been used as an indicator for carcinogenic PAHs.3,4 The metabolic activation of B[a]P by cytochromes P450 produces 7,8‐dihydroxy‐9,10‐epoxy‐7,8,9,10‐tetrahydrobenzo[a]pyrene (BPDE), the ultimate carcinogenic form that can bind covalently to DNA and proteins.5 Therefore, both DNA and protein adducts are thought to be biologically effective dose biomarkers of PAHs.6 There are a number of published reports on DNA adducts in workers exposed to PAHs.7,8 Although DNA adducts determined using DNA from white blood cells may not reflect the levels of DNA damage in the target tissues,9 DNA from the target tissues is usually not readily accessible in human biomonitoring. The albumin adducts in blood are considered a surrogate biomarker of the effective dose of exposure and are not considered to be directly involved in carcinogenesis,10 because they represent only one month exposure within the half‐life of the albumin.11
The carcinogenicity induced by PAHs compounds is believed to be initiated by DNA damage.12 A wide variety of non‐bulky base damage and single‐strand breaks are formed during metabolic activation of PAHs and involved in PAHs carcinogenesis,13,14 and these types of DNA damage can be detected by comet assay and used as a marker of early biological effects of DNA‐damaging agents in the living environments and occupational workplaces.15,16,17 Specifically, the alkaline comet assay (pH>13) can detect DNA strand breaks, alkali‐labile sites, and incomplete excision repair sites.18 However, the published results of the relation between COE exposure and DNA damage in the lymphocytes measured by comet assay are not consistent.19,20,21,22,23 For example, some investigations have shown that there was a significant increase in DNA damage in workers exposed to COE compared with unexposed controls.19,20,22 However, others21,23 did not find any effect of occupational exposure on the levels of DNA damage measured with the comet assay, possibly due to small sample sizes in these studies.
In the exposure‐to‐disease pathogenic pathway, biomarkers that can provide information of exposure to carcinogenic agents (biomarkers of exposure) and early changes caused by the agents (biomarkers of effect) are needed in epidemiological studies of cancer risk. However, the levels of exposure biomarkers and their associations with early biological effects in coke oven workers are not well documented. Some authors have reported significant association between biomarkers of internal dose (1‐hydroxypyrene) and effect biomarkers (comet assay, sister chromatid exchanges, micronuclei, chromosomal aberrations, and 8‐oxo‐7, 8‐dihydro‐2'‐deoxyguanosine) for coke oven workers exposed to PAHs,20,22,23 but these were not confirmed by other studies.21,26 One reason for this inconsistency is the levels of 1‐hydroxypyrene revealing recent exposure to COE or different sample size (from 83 to 217 subjects included). A positive association between biologically effective‐dose biomarkers (aromatic‐DNA adducts) with effect biomarkers (8‐oxo‐7, 8‐dihydro‐2'‐deoxyguanosine) among 149 coke oven workers was previously reported.26 To our knowledge, there was no reported investigation on the correlation between BPDE‐Alb adduct concentrations and DNA damage in lymphocytes in coke oven workers, although these two biomarkers were individually applied in some occupational biomonitoring. Therefore, we further assessed whether occupational exposure to COE resulted in high concentrations of BPDE–Alb adducts and DNA damage and their possible correlation in 207 male workers exposed to COE and 102 male unexposed controls.
Materials and methods
Study design and study population
A total of 309 male subjects were recruited from a steel plant in Taiyuan, northern China, of whom 207 workers who have worked on top‐, side‐ and bottom‐oven in the coke oven plant, which led to regular exposure to COE, and these workers had been employed for at least six months. The other 102 subjects with no work‐related PAHs exposure in their workplaces, such as maintenance sections, offices and the affiliated hospital of the company, were used as control subjects who were frequency matched to the exposed workers by age, sex and employment time. The subjects exposed to known mutagenic agents such as radiotherapy and chemotherapy in the last three months were excluded. A pre‐tested questionnaire on demographic characteristics, smoking history, alcohol consumption, history of occupational exposure and family medical history was administered in person by trained interviewers. Individuals who had smoked >3 months in their lifetime were considered smokers. Among these smokers, individuals who still smoked at the time of interview were classified as current smokers and the others non‐current smokers. As an indicator of cumulative smoking exposure, pack‐years were also calculated as average number of packs smoked per day multiplied by years of smoking. Individuals who drank more than twice a week in the last six months were classified as alcohol users.
After all participants signed the informed consent, 5 ml of venous blood were obtained from each subject in the morning after overnight fasting. Plasma was separated from 4 ml heparinised whole blood by centrifugation and stored at –80°C for detecting BPDE‐Alb adduct concentrations. After fresh blood lymphocytes were isolated from 1‐ml heparinised whole blood, the comet assay was carried out within three hours. All samples were analysed without knowing the subject status.
Airborne PAHs monitoring
We selected four working sites for exposed group and three working sites for control group, where collected airborne samples three times consecutively, with the average flow rate of 2.0 l/min (Gilian HFS‐513 air sampling pumps, Sensidyne, Inc, USA) for 2–5 h (240–600 l/sample). Particulate PAHs were collected on Teflon filters (pore size 2.0 μm, Advantec, Tokyo, Japan) in series with XAD‐2 column (Supelco, Bellefonte, PA, USA) in sorbent tubes to collect the vapour phase, then stored in the dark at –20°C. For extraction, the Teflon filter was covered with acetonitrile/methanol (60/40 v/v), treated in an ultrasonic bath for 60 min and shaken for 30 min. The XAD‐2 tube was eluted with acetonitrile/methanol (60/40 v/v) and dichloromethane. Subsequently, XAD‐2 material from sorbent tubes was extracted, mixed with both acetonitrile (2 ml) and dichloromethane (2 ml), and shaken again. The original filter extract and the extracts combined to the XAD‐2 material were dried under vacuum. The residues were redissolved with actetonitrile in a 25 μl aliquot. Quantitative chemical analysis of eight carcinogenic PAHs (B[a]P, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, dibenz[a,h]anthracene, benzo[g,h,i]perylene, indeno[1,2,3‐cd]pyrene) were performed by high performance liquid chromatography (Waters Corp, Milford, MA, USA) with fluorescence detectors according to the methods 5506 published by the National Institute for Occupational Safety and Health.27
Determination of plasma BPDE‐Alb adduct concentrations
Benzo[a]pyrene‐r‐7, t‐8, t‐9,c‐10‐tetrahydrotetrol (BPDE‐I) were purchased from the National Cancer Institute, Chemical Carcinogen Repository (Midwest Research Institute, Kansas City, MO, USA). Albumin was isolated from 2 ml plasma as previously described.28,29 Albumin was isolated from 2 ml plasma after precipitation of immunoglobulins with 2 ml saturated ammonium sulfate. The supernatant was acidified with acetic acid and left at 4°C overnight. The precipitated albumin was collected by centrifugation (3000 g, 30 min 4°C) and resuspended in 10 mM Tris and 1 mM EDTA (pH 8.0). The albumin concentrations of each plasma sample were determined with a Bio‐Rad protein assay kit (Hercules, CA, USA). The samples were cleaved under vacuum conditions at pH 11 and 90°C for 2 h. The resulting solution was applied to C18 solid phase extraction (SPE) cartridges (Agilent Technologies, USA) and washed with 5 ml methanol, then evaporated under a vacuum, and dissolved in 500 μl of PBS. The level of BPDE bound to plasma albumin was determined by reverse phase high performance liquid chromatography with fluorescence detector (Waters Corp, Milford, MA, USA), which was used for the quantitative analysis of BPDE‐Alb adduct concentrations using a previously published method29 with minor modifications. The excitation wavelength was 341 nm and the emission was measured at 381 nm. BPDE were separated by a linear gradient of methanol and water, 30% to 100% methanol in 17 min, 10 min at 100% methanol and then 19 min at 30% methanol before the next injection. Identification and quantification of BPDE isomers was based on retention time and peak‐area measured using a five‐point calibration curve constructed by measuring freshly derivatised standards with BPDE. Each standard was prepared singly and run in duplicate. The levels of adducts were expressed as fmol BPDE equivalents per microgram albumin.
The alkaline comet assay in peripheral blood lymphocytes
The alkaline comet assay was performed according to the method of Singh et al30 with minor modifications. Two slides were prepared, and each of about 50 cells was randomly chosen and scored microscopically and in a blinded manner. Measurements were made using an image analysis system (version 1.0, IMI comet analysis software, China).31 Because the levels of tail length and Olive tail moment (Olive TM) of cells were highly correlated, the levels of DNA damage were expressed as Olive TM and were defined by the percentage of DNA in the tail multiplied by the length between the centre of the head and tail.32
Statistical analysis
Statistical analysis was done by SPSS 11.5 software (SPSS 11.5. SPSS Inc, Chicago, IL, USA). For our statistical calculations, results lower than the analytical limit of detection have been set to
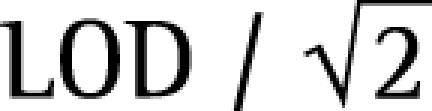 |
of the detection limit.33 Normal distribution test was examined using the Shapiro‐Wilk normality test. The mean values of age, employment time and Olive TM levels in the exposed and control groups were compared using the Student t test. The Olive TM values were normalised by natural logarithm transformation. Chi‐square tests were used to compare the frequencies of current smokers and alcohol users between the exposed and control groups. Variables not fitting the normal distribution were compared using non‐parametric tests: the Mann‐Whitney U test was used to evaluate pack‐years and BPDE‐Alb adduct concentrations between the exposed and control groups. The data were presented as median and interquartile range. Correlations were evaluated using the Spearman rank test, and r was calculated to evaluate the closeness of relation between two continuous variables (BPDE‐Alb adduct concentrations and Olive TM levels). The overall subjects were stratified into two subgroups according to the median values of BPDE‐Alb adduct concentrations and mean Olive TM levels, the association between exposure to COE and BPDE‐Alb adducts and DNA damage was investigated using a logistic regression model with adjustment for possible confounders (employment time, pack‐years, and alcohol users). p<0.05 was set as the criterion for the significance of a test.
Results
Airborne PAHs monitoring
As shown in table 1, the results of airborne monitoring showed that the median value of the sum of eight carcinogenic PAHs in the air of the work places was 0.852 μg/m3 (range 0.259–3.330 μg/m3) for the exposed group and 0.306 μg/m3 (range 0.233–0.350 μg/m3) for the control group, the difference was statistically significant (p = 0.008). The airborne median level of B[a]P alone was 0.100 μg/m3 (range 0.028–0.360 μg/m3) for the exposed group and 0.040 μg/m3 (range 0.020–0.060 μg/m3) for the control group, the difference was also statistically significant (p = 0.014).
Table 1 Selected characteristics of the exposed and control groups in a steel plant in northern China.
| Variables | Control group | Exposed group | p Value |
|---|---|---|---|
| Number of subjects | 102 | 207 | |
| Age (years, mean (SD)) | 37.0 (5.0) | 37.5 (6.4) | 0.471* |
| Employment time (years, mean (SD)) | 16.4 (6.3) | 15.9 (7.5) | 0.565* |
| Current smokers, yes (%) | 80 (78.4%) | 166 (80.2%) | 0.719** |
| Pack‐years (years, mean (SD)) | 13.0 (14.4) | 15.6 (14.0) | 0.037*** |
| Alcohol users, yes (%) | 50 (49.0%) | 104 (50.2%) | 0.903** |
| Eight carcinogenic PAHs (median levels, range) | 0.306 (0.233–0.350) | 0.852 (0.259–3.330) | 0.008*** |
| B[a]P (median levels, range) | 0.040 (0.020–0.060) | 0.100 (0.028–0.360) | 0.014*** |
*Student t test for difference in quantitative measurements between the exposed and control groups.
**χ2 tests for differences in the distribution between the exposed and control groups.
***Mann‐Whitney U test for differences between the exposed and control groups.
Main characteristics of study subjects
The characteristics of the exposed and control groups are summarised in table 1. The distributions of age and employment time were not significantly different between the exposed and control groups, nor were percentages of current smokers and alcohol users between the two groups as a result of frequency matching. The subjects in the exposed group had smoked more pack‐years than that in the control group (15.6 (14.0) vs 13.0 (14.4) pack‐years, p = 0.037).
Concentrations of BPDE‐Alb adduct
BPDE‐Alb adduct concentrations between the exposed and control groups are shown in table 2. The median concentration of BPDE‐Alb adducts in exposed group (34.36, 25th–75th: 10.89–64.48 fmol/mg albumin) was significantly higher than those in control group (21.90, 5.02–46.52 fmol/mg albumin, p = 0.012). The effect of occupational exposure to COE on BPDE‐Alb adducts was assessed using a logistic regression model with adjustment for possible confounders (that is, employment time, pack‐years, and alcohol users) are shown in table 3. For the exposed group, the odds ratio of having higher BPDE‐Alb adducts compared to the control group was 1.72 (95% CI 1.06–2.81, p = 0.028). No significant modification effects of any confounders in the logistic regression model were found as assessed by the analysis of interactions.
Table 2 Comparison of BPDE‐Alb adduct concentrations and Olive tail moment levels between the exposed and control groups.
| Variables | Control group | Exposed group | p Value |
|---|---|---|---|
| BPDE‐Alb adduct concentrations(fmol/mg, median, 25th–75th) | 21.90 (5.02–46.52) | 34.36 (10.69–64.48) | 0.012* |
| Olive TM levels (mean (SD))† | 0.63 (0.93) | 1.20 (1.10) | 0.000** |
*Mann‐Whitney U test for differences between the exposed and control groups.
**Student t test for difference between the exposed and control groups.
†Calculated from ln‐transformed values.
Table 3 Multivariate logistic regression analysis of BPDE‐Alb adduct concentrations and Olive tail moment levels with adjustment for confounding factors.
| Variables included in the model | BPDE‐Alb adduct concentrations* | Olive TM levels† | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Exposure to COE‡ | 1.72 (1.06 to 2.81) | 0.028 | 1.96 (1.20 to 3.19) | 0.007 |
| Employment time§ | 1.08 (0.65 to 1.77) | 0.774 | 0.96 (0.58 to 1.58) | 0.866 |
| Pack‐years¶ | 1.31 (0.78 to 2.19) | 0.302 | 0.81 (0.48 to 1.35) | 0.414 |
| Alcohol users** | 0.92 (0.58 to 1.45) | 0.709 | 0.79 (0.50 to 1.25) | 0.306 |
*BPDE‐Alb adduct concentrations, >28.64 (1), ⩽28.64 (0).
†Olive tail moment levels (calculated from ln‐transformed values), >1.01 (1), ⩽1.01 (0).
‡COE exposed group (1), control group (0).
§Employment time: >16.0 (1), ⩽16.0 (0).
¶Pack‐years: >15.6 (1), ⩽15.6 (0).
**Alcohol users: Yes (1), No (0).
Olive tail moment levels in the lymphocytes
The levels of DNA damage were detected by the comet assay, measured as Olive TM. The mean Olive TM levels (natural logarithm transformed values, shown in table 2) in the exposed group (1.20 (1.10)) was significantly higher than that of the control group (0.63 (0.93), p = 0.000). The OR for COE exposure associated with Olive TM levels was also assessed using a logistic regression model with adjustment for possible confounders (that is, employment time, pack‐years, and alcohol users). The COE exposure was associated with a significantly increased risk of having high Olive TM levels (OR 1.96; 95% CI 1.20 to 3.19, p = 0.007, table 3).
Correlations between BPDE‐Alb adduct concentrations and Olive tail moment levels
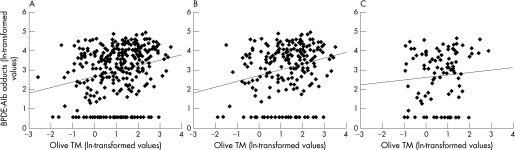
We also assessed the relationbetween the BPDE‐Alb adduct concentrations and the Olive TM levels using the Spearman rank correlation analysis (fig 1). There was a significantly positive correlation between the BPDE‐Alb adduct concentrations and the Olive TM in all subjects (Spearman's correlation, r = 0.235; p = 0.000; fig 1A), a significantly positive correlation in the exposed group (r = 0.235, p = 0.001; fig 1B), but no significant correlation in control group (r = 0.093, p = 0.353; fig 1C).
Figure 1 Scatter plotting and fitted regression line between ln‐transformed BPDE‐Alb adduct concentrations and ln‐transformed Olive TM levels (Spearman rank test). (A) in all subjects (r = 0.235, p = 0.000, n = 309); (B) in exposed group (r = 0.235, p = 0.001, n = 207); (C) in control group (r = 0.093, p = 0.353, n = 102).
Discussion
Our results showed that the exposure to COE in the workplace resulted in a significant increase in the concentrations of BPDE‐Alb adducts in the exposed group compared with the control group, and this increase was not affected by employment time, pack‐years and alcohol users.
The finding of an increase in BPDE‐Alb adducts in workers due to exposure to COE in present study is consistent with other investigations,34,35,36 although Omland et al37 and Kure et al38 showed no statistical significance in the levels of B[a]P‐albumin adducts between the exposed and control groups and no contribution of occupational exposure to COE to the formation of adducts. In addition, some results from other studies also showed that the BPDE protein adducts in workers exposed to COE were not affected by smoking,34,37,39 probably because the exposure to COE in coke oven workers is overwhelmingly high compared with exposure to smoking. For example, Rustemeier et al40 reported that B[a]P concentration in mainstream cigarette smoke is 5.10 (0.31) ng/cigarette, which is very low compared with our airborne biomonitoring data for the exposed group at the steel plant. Nevertheless, the reasons for the reported inconsistency may be due to the size of samples, methods of sample collection and detection, and the complexity of working environments.
We found that the Olive TM levels in the lymphocytes were significantly higher in the exposed group than in the control group after adjustment for smoking and other confounding factors. These results suggest that exposure to COE is an independent contributor for DNA damage in the exposed group. Some previous studies showed that there were significantly more DNA strand breaks in the exposed group than in the control group,19,20,21,23 but others performed under same alkaline conditions did not,22,24 which may be due to the sample size (the totals were 100 and 72, respectively) and the complexity of working environments (no airborne PAHs monitoring data were available).
Very interestingly, our results showed that there was a significant correlation between the concentrations of BPDE‐Alb adduct and Olive TM levels in the exposed group but not in control group, and, apparently, the positive correlation in all subjects was due to the contribution from the exposed group. Zhang et al26 reported a weak significant correlation (r = 0.19, p = 0.03) between the effect biomarker (8‐oxo‐7, 8‐dihydro‐2'‐deoxyguanosine) and the biologically effective dose biomarker (aromatic DNA adducts) among 119 coke oven workers and 38 controls. Our results showed that the concentrations of BPDE‐Alb adducts were correlated to the levels of DNA damage and that there was an association between exposure to COE and the levels of DNA damage in coke oven workers. The possible reasons of significant but relatively weak correlation between these two biomarkers in the exposed group are as follows: (1) biomarkers of exposure and effect are parameters that can be measured at different time windows, and there may be a time lag between exposure and effect. (2) BPDE‐Alb adduct may be chemical‐specific and caused by the metabolic activation of B[a]P. B[a]P is just one genotoxic substance in a complex mixture of PAHs. Biomarkers of effect such as DNA damage may be non‐specific as other PAHs may be responsible for the DNA damage. This is because other PAHs resulted in the formation of DNA damage was not detectable through BPDE‐Alb adducts or both B[a]P and other PAHs had caused detectable DNA damage in lymphocytes. (3) The genetic polymorphisms that cause variation in enzyme activities of B[a]P metabolism may be involved in the large variation of BPDE‐Alb adduct.2,11,24,36 Furthermore, variation in many DNA repair genes may also contribute to the difference of DNA damage levels.8,20 As for no statistically significant correlation in the control group, a possible explanation was that other factors such as smoking, diet, cooking and other environmental air pollution might contribute more to the levels of DNA damage than the low or undetectable exposure levels at the workplace.
In conclusion, these data reinforce the notion that plasma BPDE‐Alb adducts and DNA damage in peripheral blood lymphocytes are useful exposure and effect biomarkers to monitor COE in the workplace. A positive correlation between the levels of plasma BPDE‐Alb adducts and DNA damage levels suggests that the formation of early biological effects (DNA damage) was associated with biologically effective dose (plasma BPDE‐Alb adduct), which may provide a link between exposure to COE and the mechanisms of BPDE‐induced carcinogenesis. The two types of biomarkers evaluated here should provide guidance in the inclusion of such biomarkers in specific epidemiological studies in these workers. Although there were significant differences of adducts and DNA damage between the control and exposed groups, the background levels of protein adducts and DNA damage in the two groups were rather high, so it will be necessary to validate this result using a larger sample size or more sensitive methods in the future. Furthermore, our attempt to elucidate these correlations may be limited by the complexity of the COE, inter‐individual genetic variations in key enzymes related to metabolism of PAHs and DNA repair, and the influence of various uncontrolled confounding factors in the working and living environments.
Main message
Increased concentrations of BPDE‐Alb adducts are significantly positively related to lymphocyte DNA damage levels in workers exposed to coke oven emissions.
Policy implication
Both BPDE‐Alb adducts and DNA damage are useful for monitoring occupational exposure to coke oven emissions.
Acknowledgements
We thank all individuals who volunteered to participate in this study and the members of the medical personnel of Centers for Disease Control and Prevention, Taiyuan Steel and Iron Limited Co.
Abbreviations
B[a]P - benzo[a]pyrene
BPDE - 7,8‐dihydroxy‐9,10‐epoxy‐7,8,9,10‐tetrahydrobenzo[a]pyrene
BPDE‐Alb adduct - benzo[a]pyrene‐r‐7, t‐8, t‐9,c‐10‐tetrahydrotetrol‐albumin adduct
COE - coke oven emissions
IARC - International Agency for Research on Cancer
Olive TM - Olive tail moment
PAHs - polycyclic aromatic hydrocarbons
Footnotes
Funding: This study was partly supported by National Key Basic Research and Development Program of China (No 2002CB512905) and the National Natural Science Foundation of China (No 30371204 and 30525031).
Competing interests: None.
Ethics approval: The research protocol was approved by the Ethics and Human Subject Committee of Tongji Medical College.
References
- 1.World Health Organization International Agency for Research on Cancer Polycyclic aromatic hydrocarbons. Industrial exposure in aluminium production, coal gasification, coke production, and iron and steel founding. IARC Monographs 198434 [Google Scholar]
- 2.World Health Organization International Agency for Research on Cancer IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans. IARC Monographs 19877(Suppl) [Google Scholar]
- 3.World Health Organization International Agency for Research on Cancer Polynuclear aromatic compounds. Chemical, environmental and experimental data. IARC Monographs 198332 [PubMed] [Google Scholar]
- 4.World Health Organization International Agency for Research on Cancer Polynuclear aromatic compounds. Industrial exposures in aluminium production, coal gasification, coke production, and iron and steel founding. IARC Monographs,198334 [Google Scholar]
- 5.Day B W, Skipper P L, Zaia J.et al Enantiospecificity of covalent adduct formation by benzo[a]pyrene anti‐diol epoxide with human serum albumin. Chem Res Toxico 19947829–835. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen M, Autrup H, Moller P.et al Linking exposure to environmental pollutants with biological effects. Mutat Res 2003544255–271. [DOI] [PubMed] [Google Scholar]
- 7.Rojas M, Alexandrov K, van Schooten F J.et al Validation of a new fluorometric assay for benzo[a]pyrene diolepoxide‐DNA adducts in human white blood cells: comparisons with 32P‐postlabeling and ELISA. Carcinogenesis 199415557–560. [DOI] [PubMed] [Google Scholar]
- 8.Pastorelli R, Cerri A, Mezzetti M.et al Effect of DNA repair gene polymorphisms on BPDE‐DNA adducts in human lymphocytes. Int J Cancer 20021009–13. [DOI] [PubMed] [Google Scholar]
- 9.Hemminki K, Koskinen M, Zhao C. DNA adducts as a marker for cancer risk? Int J Cancer 200192923–926. [DOI] [PubMed] [Google Scholar]
- 10.Meyer M J, Bechtold W E. Protein adduct biomarkers: state of the art. Environ Health Perspect 1996104879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Autrup H, Vestergaard A B, Okkels H. Transplacental transfer of environmental genotoxins: polycyclic aromatic hydrocarbon‐albumin in non‐smoking women, and the effect of maternal GSTM1 genotype. Carcinogenesis 1995161305–1309. [DOI] [PubMed] [Google Scholar]
- 12.Conney A H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res 1982424875–4917. [PubMed] [Google Scholar]
- 13.Frenkel K. Carcinogen‐mediated oxidant formation and oxidative DNA damage. Pharmacol Ther 199253127–166. [DOI] [PubMed] [Google Scholar]
- 14.Pryor W A. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect 1997105(Suppl 4)875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairbairn D W, Olive P L, O'Neill K L. The comet assay: a comprehensive review. Mutat Res 199533937–59. [DOI] [PubMed] [Google Scholar]
- 16.Mussali‐Galante P, Avila‐Costa M R, Pinon‐Zarate G.et al DNA damage as an early biomarker of effect in human health. Toxicol Ind Health 200521155–166. [DOI] [PubMed] [Google Scholar]
- 17.Kassie F, Parzefall W, Knasmuller S. Single cell gel electrophoresis assay: a new technique for human biomonitoring studies. Mutat Res 200046313–31. [DOI] [PubMed] [Google Scholar]
- 18.Tice R R, Agurell E, Anderson D.et al Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 200035206–221. [DOI] [PubMed] [Google Scholar]
- 19.Popp W, Vahrenholz C, Schell C.et al DNA single strand breakage, DNA adducts, and sister chromatid exchange in lymphocytes and phenanthrene and pyrene metabolites in urine of coke oven workers. Occup Environ Med 199754176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng S, Cheng J, Pan Z.et al Associations between XRCC1 and ERCC2 polymorphisms and DNA damage in peripheral blood lymphocyte among coke oven workers. Biomarkers 20049395–406. [DOI] [PubMed] [Google Scholar]
- 21.Marczynski B, Rihs H P, Rossbach B.et al Analysis of 8‐oxo‐7,8‐dihydro‐2'‐deoxyguanosine and DNA strand breaks in white blood cells of occupationally exposed workers: comparison with ambient monitoring, urinary metabolites and enzyme polymorphisms. Carcinogenesis 200223273–281. [DOI] [PubMed] [Google Scholar]
- 22.Siwinska E, Mielzynska D, Kapka L. Association between urinary 1‐hydroxypyrene and genotoxic effects in coke oven workers. Occup Environ Med 200461e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao C, Chen S, Li J.et al Association of HSP70 and genotoxic damage in lymphocytes of workers exposed to coke‐oven emission. Cell Stress Chaperones 20027396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Delft J H, Steenwinkel M S, van Asten J G.et al Biological monitoring the exposure to polycyclic aromatic hydrocarbons of coke oven workers in relation to smoking and genetic polymorphisms for GSTM1 and GSTT1. Ann Occup Hyg 200145395–408. [PubMed] [Google Scholar]
- 25.Wu M T, Pan C H, Chen C Y.et al Lack of modulating influence of GSTM1 and GSTT1 polymorphisms on urinary biomonitoring markers in coke‐oven workers. Am J Ind Med 200446112–119. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Ichiba M, Hanaoka T.et al Leukocyte 8‐hydroxydeoxyguanosine and aromatic DNA adduct in coke‐oven workers with polycyclic aromatic hydrocarbon exposure. Int Arch Occup Environ Health 200376499–504. [DOI] [PubMed] [Google Scholar]
- 27.National Institute of Occupational Safety, Health (NIOSH) Polynuclear aromatic hydrocarbons by HPLC method 5506. In: NIOSH manual of analytical methods. Fourth edition. Cincinnati, OH: US Department of Health and Human Services, 1998
- 28.Frank S, Renner T, Ruppert T.et al Determination of albumin adducts of (+)‐anti‐benzo[a]pyrene‐diol‐epoxide using an high‐performance liquid chromatographic column switching technique for sample preparation and gas chromatography‐mass spectrometry for the final detection. J Chromatogr B Biomed Sci Appl 1998713331–337. [DOI] [PubMed] [Google Scholar]
- 29.Islam G A, Greibrokk T, Harvey R G.et al HPLC analysis of benzo[a]pyrene‐albumin adducts in benzo[a]pyrene exposed rats. Detection of cis‐tetrols arising from hydrolysis of adducts of anti‐ and syn‐BPDE‐III with proteins. Chem Biol Interact 1999123133–148. [DOI] [PubMed] [Google Scholar]
- 30.Singh N P, McCoy M T, Tice R R.et al A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988175184–191. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Z, Zhuang Z, Huang Y.et al Design and application of image analysis system to single cell gel electrophoresis. [In Chinese]. Chin J Ind Hyg Occup Dis 200119298–300. [Google Scholar]
- 32.Olive P L, Banath J P, Durand R E. Heterogeneity in radiation‐induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res 199012286–94. [PubMed] [Google Scholar]
- 33.Hornung R W, Reed L D. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990546–51. [Google Scholar]
- 34.Sherson D, Sabro P, Sigsgaard T.et al Biological monitoring of foundry workers exposed to polycyclic aromatic hydrocarbons. Br J Ind Med 199047448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tas S, Buchet J P, Lauwerys R. Determinants of benzo[a]pyrene diol epoxide adducts to albumin in workers exposed to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health 199466343–348. [DOI] [PubMed] [Google Scholar]
- 36.Pastorelli R, Cerri A, Minoia C.et al Benzo(a)pyrene diolepoxide adducts to albumin in workers exposed to polycyclic aromatic hydrocarbons: association with specific CYP1A1, GSTM1, GSTP1 and EHPX genotypes. Biomarkers 20016357–374. [DOI] [PubMed] [Google Scholar]
- 37.Omland O, Sherson D, Hansen A M.et al Exposure of iron foundry workers to polycyclic aromatic hydrocarbons: benzo(a)pyrene‐albumin adducts and 1‐hydroxypyrene as biomarkers for exposure. Occup Environ Med 199451513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kure E H, Andreassen A, Ovrebo S.et al Benzo(a)pyrene‐albumin adducts in humans exposed to polycyclic aromatic hydrocarbons in an industrial area of Poland. Occup Environ Med 199754662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozbal C C, Skipper P L, Yu M C.et al Quantification of (7S,8R)‐dihydroxy‐(9R,10S)‐epoxy‐7,8,9,10‐tetrahydrobenzo[a]pyrene adducts in human serum albumin by laser‐induced fluorescence: implications for the in vivo metabolism of benzo[a]pyrene. Cancer Epidemiol Biomarkers Prev 20009733–739. [PubMed] [Google Scholar]
- 40.Rustemeier K, Stabbert R, Haussmann H J.et al Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem Toxicol 20024093–104. [DOI] [PubMed] [Google Scholar]