Abstract
Background
Farming has been associated with respiratory symptoms and with protection against atopy. To date, effects of organic farming on respiratory health have not been studied.
Aims
To (1) compare hay fever and asthma‐like symptoms in organic and conventional farmers and (2) assess associations between current and childhood farm exposures and respiratory health effects by conducting a survey.
Methods
Questionnaire data from 1205 conventional and 593 organic farmers were evaluated. Associations between health effects and farm exposures were assessed by logistic regression analyses.
Results
Organic farmers reported less wheezing with shortness of breath and slightly more hay fever than conventional farmers. However, organic farming was not an independent determinant of hay fever when adjusted for farming practices and potential confounders. Livestock farmers who grew up on a farm had a threefold lower prevalence of hay fever than crop farmers without a farm childhood (odds ratio (OR) 0.3, 95% confidence interval (CI) 0.1 to 0.5). Both crop farmers who grew up on a farm and livestock farmers who did not grow up on a farm had a reduced prevalence, although less pronounced and not statistically significant. Use of disinfectants containing quaternary ammonium compounds was positively related to hay fever (OR 2.1, 95% CI 1 to 4.4). No effects of farming practices were found for asthma.
Conclusions
Our study adds to the evidence that a farm childhood in combination with current livestock farming protects against allergic disorders. This effect was found for both organic and conventional farmers.
Farmers have been shown to have an increased risk of respiratory diseases, including chronic obstructive pulmonary disease, accelerated lung function decline and organic dust toxic syndrome.1,2,3,4,5 Conversely, living on a farm during childhood has been associated with a reduced risk for atopic sensitisation, allergic asthma and hay fever as shown in children, adolescents and young adults.6,7,8,9,10,11,12,13 Recent studies on adult farmers have shown that protection against atopy and atopic asthma may continue into adulthood.14,15,16 Exposure to livestock and microbial agents, in particular bacterial endotoxin, has been suggested to have a critical role. Current exposure to endotoxins may also protect highly exposed adult pig farmers against atopic sensitisation.17 In the same study, however, endotoxin exposure was also positively associated with respiratory symptoms, bronchial hyper‐responsiveness and a lower lung function.
To date, effects of organic farming on respiratory health have not been studied. In The Netherlands, organic farming has grown considerably in the past few years,18 and a similar development has occurred in other Western countries. The principal features that distinguish organic farming from conventional farming are complete rejection of the use of chemical pesticides, artificial fertilisers and genetically modified organisms.19 Organic livestock housing must meet strict criteria, and livestock farmers are not allowed to carry out practices such as tail docking, cutting of teeth and dehorning unless it is necessary for safety or welfare reasons. Furthermore, certain disinfectants with established respiratory and immunological effects such as chloramine‐T and quaternary ammonium compounds (QACs) are prohibited.19 Consequently, organic and conventional farmers are likely to have different exposure patterns for both chemical and biological compounds. In addition, organic farmers may also differ from conventional farmers in socioeconomic status and lifestyle factors such as education, farm childhood and diet. Some organic farmers may have also adopted an anthroposophic lifestyle, which has been associated with lower occurrence of childhood atopy.13,20
In this study, we conducted a questionnaire survey to compare the prevalence of respiratory symptoms among adult organic farmers and conventional farmers. Symptoms in farmers were compared with a general non‐farming population. Furthermore, we investigated to what extent farming exposures during childhood combined with current farming practices affect the prevalence of asthma‐like symptoms and hay fever in organic and conventional farmers.
Methods
Study population
Questionnaires for both principal farm operators and also their spouses when working on the farm were sent to 1013 organic and 1846 conventional farms in March 2001. Organic farms were selected from the records of the inspection body for organic production in The Netherlands (Skal, Zwolle, The Netherlands). All companies certified by Skal were selected, excluding those owned by a non‐profit organisation, those in transition from conventional to organic production and those processing only agricultural products. Conventional farms were selected using a commercial database (Prosu, Dronten, The Netherlands). Questionnaires were sent to all mid‐sized pig farms (100–200 sows or 400–600 finishing pigs; n = 617), dairy farms (around 50 cows; n = 647) and crop farms (between 22 and 45 ha; n = 582) from four central and eastern provinces.
A major outbreak of foot and mouth disease occurred a few weeks after the questionnaire had been mailed,21 which affected our response rates. Between April and June 2001, we attempted to remind all farmers by telephone. Those who declined to complete the questionnaire were requested to answer a limited number of questions on respiratory symptoms and allergy to test for non‐response bias. However, telephone reminders were not effective as many farmers were distressed about the foot and mouth epidemic and unwilling to cooperate. Therefore, telephone reminders were limited to all organic farmers and approximately half of the conventional farmers (mainly crop farmers). Of the 2859 farm owners initially approached, 96 replied that they had left farming and were excluded from the study. A total of 1923 questionnaires were returned, 1054 by principal farmers (response rate 38.1%) and 869 by spouses who were working on the farm. Hereafter, all responders are called farmers. Response rates for organic and non‐organic farms were comparable (37.9% and 38.3%, respectively). Responders were excluded if they were aged >70 years (n = 12), or if their production was only partly organic, or in transition from conventional to organic production (n = 113). Altogether, data from 1798 farmers (975 men and 823 women) were used for statistical analysis.
Of these, 1557 enrolled before the telephone reminder and 241 responded after the reminder. Another 105 farmers answered a few questions on symptoms by telephone. Information on lack of response was obtained from 168 farms, either by telephone or because the questionnaire was returned with a reason for lack of response. The main reasons for not participating included a lack of time or lack of interest. The foot and mouth epidemic was another commonly mentioned reason for not participating.
Items regarding general respiratory symptoms were compared with data from the Dutch part of the European Community Respiratory Health Survey (ECRHS).22,23 In The Netherlands, subjects aged 20–70 years were investigated (those aged 20–44 years were included in the ECRHS). Twenty nine agricultural workers and three subjects aged >70 years were excluded from this general population sample, and data on the remaining 2679 subjects were used.
Questionnaire
The questionnaire consisted of questions on respiratory symptoms, family history of asthma or allergy, personal characteristics, occupational characteristics and smoking habits. The questionnaire for the principal farmer also included questions about farm characteristics. Questions on respiratory symptoms were adopted from the Dutch version of the ECRHS Questionnaire.22,24 According to the ECRHS definition, asthma was defined as a positive response to any of the following questions: “Have you had an attack of asthma in the last 12 months?”, “Have you been woken by an attack of shortness of breath at any time in the last 12 months?” and “Are you currently taking any medicine for asthma?24 A positive response to the question “ Have you ever had any allergies?” was followed by a list of possible allergens (house dust, food, animals, pollen or others) and symptoms (sneezing or runny nose, dyspnoea, itchy skin, or itchy or watery eyes). Hay fever was defined as self‐reported pollen allergy accompanied by itchy or watery eyes or sneezing.
Statistical analysis
Data were analysed using SAS statistical software V.8.2. The crude prevalence of respiratory symptoms was compared between early and late responders (those who returned a questionnaire after the telephone reminder or answered a limited number of questions by telephone). The late response group included more organic farmers and more crop farmers, therefore when comparing early and late responses, weighted prevalences were also calculated to correct for differences between the initial sample and late‐response group—that is, the prevalence of symptoms among organic/conventional farmers or crop/livestock farmers in the late‐response group was given the weight of their proportion in the early‐response group. The prevalence of respiratory symptoms was compared between farmers and a general non‐farming Dutch population sample by logistic regression analysis, adjusting for age, sex and smoking habits. In further analyses, we studied farming characteristics in association with asthma and hay fever in univariate logistic regression models for organic farmers and conventional farmers separately, and for all farmers together. All variables that were associated with the outcome variables in the univariate models (p<0.2), along with age, sex and smoking habits, were included in the multiple regression models. As 97% of principal farmers were men, we only adjusted for sex and not for principal farmer/spouse status. We also repeated the analyses by using logistic regression with generalised estimating equations to adjust for possible correlation in each household (farm was included as cluster). Estimates and standard errors were not different from those obtained by the initial models. Variables not originally selected from the univariate models were added one at a time into the model to assess significance in the multiple regression models. Goodness of fit of the multiple regression models was assessed by the Hosmer–Lemeshow test.
Results
Table 1 summarises the demographic and farming characteristics of 1205 conventional and 593 organic farmers. Organic farmers were slightly younger, had been working as farmers for a shorter period of time, had a higher level of education and had lived on a farm during childhood less often than conventional farmers (p<0.05; t test, χ2 test). Hay fever in parents or siblings was reported more commonly by organic farmers (p<0.05; χ2 test), whereas the prevalence of asthma in parents or siblings was equal among both groups. In addition, differences existed between both farming populations with respect to farm type (livestock and crop types) and disinfectant use. Female farmers less often grew up on a farm than male farmers (59% v 91%), and 65% of women worked part time on the farm when compared with 21% of men.
Table 1 Demographic and farming characteristics of conventional farmers and organic farmers.
| Conventional farmers (n = 1205) | Organic farmers (n = 593) | |
|---|---|---|
| Male, n (%) | 658 (54.6) | 317 (53.5) |
| Mean (SD) age, years | 45.5 (9.7) | 44.2 (8.4) |
| Mean (SD) years of farm work | 21.8 (10.8) | 16.6 (9.9) |
| Education, n (%) | ||
| Low or medium | 965 (80.8) | 325 (55.5) |
| High (at least higher secondary education) | 229 (19.2) | 261 (44.5) |
| Smoking habits, n (%) | ||
| Current | 228 (19.1) | 91 (15.4) |
| Ever | 380 (31.8) | 210 (35.6) |
| Asthma in parents or siblings, n (%) | 193 (16.1) | 96 (16.3) |
| Hay fever in parents or siblings, n (%) | 204 (17.1) | 130 (22) |
| Childhood farming environment, n (%) | ||
| No | 202 (16.8) | 226 (38.1) |
| Crops only | 234 (19.4) | 80 (13.5) |
| Livestock | 769 (63.8) | 287 (48.5) |
| Current farm type, n (%) | ||
| Crops only | 428 (35.5) | 201 (33.9) |
| Livestock and crops | 137 (11.4) | 121 (20.4) |
| Livestock only | 640 (53.1) | 271 (45.7) |
| Farm childhood, adulthood livestock farming, n (%) | ||
| No, No | 86 (7.1) | 98 (16.5) |
| No, Yes | 116 (9.6) | 128 (21.6) |
| Yes, No | 342 (28.4) | 103 (17.4) |
| Yes, Yes | 661 (54.9) | 264 (44.6) |
| Livestock type, n (%) | ||
| Dairy | 558 (46.3) | 254 (42.8) |
| Pigs | 372 (30.9) | 43 (7.3) |
| Sheep | 79 (6.6) | 90 (15.2) |
| Poultry | 33 (2.7) | 47 (7.9) |
| Beef or veal | 33 (2.7) | 43 (7.3) |
| Goats | 8 (0.7) | 39 (6.6) |
| Crop type, n (%) | ||
| Arable farming | 548 (45.5) | 167 (28.2) |
| Horticulture | 120 (10) | 256 (43.2) |
| Disinfectant use, n (%) | ||
| QACs | 183 (15.2) | 34 (5.7) |
| Other disinfectants | 541 (44.9) | 197 (33.2) |
QACs, quaternary ammonium compounds.
Early or late response was not associated with the prevalence of self‐reported respiratory symptoms or allergy, both before and after adjustment for the proportion of organic farmers or crop farmers in the late response group (table 2; p>0.1, χ2 test).
Table 2 Prevalence of respiratory symptoms in 1557 farmers who responded early, and in 346 farmers who returned a questionnaire after the telephone reminder or answered a few questions by telephone (late‐response group).
| Early response | Late response | Late response* | Late response† | |
|---|---|---|---|---|
| Daily cough up phlegm | 8.6 | 9.0 | 9.1 | 9.4 |
| Woken due to shortness of breath | 2.1 | 3.2 | 3.6 | 2.4 |
| Wheezing | 11.5 | 11.1 | 11.8 | 10.0 |
| Episode of asthma last year | 1.8 | 2.1 | 2.4 | 1.6 |
| Use of drugs for asthma | 3.2 | 4.1 | 4.1 | 3.5 |
| Asthma (ECRHS) | 4.6 | 5.9 | 6.2 | 4.4 |
| Any allergy | 24.0 | 25.8 | 25.4 | 25.1 |
| Hay fever | 7.6 | 7.9 | 7.8 | 7.5 |
ECRHS, European Community Respiratory Health Survey.
p>0.1 for all symptoms; χ2 test.
Values are expressed in percentage.
*Weighted for proportion of organic farmers in early‐response group.
†Weighted for proportion of livestock farmers in early‐response group.
Mean age was comparable in farmers and in the general population (45.1 v 45.4 years), but among the general population there were more current smokers (36.7% v 17.9%) and women (49.7% v 45.8%). Farmers reported significantly less often that they had woken up due to cough or shortness of breath, wheezing, wheezing with shortness of breath, wheezing without a cold and asthma (adjusted odds ratios (OR) between 0.2 and 0.6; table 3). Adjustment for age, smoking habits and sex did not change the results. Almost all asthma symptoms were less prevalent in organic farmers than in conventional farmers. This difference was significant for waking up due to shortness of breath (OR 0.4, 95% confidence interval (CI) 0.2 to 0.9) and wheezing with shortness of breath (OR 0.7, 95% CI 0.4 to 1).
Table 3 Prevalence (%) and adjusted OR of respiratory symptoms between farmers (and farmer subpopulations) and a general non‐farming Dutch population sample of the European Community Respiratory Health Survey.
| General population (n = 2679), | All farmers (n = 1798) | Conventional farmers (n = 1205) | Organic farmers (n = 593) | ||||
|---|---|---|---|---|---|---|---|
| % | % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) | |
| Cough symptoms | |||||||
| Daily cough | 15.8 | 12.7 | 0.9 (0.8 to 1.1) | 13.1 | 0.9 (0.8 to 1.2) | 11.7 | 0.9 (0.7 to 1.2) |
| Daily cough up phlegm | 9.9 | 8.6 | 0.9 (0.7 to 1.1) | 8.5 | 0.9 (0.7 to 1.2) | 8.7 | 0.9 (0.7 to 1.3) |
| Woken due to cough | 32.0 | 17.1 | 0.5 (0.4 to 0.6) | 17.7 | 0.5 (0.4 to 0.6) | 16.1 | 0.5 (0.4 to 0.6) |
| Shortness of breath, wheezing | |||||||
| Woken due to SOB | 6.3 | 2.4 | 0.4 (0.3 to 0.5) | 2.9 | 0.4 (0.3 to 0.7) | 1.4 | 0.2 (0.1 to 0.4)* |
| Wheezing | 23.7 | 11.7 | 0.5 (0.4 to 0.6) | 12.3 | 0.5 (0.4 to 0.6) | 10.6 | 0.5 (0.4 to 0.6) |
| Wheezing with SOB | 15.7 | 7.7 | 0.5 (0.4 to 0.6) | 8.6 | 0.5 (0.4 to 0.7) | 5.8 | 0.4 (0.3 to 0.6)* |
| Wheezing without a cold | 13.0 | 5.5 | 0.4 (0.4 to 0.6) | 5.5 | 0.4 (0.3 to 0.6) | 5.3 | 0.4 (0.3 to 0.7) |
| Asthma | |||||||
| Asthma diagnosed by doctor | 4.7 | 5.5 | 1.0 (0.8 to 1.4) | 6.0 | 1.1 (0.8 to 1.6) | 4.4 | 0.9 (0.5 to 1.3) |
| Episode of asthma last year | 1.6 | 1.9 | 1.2 (0.7 to 1.9) | 2.1 | 1.3 (0.8 to 2.2) | 1.5 | 1.0 (0.5 to 2.2) |
| Use of drugs for asthma | 2.5 | 3.3 | 1.2 (0.8 to 1.7) | 3.8 | 1.4 (0.9 to 2.0) | 2.4 | 0.8 (0.5 to 1.6) |
| Asthma (ECRHS) | 7.6 | 4.7 | 0.6 (0.4 to 0.8) | 5.2 | 0.6 (0.5 to 0.9) | 3.9 | 0.5 (0.3 to 0.8) |
ECRHS, European Community Respiratory Health Survey; SOB, shortness of breath.
Data are presented as OR for farmers versus general population with 95% CI, adjusted for age, smoking habits and sex.
*Prevalence of symptoms differs significantly between conventional and organic farmers (p<0.05).
Hay fever was reported more commonly among organic farmers than among conventional farmers (9.3% v 6.9%), although this difference was only of borderline statistical significance (univariate analysis, table 4). Current livestock farming and childhood–farming environment (both crop and livestock) were inversely associated with hay fever, whereas hay fever heredity (hay fever in parents or siblings) strongly increased the risk. Asthma heredity and QAC or other disinfectant use were associated with an increased risk of asthma. Stratified analysis by conventional and organic farming showed only minor differences between organic and conventional farmers with regard to risk factors both for hay fever and asthma (table 4). The prevalence of hay fever was significantly lower in conventional pig farmers (OR 0.6, 95% CI 0.3 to 1), whereas this was not the case for the small group of organic pig farmers. Other specific types of livestock farming were not associated with hay fever or asthma in univariate analyses (data not shown).
Table 4 Univariate models for hay fever and asthma (ECRHS definition) in conventional farmers (n = 1205) and organic farmers (n = 593) (stratified analysis), and in all farmers (n = 1798).
| Hay fever, crude OR (95% CI) | Asthma, crude OR (95% CI) | |||||
|---|---|---|---|---|---|---|
| All farmers | Conventional farmers | Organic farmers | All farmers | Conventional farmers | Organic farmers | |
| Organic farming | ||||||
| No | 1 | 1 | — | — | ||
| Yes | 1.4 (1.0 to 2.0) | — | — | 0.7 (0.5 to 1.2) | — | — |
| Current farm type | ||||||
| Crops only | 1 | 1 | 1 | 1 | 1 | 1 |
| Livestock | 0.7 (0.5 to 1.0) | 0.7 (0.5 to 1.2) | 0.7 (0.4 to 1.2) | 1.5 (0.9 to 2.4) | 1.5 (0.8 to 2.6) | 1.5 (0.6 to 3.8) |
| Childhood farming environment | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Crops | 0.4 (0.3 to 0.7) | 0.5 (0.2 to 0.9) | 0.3 (0.1 to 1.0) | 0.5 (0.2 to 1.1) | 0.6 (0.2 to 1.6) | 0.2 (0.0 to 1.8) |
| Livestock | 0.4 (0.3 to 0.6) | 0.3 (0.2 to 0.6) | 0.5 (0.3 to 0.9) | 0.9 (0.6 to 1.5) | 1.0 (0.5 to 2.0) | 0.6 (0.3 to 1.5) |
| Disinfectant use | ||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 |
| QACs | 1.0 (0.6 to 1.8) | 1.2 (0.6 to 2.2) | 0.7 (0.2 to 2.9) | 1.9 (1.0 to 3.6) | 2.1 (1.0 to 4.4) | NE |
| Other disinfectants | 0.9 (0.6 to 1.3) | 0.8 (0.5 to 1.2) | 1.3 (0.8 to 2.4) | 1.6 (1.0 to 2.6) | 1.5 (0.8 to 2.7) | 1.7 (0.7 to 4.0) |
| Asthma in parents or siblings | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 1.9 (1.3 to 2.9) | 1.6 (0.9 to 2.7) | 2.6 (1.4 to 4.8) | 3.8 (2.4 to 6.0) | 4.5 (2.7 to 7.6) | 2.4 (0.9 to 5.9) |
| Hay fever in parents or siblings | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Yes | 4.7 (3.3 to 6.8) | 5.4 (3.4 to 8.5) | 3.7 (2.1 to 6.6) | 2.1 (1.3 to 3.3) | 1.8 (1.0 to 3.3) | 2.9 (1.2 to 6.7) |
NE, not estimable
– Not included (as it is not estimable in the subgroups)
QACs, quaternary ammonium compounds.
The association between organic farming and hay fever decreased after adjustment for confounders in a multiple logistic regression model (table 5). The same model confirmed that currently keeping livestock and growing up on a crop farm or livestock farm were associated with a two times lower prevalence of hay fever. Use of QACs was associated with a two times higher prevalence of hay fever. Farming characteristics were not significantly associated with asthma in the multiple regression model. Potential confounders such as level of education and working part‐time, and other farming characteristics such as pig farming were also considered in the multiple regression models, but none of these were related to hay fever or asthma and they were therefore not included in the final model. Hay fever and asthma were approximately four times more prevalent in farmers with a family history of hay fever or asthma. Female farmers had somewhat less hay fever and asthma than male farmers, but differences were not significant (OR 0.9, 95% CI 0.6 to 1.3 and OR 0.7, 95% CI 0.4 to 1.2, respectively). Removing family history variables, age, smoking habits or sex from the model did not influence the results. The Hosmer–Lemeshow test showed adequate fit for both models (p>0.2).
Table 5 Multiple logistic regression analysis of hay fever and asthma (European Community Respiratory Health Survey definition) in farmers (n = 1798).
| Hay fever, adjusted OR (95% CI) | Asthma, adjusted OR (95% CI) | |
|---|---|---|
| Organic farming | ||
| No | 1 | 1 |
| Yes | 1.2 (0.8 to 1.7) | 0.7 (0.4 to 1.2) |
| Current farm type | ||
| Crops only | 1 | 1 |
| Livestock | 0.5 (0.3 to 0.9) | 1 (0.5 to 2.2) |
| Childhood farming environment | ||
| No | 1 | 1 |
| Crops only | 0.5 (0.3 to 0.9) | 0.5 (0.2 to 1.2) |
| Livestock | 0.4 (0.3 to 0.7) | 0.6 (0.4 to 1.2) |
| Disinfectant use | ||
| None | 1 | 1 |
| QACs | 2.1 (1 to 4.4) | 1.7 (0.7 to 3.9) |
| Other disinfectants | 1.5 (0.9 to 2.8) | 1.4 (0.7 to 2.9) |
| Hay fever in parents or siblings | ||
| No | 1 | |
| Yes | 4.4 (3 to 6.3) | — |
| Asthma in parents or siblings | ||
| No | 1 | |
| Yes | — | 3.6 (2.3 to 5.7) |
QAC, quaternary ammonium compounds.
OR are adjusted for age, smoking habits, sex and all other variables in the model.
– Variable not included in the model.
Asthma‐like symptoms that differed significantly between organic and conventional farmers (waking up due to shortness of breath and wheezing with shortness of breath) were studied in the same multiple regression models. Only wheezing with shortness of breath was significantly less prevalent in organic farmers after adjustment for potential confounders (OR 0.6, 95% CI 0.4 to 0.9). Asthma heredity was a strong determinant for these and other asthma‐like symptoms, such as wheezing and asthma diagnosed by doctor (OR 3.1–4.2). No other clear determinants for asthma‐like symptoms were identified.
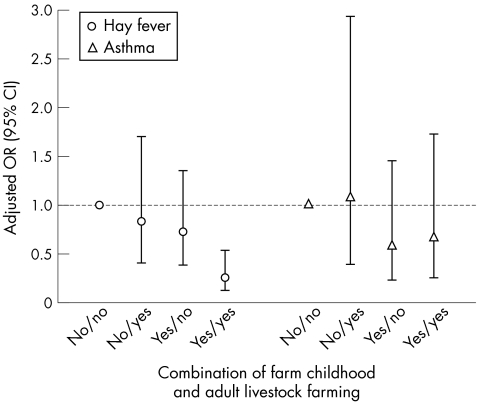
Finally, we investigated whether current farming practices and farm childhood were independently associated with respiratory or allergic outcomes. Figure 1 presents a regression model that includes a variable combining farm childhood (yes or no) and adulthood livestock farming (yes or no), along with all other variables that were included in the earlier model. Hay fever was significantly less prevalent in livestock farmers who grew up on a farm, compared with crop farmers who did not grow up on a farm (OR 0.3, 95% CI 0.1 to 0.5). The effect of farm childhood in combination with current livestock farming is stronger than the addition of both separate effects—that is, significant interaction (p<0.03) between farm childhood and current livestock farming. Living on a farm during childhood without adulthood livestock farming, and vice versa, was also associated with a lower prevalence of hay fever, but this was less pronounced and not statistically significant. No significant differences were found for asthma.
Figure 1 Odds ratios (OR; 95% CI) for hay fever and asthma (European Community Respiratory Health Survey definition) in farmers (n = 1798) by farm childhood and adult livestock farming. ORs are adjusted by logistic regression for age, smoking habits, sex, organic farming, disinfectant use and hay fever or asthma in family members.
Discussion
Organic and conventional farmers reported significantly less asthma‐like symptoms in comparison to a general Dutch population. The adjusted prevalence of wheezing with shortness of breath was lower in organic farmers than in conventional farmers, suggesting a lower risk of asthma‐like symptoms in organic farmers. In conventional and organic farmers with both a farm childhood and current livestock exposure, a strongly decreased prevalence of hay fever was seen, compared with crop farmers who did not grow up on a farm. The prevalence of hay fever seemed slightly higher in organic farmers than in conventional farmers. However, this difference disappeared after adjustment for other variables such as having lived on a farm during childhood, which was less often the case in organic farmers. Effects of farm childhood and farming characteristics on asthma and asthma‐like symptoms were small and mostly non‐significant.
A limitation of our study was the low response rate of <40%. An important reason for this low response was the foot and mouth disease epidemic that occurred shortly after the questionnaires had been mailed. The serious nature of the epidemic and the psychological effect this had on many of the study participants prevented us from conducting a more rigorous follow‐up that would involve multiple reminders and telephone interviews. The non‐response owing to this epidemic is unlikely to have significantly biased our results as it is not expected to be associated with asthma and/or hay fever symptoms in the participants. To test this, we compared early responders (n = 1557) with late responders and non‐responders who completed a few questions on asthma symptoms by telephone (n = 346). These results showed no significant differences in the prevalence of symptoms (table 2), suggesting that self‐selection and subsequent bias was indeed minimal. However, we cannot exclude the possibility that some bias has occurred because of lack of response. Nonetheless, this is unlikely to explain the protective effects observed in farmers compared with the general population as it implies that the high lack of response in the farming population would have led to an overestimation of the prevalence of symptoms in that population and consequently to an underestimation of the protective effect. In our study, non‐response bias would thus have resulted in a bias towards the null for the comparison between the farming and the general population, and the reported protective effects are therefore a conservative estimate. For bias to explain the differences in the prevalence of symptoms in the group of farmers, lack of response should have resulted in an overestimation of symptoms in crop farmers without a farm childhood (or an underestimation of symptoms in livestock farmers and farmers who grew up on a farm), which is unlikely. Similarly, it is unlikely that non‐response resulted in an overestimation of the prevalence of hay fever in those farmers who reported using disinfectants, particularly because, as mentioned above, there were no indications that symptom reports were different between early responders, and late responders and non‐responders.
Another limitation was that only self‐administered questionnaire data on health outcomes and farming exposures were used, and no objective data on dust, endotoxin, muramic acid, fungal glucans, ammonia, etc, were collected. On the other hand, self‐reported hay fever symptoms have been shown to be strongly associated with more objective skin‐prick tests or specific radio‐allergosorbent tests in Dutch populations,23,25 and questions on asthma and other respiratory symptoms were derived from the validated and widely used ECRHS Questionnaire. It could be argued that it may not be appropriate to use the ECRHS population as it was studied 9 years before the farming population. However, a more recent general population study on chronic diseases was undertaken in The Netherlands between 1993 and 1997, and only relatively small differences regarding the prevalence of asthma or chronic obstructive pulmonary disease symptoms were found between both population studies.26 Nevertheless, we cannot exclude the possibility that changes in the prevalence of respiratoy symptoms occurred in the Dutch population between 1997 and 2001, leading to underestimation or overestimation of the difference between farmers and the general population. The ECRHS Questionnaire was not specifically validated for farmers, but the same questionnaire has been used in many other occupational groups including both white‐collar and blue‐collar workers. Moreover, it has been suggested that farmers might under‐report symptoms.16 However, the evidence for this is very weak. Also, a recent study on farmers' and control children in Europe concluded that the reliability of questionnaire responses on asthma and wheezing was comparable between farmers' and control children.27
Use of disinfectants, particularly QACs, was an independent risk factor for hay fever, which is in accordance with an earlier study on Dutch pig farmers that showed a high prevalence of IgE sensitisation to common allergens in farmers using QACs.28 Atopic pig farmers who used QACs or were exposed to high levels of endotoxin had a higher risk of asthma‐like symptoms28; moreover, the use of QACs and other disinfectants was associated with mild bronchial hyper‐responsiveness.29 In our study, asthma was increased in farmers using QACs and other disinfectants; however, it did not seem to be a significant risk factor for asthma in the multiple regression analysis. As organic farmers are not allowed to use disinfectants such as QACs and chloramine‐T, they may be protected against disinfectant‐induced atopy or asthma. However, longitudinal studies are necessary to study such effects in more detail.
Interestingly, the reduced risk for hay fever was similar for farmers who were raised on a livestock farm or on a crop farm. Previous studies on farm children focused mainly on livestock farms, as increased bacterial endotoxin levels in house dust were found in households where children had regular contact with farm animals.6,30,31 Children from non‐farming families (with or without contact with livestock) served as reference groups; information on exposure for crop farmers and their children is therefore scarce. A comprehensive study among Dutch farmers and other agricultural workers has shown that endotoxin exposure levels >200 EU/m3 are often present during crop production,32 which might suggest that farmers who were raised on a crop farm benefited from microbial exposures during childhood, similar to those raised on a livestock farm. Nonetheless, we cannot rule out the possibility that farmers who were raised on a crop farm also had regular contact with livestock as a child. Recall bias regarding the type of farm during childhood could also have affected the association, although it is unlikely that the observed protection is entirely attributable to bias.
Our findings on hay fever confirm previous results of a Finnish study showing a similarly decreased OR for pollen sensitisation in women who lived on a farm during childhood.14 A study among adult Norwegian farmers showed a reduced prevalence of atopic asthma in farmers with two or more types of livestock, whereas non‐atopic asthma was increased in pig farmers and in farmers with two or more types of livestock.15 In the same study, exposure to endotoxin and fungal spores during farm work was inversely associated with atopic asthma and positively with non‐atopic asthma. In our study, we observed a negative association between hay fever, an atopic condition, and livestock farming, confirming these results to a certain extent, but as serum IgE or skin‐prick test data were not available, we could not differentiate between atopic and non‐atopic asthma. In this study, only 21 of 85 farmers with asthma reported hay fever, which suggests that non‐allergic asthma may be quite common in our farming population. However, as the numbers are too small, we did not calculate ORs for asthma after stratifying for reported hay fever. In the Norwegian study, 80% of the farmers with asthma had a non‐atopic phenotype, which the authors attributed to high levels of microbial exposures such as endotoxins that can cause non‐allergic, non‐eosinophilic inflammatory responses leading to reversible airway obstruction.33,34 These findings are supported by a recent study on Swedish farmers that found an increased risk for adult‐onset asthma despite a low prevalence during childhood.35
Farmers with respiratory health problems may seek a job outside agriculture leading to a “healthy farmer effect”, which could (at least partly) explain the differences between farmers and the general population in our and other similar studies.36,37 Similarly, young adults with asthma or allergy may not take over their parents' farm, and adults without a farm childhood may only start farming if they have no health problems.38,39 To what extent such selection processes play a part in this study is difficult to establish, but a healthy farmer effect and selection into the population over multiple generations cannot be excluded. Nonetheless, differential avoidance of livestock farming because of hay fever does not seem to be likely—that is, in our study 23 principal farmers reported that they had changed production of their farm because of respiratory health reasons, and only three of them (two livestock farmers and one crop farmer) had hay fever. Farmers who grew up on a farm less often had a family history of hay fever (16.7% v 25.1%), which suggests that selection processes over time might have occurred. However, farm childhood was a protective factor for hay fever independently of family history. Thus, our data seem to support the growing evidence that a farm childhood continues to protect against atopic disease in adult farmers.
Main messages
Wheezing with shortness of breath was less prevalent in organic farmers than in conventional farmers, suggesting a lower risk of asthma‐like symptoms in organic farmers.
The prevalence of hay fever is strongly decreased in conventional and organic livestock farmers who have lived on a farm during childhood compared with crop farmers without a farm chidhood.
Policy implications
Although farmers may be protected against allergies and hay fever, it is well known that high exposures to organic dust and pesticides may cause chronic respiratory health effects. The results of our study and those of others showing protective effects of farming should therefore not be used as an argument to relax existing policies to lower current exposure levels in the farming environment.
In conclusion, a lower prevalence of asthma‐like symptoms was found in organic farmers than in conventional farmers. Living on a farm during childhood, combined with current livestock farming, is associated with a lower prevalence of hay fever in both conventional and organic farmers.
Acknowledgements
We thank all farmers for their participation. We are grateful to Mirjam Matze (Louis Bolk Institute, Driebergen, The Netherlands) for advice, and to the Department of Epidemiology, University of Groningen, for providing data from the Dutch contribution to the European Community Respiratory Health Survey.
Abbreviations
ECRHS - European Community Respiratory Health Survey
QACs - quaternary ammonium compounds
Footnotes
Funding: JD is supported by a Sir Charles Hercus Research Fellowship from the Health Research Council of New Zealand.
Competing interests: None.
References
- 1.Dalphin J C, Dubiez A, Monnet E.et al Prevalence of asthma and respiratory symptoms in dairy farmers in the French province of the Doubs. Am J Respir Crit Care Med 19981581493–1498. [DOI] [PubMed] [Google Scholar]
- 2.Danuser B, Weber C, Kunzli N.et al Respiratory symptoms in Swiss farmers: an epidemiological study of risk factors. Am J Ind Med 200139410–418. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang P F, van der Gulden J W, Folgering H.et al Endotoxin exposure as a major determinant of lung function decline in pig farmers. Am J Respir Crit Care Med 199815715–18. [DOI] [PubMed] [Google Scholar]
- 4.Monso E, Magarolas R, Radon K.et al Respiratory symptoms of obstructive lung disease in European crop farmers. Am J Respir Crit Care Med 20001621246–1250. [DOI] [PubMed] [Google Scholar]
- 5.Rask‐Andersen A. Organic dust toxic syndrome among farmers. Br J Ind Med 198946233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riedler J, Braun‐Fahrlander C, Eder W.et al Exposure to farming in early life and development of asthma and allergy: a cross‐sectional survey. Lancet 20013581129–1133. [DOI] [PubMed] [Google Scholar]
- 7.Von Ehrenstein O S, Von Mutius E, Illi S.et al Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy 200030187–193. [DOI] [PubMed] [Google Scholar]
- 8.Braback L, Hjern A, Rasmussen F. Trends in asthma, allergic rhinitis and eczema among Swedish conscripts from farming and non‐farming environments. A nationwide study over three decades. Clin Exp Allergy 20043438–43. [DOI] [PubMed] [Google Scholar]
- 9.Ernst P, Cormier Y. Relative scarcity of asthma and atopy among rural adolescents raised on a farm. Am J Respir Crit Care Med 20001611563–1566. [DOI] [PubMed] [Google Scholar]
- 10.Kilpelainen M, Terho E O, Helenius H.et al Childhood farm environment and asthma and sensitization in young adulthood. Allergy 2002571130–1135. [DOI] [PubMed] [Google Scholar]
- 11.Portengen L, Sigsgaard T, Omland O.et al Low prevalence of atopy in young Danish farmers and farming students born and raised on a farm. Clin Exp Allergy 200232247–253. [DOI] [PubMed] [Google Scholar]
- 12.Leynaert B, Neukirch C, Jarvis D.et al Does living on a farm during childhood protect against asthma, allergic rhinitis, and atopy in adulthood? Am J Respir Crit Care Med 20011641829–1834. [DOI] [PubMed] [Google Scholar]
- 13.Alfven T, Braun‐Fahrlander C, Brunekreef B.et al Allergic diseases and atopic sensitization in children related to farming and anthroposophic lifestyle—the PARSIFAL Study. Allergy 200661414–421. [DOI] [PubMed] [Google Scholar]
- 14.Koskela H O, Happonen K K, Remes S T.et al Effect of farming environment on sensitisation to allergens continues after childhood. Occup Environ Med 200562607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eduard W, Douwes J, Omenaas E.et al Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax 200459381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radon K, Schulze A, Nowak D. Inverse association between farm animal contact and respiratory allergies in adulthood: protection, underreporting or selection? Allergy 200661443–446. [DOI] [PubMed] [Google Scholar]
- 17.Portengen L, Preller L, Tielen M.et al Endotoxin exposure and atopic sensitization in adult pig farmers. J Allergy Clin Immunol 2005115797–802. [DOI] [PubMed] [Google Scholar]
- 18.Dutch Ministry of Agriculture NaFQ Dutch Policy Document on Organic Agriculture 2005–2007. The Netherlands: Dutch Ministry of Agriculture NaFQ, 2004
- 19.European Council Council Regulation (EEC) No 2092/91 of 24 June 1991 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs. 2005
- 20.Alm J S, Swartz J, Lilja G.et al Atopy in children of families with an anthroposophic lifestyle. Lancet 19993531485–1488. [DOI] [PubMed] [Google Scholar]
- 21.Bouma A, Elbers A R, Dekker A.et al The foot‐and‐mouth disease epidemic in The Netherlands in 2001. Prev Vet Med 200357155–166. [DOI] [PubMed] [Google Scholar]
- 22.Rijcken B, Kerkhof M, de Graaf A.et alEuropees luchtweg onderzoek Nederland. Groningen: Stichting drukkerij Regenboog, 1996
- 23.Droste J H, Kerhof M, de Monchy J G.et al Association of skin test reactivity, specific IgE, total IgE, and eosinophils with nasal symptoms in a community‐based population study. The Dutch ECRHS Group. J Allergy Clin Immunol 199697922–932. [DOI] [PubMed] [Google Scholar]
- 24.Burney P G, Luczynska C, Chinn S.et al The European Community Respiratory Health Survey. Eur Respir J 19947954–960. [DOI] [PubMed] [Google Scholar]
- 25.Crobach M J, Hermans J, Kaptein A A.et al The diagnosis of allergic rhinitis: how to combine the medical history with the results of radioallergosorbent tests and skin prick tests. Scand J Prim Health Care 19981630–36. [DOI] [PubMed] [Google Scholar]
- 26.Tabak C, Smit H A. Bronchial symptoms and obstruction: recent prevalence and short‐term trends (1993–1997) in adults in the Netherlands. Ned Tijdschr Geneeskd 20011452429–2434. [PubMed] [Google Scholar]
- 27.Ublagger E, Schreuer M, Eder W.et al Validation of questions on asthma and wheeze in farming and anthroposophic children. Clin Exp Allergy 2005351033–1039. [DOI] [PubMed] [Google Scholar]
- 28.Preller L, Doekes G, Heederik D.et al Disinfectant use as a risk factor for atopic sensitization and symptoms consistent with asthma: an epidemiological study. Eur Respir J 199691407–1413. [DOI] [PubMed] [Google Scholar]
- 29.Vogelzang P F, van der Gulden J W, Preller L.et al Bronchial hyperresponsiveness and exposure in pig farmers. Int Arch Occup Environ Health 199770327–333. [DOI] [PubMed] [Google Scholar]
- 30.von Mutius E, Braun‐Fahrlander C, Schierl R.et al Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy 2000301230–1234. [DOI] [PubMed] [Google Scholar]
- 31.Schram D, Doekes G, Boeve M.et al Bacterial and fungal components in house dust of farm children, Rudolf Steiner school children and reference children—the PARSIFAL Study. Allergy 200560611–618. [DOI] [PubMed] [Google Scholar]
- 32.Spaan S, Wouters I M, Oosting I.et al Exposure to inhalable dust and endotoxins in agricultural industries. J Environ Monit 2006863–72. [DOI] [PubMed] [Google Scholar]
- 33.Douwes J, Gibson P, Pekkanen J.et al Non‐eosinophilic asthma: importance and possible mechanisms. Thorax 200257643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douwes J, Pearce N, Heederik D. Does environmental endotoxin exposure prevent asthma? Thorax 20025786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lembke B, Janson C, Norback D.et al High risk of adult‐onset asthma and work‐related wheeze in farmers despite low prevalence of asthma in young farmers. Int J Tuberc Lung Dis 200481285–1291. [PubMed] [Google Scholar]
- 36.Radon K, Danuser B, Iversen M.et al Respiratory symptoms in European animal farmers. Eur Respir J 200117747–754. [DOI] [PubMed] [Google Scholar]
- 37.Eduard W, Omenaas E, Bakke P S.et al Atopic and non‐atopic asthma in a farming and a general population. Am J Ind Med 200446396–399. [DOI] [PubMed] [Google Scholar]
- 38.Vogelzang P F, van der Gulden J W, Tielen M J.et al Health‐based selection for asthma, but not for chronic bronchitis, in pig farmers: an evidence‐based hypothesis. Eur Respir J 199913187–189. [DOI] [PubMed] [Google Scholar]
- 39.Braback L, Hjern A, Rasmussen F. Selective migration contributes to a healthy worker effect in the farming population. J Clin Epidemiol 200659102–103. [DOI] [PubMed] [Google Scholar]