Abstract
Background
The association between coal tar‐derived substances, a complex mixture of polycyclic aromatic hydrocarbons, and cancer is well established. However, the specific aetiological agents are unknown.
Objective
To compare the dose–response relationships for two common measures of coal tar‐derived substances, benzene‐soluble material (BSM) and benzo(a)pyrene (BaP), and to evaluate which among these is more strongly related to the health outcomes.
Methods
The study population consisted of 6423 men with ⩾3 years of work experience at an aluminium smelter (1954–97). Three health outcomes identified from national mortality and cancer databases were evaluated: incidence of bladder cancer (n = 90), incidence of lung cancer (n = 147) and mortality due to acute myocardial infarction (AMI, n = 184). The shape, magnitude and precision of the dose–response relationships and cumulative exposure levels for BSM and BaP were evaluated. Two model structures were assessed, where 1n(relative risk) increased with cumulative exposure (log‐linear model) or with log‐transformed cumulative exposure (log–log model).
Results
The BaP and BSM cumulative exposure metrics were highly correlated (r = 0.94). The increase in model precision using BaP over BSM was 14% for bladder cancer and 5% for lung cancer; no difference was observed for AMI. The log‐linear BaP model provided the best fit for bladder cancer. The log–log dose–response models, where risk of disease plateaus at high exposure levels, were the best‐fitting models for lung cancer and AMI.
Conclusion
BaP and BSM were both strongly associated with bladder and lung cancer and modestly associated with AMI. Similar conclusions regarding the associations could be made regardless of the exposure metric.
The primary exposure of aluminium smelting cohort to coal tar‐derived substances has been associated with increased risk of bladder and lung cancer.1,2,3,4,5,6,7,8 Coal tar‐derived substances are a complex mixture that includes over 100 polycyclic aromatic hydrocarbons (PAHs), several of which are known carcinogens.9,10 The mixture may also contain very low levels of amino‐PAHs and nitro‐PAHs, which are also known bladder carcinogens.11,12 Exposure to PAHs has also been associated with cardiovascular disease mortality within aluminium smelter cohorts and within other industries with PAH exposure,6,13 but only a recent study of male asphalt workers has demonstrated a consistent dose–response relationship.14 Although PAHs are suspected, the specific causal agents of the increased risk of cancer and cardiovascular disease in aluminium smelters are not known.9,14,15,16,17
The occurrence of exposure to PAHs as a mixture creates significant challenges for exposure assessment in epidemiological studies. One challenge is in choosing an appropriate indicator of the carcinogenic or toxic potential of the mixture. Epidemiological studies have used various indicators, including measurements of individual hydrocarbons of the mixture, to describe exposure to PAHs and to examine dose–response relationships. These measures have included benzene‐soluble materials (BSMs), total particulate PAHs and benzo(a)pyrene (BaP), owing to routine monitoring of these components at most smelters.18 Of these metrics, BaP has been proposed to be a more specific indicator of exposure to PAHs as a class of carcinogens, and thus a better indicator of the carcinogenic potential of coal tar‐derived substances than BSM.16,17 Initial comparisons between BaP and BSM as exposure indices in aluminium smelter studies have found that BaP provided a slight improvement in the dose–response relationship for bladder cancer,3,17 whereas BSM provided a better‐fitting dose–response relationship for lung cancer.2
A recent follow‐up health study at a vertical stud Söderberg aluminium smelter provided an opportunity to compare exposure indices of BSM and BaP quantitatively in analyses of mortality and cancer incidence. In contrast with other aluminium smelter studies that used the work‐area‐specific relationship between BaP and BSM to derive estimates of exposure to BaP,2,3 the BaP exposure index used here was derived independently of BSM exposure levels.19 In this study, our objective was to examine the dose–response relationships to determine which of the exposure indices, BSM or BaP, provided a better marker for the causal component(s) of coal tar‐derived substances. The shape of the dose–response relationship was examined using both a log‐linear model (ln(relative risk (RR)) = β*cumulative exposure) and a log–log model (ln(RR) = β*ln(cumulative exposure+1)) structure. The focus of the dose–response relationships presented in this paper was on bladder cancer incidence, lung cancer incidence, and mortality due to AMI, because a substantial number of cases were available for examining the slope of dose–response relationships and monotonically increasing risks have been observed in categorical analyses.1
Methods
Study population
This study extended follow‐up by 12 years on a previous retrospective epidemiological study of workers in an aluminium smelter.8 This updated cohort included all male workers with ⩾3 years of work experience at the aluminium smelter or its power‐generating station since operation began in 1954 through 1997 (n = 6423), and is further described by Spinelli et al.1 The cohort members' work histories up to 31 December 1997 (job title, department, starting date and stopping date for each job assignment) were obtained from company records. Cohort members were linked using probabilistic linkage techniques to the National Mortality Database (1954–99) to ascertain their cause of death and to the National Cancer Registry (1969–99) to identify cancer diagnoses. Vital status was ascertained through linkage to the British Columbia (BC) Client Registry administered by the BC Ministry of Health (records of healthcare recipients on the provincial medical system since 1984) to determine their last known dates of residing in BC. Vital status was also obtained using active follow up from the original study; pension records, advertisements in company newsletter, union lists, and contact of last employers and family members supplemented vital status information.8 Workers were censored at their last contact dates if they did not link to the BC Client Registry and their location at last contact was that company or not in Canada, or if they had been censored in the original study before 1985 and did not link to the BC Client Registry. Workers who were not censored and did not die during the follow‐up period (1954–99) were assumed to be alive at the end of the study period of 31 December 1999. Workers with contact information (current workers and pensioners) were sent a self‐administered questionnaire requesting for information on their smoking habits. The results were supplemented with smoking information obtained from a similar mailed questionnaire to current workers in the original study.8 Further details on the smoking assessment are described in Spinelli et al.1
Exposure assessment
Exposure was evaluated using the two different measures of PAH exposure that were routinely monitored at the aluminium smelter: BSM and BaP. BSM provides a measure of the mixture and includes all components of the particulate exposure that are extractable by benzene, including PAHs. BaP is a single particulate‐phase PAH and has been found to be a good indicator of both individual and total particulate PAHs, but not necessarily of volatile PAHs.16,20 The relationship between BaP, BSM and other PAHs, has been found to vary between work areas, jobs and technological processes.3,16,20,21
Job exposure matrices with dimensions for job, department and time period for BSM and BaP were developed independently of each other using several exposure assessment approaches to maximise the use of personal exposure measurements, and has been reported previously.19 Measures of total PAH were unavailable at this smelter. Briefly, statistical models were developed to derive annual arithmetic means for each potroom (reduction plant) operation and potroom maintenance job from 1977 to 2000. For non‐potroom locations, mean exposures were directly calculated. Exposure estimates for jobs without exposure measurements were extrapolated from exposure estimates from the statistical models after adjusting for the amount of time in exposed areas. Estimating pre‐1977 exposure levels involved backwards extrapolation of 1977 exposure levels. The job exposure matrix was applied to each worker's work history record and aggregated over the worker's employment to obtain each worker's cumulative exposure.
Data analysis
The relationship between cumulative BaP and BSM exposure metrics for each worker was examined using Pearson's correlation; the partial correlation between metrics, which adjusts the correlation to account for duration of employment of each worker, was also examined. The percentage agreement between the exposure categories was calculated by dividing the total number of workers whose categorical assignments agreed between the two metrics by the total number of workers. The level of agreement between the categorical assignments of the different metrics was calculated using weighted κ values using quadratic weights where closer agreement in categories was penalised less than larger discrepancies.22
To examine the slopes and precisions of the dose–response relationships with cumulative BSM and BaP exposures, we selected diseases with a large number of cases that had significant dose–response trends in the categorical analyses published previously.1 Three diagnoses were examined: bladder cancer incidence (International Classification of Diseases (ICD)‐9: 188; 90 cases, including in‐situ cases); lung cancer incidence (ICD‐9: 162; 147 cases), and mortality due to AMI (ICD‐9: 410; 184 deaths). The cumulative exposure indices were lagged according to the best‐fitting latency periods observed in the categorical analyses. For bladder and lung cancer incidence, exposure was lagged 20 years—that is, only exposure occurring more than 20 years before diagnosis was considered. Exposure was not lagged for AMI.
Internal comparisons, using workers in the lowest exposure category as the reference group, were used to examine dose–response relationships. The Life Table Analysis System (PCLTAS, NIOSH, Cincinnati, Ohio, USA) software was used to generate files for analysis. RRs were calculated using maximum likelihood methods after adjustment for age, calendar year and smoking status using Poisson's regression (SAS V.8.0). Six exposure categories were used: an unexposed group (BSM: <0.05 mg/m3•year; BaP: <0.5 μg/m3•year), and five exposed groups with cumulative exposure cutpoints defined by distributing the remaining cases into five groups of equal size. As such, different exposure cutpoints were identified for each outcome and exposure metric. Because of the different units of the BaP and BSM exposure metrics, a case‐based definition of exposure cutpoints provided a comparison that was less sensitive to a priori cutpoints and equalised the SEs for each exposure category. This approach has previously been found to minimise bias in grouped analyses of cohort data.23 Smoking status was defined as ever (57% of workers, reference group), never (19%) or unknown (23%). A 10‐year calendar period and 5‐year age categories were used. Categories were combined when necessary to ensure all categories had a minimum of 10 cases to improve precision. 95% CIs were based on the SE of the coefficients derived from the model.
Exposure was treated as a continuous variable in Poisson regression by assigning the mean cumulative exposure to all subjects in each category. The mean cumulative exposure was weighted by the person‐years each subject contributed.23 Two dose–response model structures were assessed: the log‐linear model, where the RR, increased logarithmically with a linear increase in cumulative exposure (CE) (equation 1); the log–log model, where the RR increased logarithmically with log‐transformed cumulative exposure (equation 2).24
Log‐linear model:
Ln(RR)=β*CE (1)
Log–log model:
Ln(RR)=β*ln(CE+1) (2)
To determine which of the exposure metrics and which dose–response relationship provided the best fit for each health outcome, we compared two measures: (1) the change in the −2 log likelihood model fit statistic with and without exposure in Poisson regression, while adjusting for age, calendar year and smoking as fixed effects; (2) the precision of the dose–response relationship based on the Wald statistic (slope/SE).25
Results
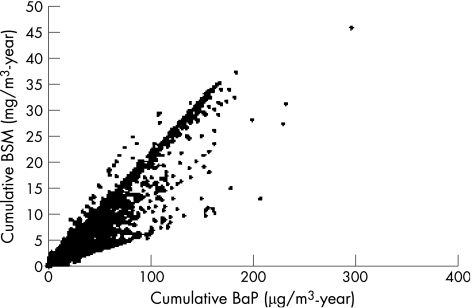
The cumulative exposure indices for BSM and BaP were very highly correlated (r = 0.94), but substantial deviations from a linear relationship were observed (fig 1). Adjusting the correlation for duration of employment did not weaken the relationship (partial correlation = 0.93) since duration of employment was only moderately correlated with cumulative exposure of either metric (r = 0.4). About half of the workers worked in non‐exposed areas of the plant. Categorisation of cumulative exposure based on the distribution of cases slightly decreased the relationship between the exposure metrics, with weighted kappa values between 0.85–0.87 and percent agreement of category assignment between 74–77%, with the slight variation depending on the health outcome.
Figure 1 Relationship between cumulative benzo(a)pyrene (BaP) and benzene‐soluble materials (BSM) exposure indices.
The median BSM cumulative exposures of the exposed workers were 2.6 and 2.4 mg/m3•year for no lag and 20‐year lag, respectively, and the maximum cumulative exposure was 46 mg/m3•year. The median BaP cumulative exposures were 20 and 18 μg/m3•year for no lag and 20‐year lag, respectively, and the maximum cumulative exposure was 300 μg/m3•year. The proportions of workers in the unexposed category with no lag were 19% and 20% for BSM and BaP, respectively. With a 20‐year lag, the proportions unexposed were 36% and 37% for BSM and BaP, respectively. The cutpoints used to define the six exposure categories were disease‐specific and are listed in table 1; the mean cumulative exposure for each category is also listed.
Table 1 Exposure category cutpoints and mean cumulative exposure for benzo(a)pyrene (BaP) and benzene‐soluble materials (BSM) by health outcome.
| Cumulative BaP (μg/m3•year) | Cumulative BSM (mg/m3•year) | |||||
|---|---|---|---|---|---|---|
| Bladder cancer incidence | Lung cancer incidence | Acute myocardial infarction | Bladder cancer incidence | Lung cancer incidence | Acute myocardial infarction | |
| Category cutpoints* | ||||||
| Unexposed | 0.50 | 0.50 | 0.50 | 0.05 | 0.05 | 0.05 |
| 20th percentile | 7.63 | 11.32 | 6.21 | 0.70 | 0.89 | 0.65 |
| 40th percentile | 19.99 | 27.25 | 20.99 | 2.14 | 3.61 | 2.43 |
| 60th percentile | 38.90 | 52.05 | 42.45 | 5.30 | 7.45 | 5.77 |
| 80th percentile | 69.61 | 81.70 | 80.61 | 11.80 | 12.41 | 12.42 |
| Mean cumulative exposure† | ||||||
| Unexposed | 0 | 0 | 0 | 0 | 0 | 0 |
| <20th percentile | 3.5 | 4.9 | 3.1 | 0.33 | 0.40 | 0.33 |
| 20–40th percentile | 13.3 | 18.4 | 13.1 | 1.33 | 1.97 | 1.44 |
| 40–60th percentile | 28.4 | 37.8 | 30.3 | 3.57 | 5.18 | 3.89 |
| 60–80th percentile | 51.6 | 64.7 | 57.7 | 7.94 | 9.49 | 8.31 |
| >80th percentile | 108.3 | 119.6 | 122.0 | 20.25 | 20.87 | 21.31 |
*Category cutpoints based on cumulative exposure of exposed cases.
†Mean cumulative exposure of all subjects, weighted by person‐years contributed by subject.
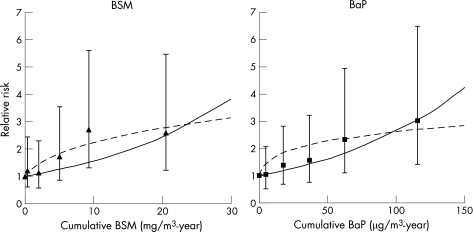
For bladder cancer incidence, both the BSM and BaP cumulative exposure metrics had a strong dose–response relationship (fig 2). The BaP metric had the highest RR (3) with the highest cumulative exposure, the most consistent monotonic increase, the largest improvement in model fit, and the most precise slope for the dose–response relationship (table 2). For BaP, the log‐linear model provided the best model fit (as measured by the change in −2 log likelihood) and the most precise exposure–response slope (B/SE). For BSM, the log–log dose–response model structure had the best model fit and model precision.
Figure 2 Log‐linear (—) and log–log (‐‐) relationships between exposure and bladder cancer incidence, 20‐year lag (BaP, benzo(a)pyrene; BSM, benzene‐soluble material).
Table 2 Model parameters and precisions for the relationships between exposure to benzo(a)pyrene (BaP) and benzene‐soluble materials (BSM) and incidence of bladder cancer, lung cancer and acute myocardial infarction.
| Cumulative BSM (mg/m3•year) | Cumulative BaP (μg/m3•year) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Change in −2 log likelihood* | B | SE | B/SE | Change in –2 log likelihood* | B | SE | B/SE | ||
| Bladder cancer incidence, 20‐year lag | |||||||||
| Log‐linear model† | 7.92 | 0.0446 | 0.0150 | 2.97 | 10.77 | 0.0095 | 0.0028 | 3.39 | |
| Log–log model‡ | 9.95 | 0.3323 | 0.1060 | 3.13 | 8.90 | 0.2082 | 0.0713 | 2.92 | |
| Lung cancer incidence, 20‐year lag | |||||||||
| Log‐linear model | 3.90 | 0.0248 | 0.0122 | 2.03 | 3.22 | 0.0039 | 0.0021 | 1.86 | |
| Log–Log model | 4.67 | 0.1744 | 0.0804 | 2.17 | 5.31 | 0.1162 | 0.0511 | 2.27 | |
| Acute myocardial infarction, no lag | |||||||||
| Log‐linear model | 1.52 | 0.0124 | 0.0098 | 1.27 | 1.32 | 0.0020 | 0.0017 | 1.18 | |
| Log–log model | 1.84 | 0.0943 | 0.0691 | 1.36 | 1.88 | 0.0611 | 0.0447 | 1.37 | |
B, slope parameter from model.
*Change in −2 log likelihood compared with the model with age, calendar year and smoking status but no exposure.
†Log‐linear: LogRR = B*CE.
‡Log–log: LogRR = B*ln(CE + 1).
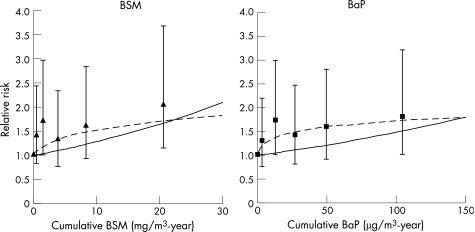
For lung cancer incidence, both the BSM and BaP cumulative exposure metrics had similar shapes for their respective dose–response relationships (fig 3). The 95% CIs for the highest cumulative exposure categories did not include one for both metrics, with RRs of 2 for BSM and 1.8 for BaP. For both metrics, the log–log model structure provided a greater improvement in model fit compared with the log‐linear model (table 2). The slope of the dose–response curve was most precise for BaP with the log–log model.
Figure 3 Log‐linear (—) and log–log (‐‐) relationships between exposure and lung cancer incidence, 20‐year lag. BaP, benzo(a)pyrene; BSM, benzene‐soluble material.
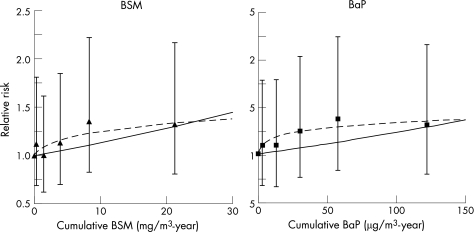
For AMI, we observed a roughly monotonically increasing risk with increasing cumulative exposure for both metrics in the categorical analyses, but the slopes of the dose–response relationships were not significant for either metric, and no individual exposure category had significantly increased risks (fig 4, table 2). The highest RR was 1.4 for both BSM and BaP. The BaP and BSM cumulative exposure metrics with the log–log model structure provided nearly identical improvements in model fit, and their slopes were equally precise.
Figure 4 Log‐linear (—) and log–log (‐‐) relationships between exposure and mortality due to acute myocardial infraction, no lag. BaP, benzo(a)pyrene; BSM, benzene‐soluble material.
Discussion
BaP cumulative exposure provided a modest improvement in model fit and model precision over BSM for bladder and lung cancer incidence. No differences were observed between metrics for AMI. Similar conclusions were made regarding associations between exposure to coal tar‐derived substances and the health outcome of interest regardless of exposure metric.
Previous aluminium smelter studies have assessed BaP exposure using the ratio of BaP to BSM, since much less sampling data are available for BaP than for BSM.2,3 These studies have accounted for work area and job differences in the BaP:BSM ratio, but have assumed through necessity that these ratios were stable throughout the study period. In this study, we assessed BaP exposure independently from BSM, and so could account for time‐varying factors that influenced BaP exposure, but were unable to account for differences in the relationship between BaP and other PAHs, which has been found to vary across time, job and work area.26 Exposure misclassification may be introduced by the use of an individual compound if its relationship with the causal components varies.
A quantitative comparison of two different exposure metrics becomes complicated when the metrics have different units of exposure or scales of exposure. To facilitate comparisons between BSM and BaP, we defined exposure categories on the basis of the cumulative exposure of the cases to minimise bias due to cutpoint selection and treated exposure as a continuous variable in Poisson's regression.23 Both the improvement in model fit and the precision of the dose–response relationship indicated that cumulative BaP exposure was the better index for all three health outcomes. However, both measures are sensitive to the units and scale of the exposure metric owing to the logarithmic relationship with RR and exposure. For the log‐linear model, changing the scale of the exposure metric (ie, dividing BaP by 10 to be on the same scale as BSM) did not change the magnitude of the improvement in model fit, but did slightly change the magnitude of the precision. The log–log model was particularly sensitive to the scale of these measures, with both the improvement in model fit and the magnitude of the precision changing with the scale of the metric. Regardless, BaP provided a better fit in all cases.
A levelling off of risk at the highest exposure categories (log–log model) has been observed in many retrospective studies. Non‐differential exposure misclassification has been shown in simulation studies to have this effect.27 The highest exposure categories were strongly influenced by pre‐1977 exposure levels, when no exposure measurements were available. While we extrapolated backwards from 1977 exposure estimates, assumptions regarding the shape of the time trend were required.19 The linear relationship between bladder cancer incidence and BaP exposure seen here provides indirect support for the validity of the BaP exposure metric. Sensitivity analyses could be conducted in future analyses to examine the impact of different assumptions regarding the backwards extrapolation.28,29 Other possible explanations for the levelling off of risk at high exposure categories include confounding by other risk factors, healthy worker survivor effect; depletion of the number of susceptible people at high exposure levels; a natural limit on RRs for diseases with high background rates; and the saturation of key disease pathways.30 Several of these reasons may be attributed to this cohort; however, these explanations would be expected to have a similar impact for both the BaP and BSM exposure indices.
Other aluminium smelter exposures—namely, benzo(b)fluoranthene and naphthalene—have been proposed as indicators of PAH. Aubin and Farant31 suggested benzo(b)fluoranthene as a more stable indicator of PAH environmental exposure because BaP levels are more influenced by environmental factors; however, it was very highly correlated with BaP (r = 0.97) within this smelter. Rappaport et al32 proposed naphthalene as a potential surrogate for occupational PAH exposure because it is the most abundant PAH and because it is present almost entirely in the gas phase and therefore can be measured easily. The relationship between BaP and naphthalene at this smelter is not known, as naphthalene has not been monitored regularly. Naphthalene has been found to be only moderately correlated (r = 0.54) with BaP at another Söderberg aluminium smelter,16 suggesting that it would be a useful indicator as it measures alternative exposures. The correlation between BaP and amino‐PAHs and nitro‐PAHs has not been reported, but amino‐PAHs and nitro‐PAH levels were very low in one aluminium smelter and were found to be well controlled by current ventilation processes.11
The consistently strong and more precise dose–response relationships observed with the BaP metric provides support for the use of BaP as a measure of exposure to the PAH mixture in examinations of cancer and heart disease in aluminium smelters. The strength of the comparison between dose–response relationships presented here was the substantial number of cases for each outcome, the examination of the continuous dose–response relationships, and the measurement‐based exposure assessment strategy that independently assessed BaP and BSM exposure levels. The levelling off of risk at high exposure categories may result from exposure misclassification, but may also result from time‐varying differences between BaP and other specific causal exposure agents, or may suggest that different causal components may be associated with the different disease outcomes. Morbidity outcomes, such as hospitalisation for respiratory or cardiovascular disease, may provide a better comparison of the usefulness of BaP and BSM as indices of exposure to coal tar‐derived substances, as there is more confidence in the exposure estimates in more recent time periods. The relationship between BaP and other coal tar constituents, and factors that influence that relationship should continue to be examined, in order to shed light on the specific causal agents.
Main messages
The occurrence of polycyclic aromatic hydrocarbons (PAHs) as a mixture creates significant challenges for exposure assessment in epidemiology, including choosing an appropriate indicator of the carcinogenic or toxic potential of the mixture.
Benzo(a)pyrene (BaP) and benzene‐soluble materials (BSMs) were both strongly associated with bladder and lung cancer, and similar conclusions regarding the associations could be made regardless of the exposure metric.
Cumulative BaP exposure provided a modest improvement in the precision of the exposure–response relationship for bladder cancer and lung cancer incidence than cumulative BSM metrics.
Evaluating the difference metrics of PAH exposure was facilitated by examining continuous exposure–response relationships and comparing the model fit and precision of the model slope.
Policy implications
Comparing exposure–response relationships using multiple quantitative exposure metrics will improve our knowledge of the aetiological agents, and can facilitate risk assessment and exposure standard setting.
Acknowledgements
We thank the joint ALCAN/Canadian Auto Workers Advisory Committee for their assistance. We also thank Barry Boudreault, Jim Thorne, Nela Walter and Mary Lo for their assistance in abstracting historical information. This research was partially supported by grants from Alcan and the WCB. Trainee support for MF was provided by the Michael Smith Foundation for Health Research and the Canadian Institutes for Health Research.
Abbreviations
AMI - acute myocardial infarction
BaP - benzo(a)pyrene
BC - British Columbia
BSM - benzene‐soluble material
ICD - International Classification of Diseases
PAH - polycyclic aromatic hydrocarbon
Footnotes
Competing interests: None declared.
References
- 1.Spinelli J J, Demers P A, Le N D.et al Cancer risk in aluminum reduction plant workers (Canada). Cancer Causes Control 200617939–948. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong B, Tremblay C, Baris D.et al Lung cancer mortality and polynuclear aromatic hydrocarbons: a case–cohort study of aluminum production workers in Arvida, Quebec, Canada. Am J Epidemiol 1994139250–262. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong B G, Tremblay C G, Cyr D.et al Estimating the relationship between exposure to tar volatiles and the incidence of bladder cancer in aluminum smelter workers. Scand J Work Environ Health 198612486–493. [DOI] [PubMed] [Google Scholar]
- 4.Romundstad P, Andersen A, Haldorsen T. Cancer incidence among workers in six Norwegian aluminum plants. Scand J Work Environ Health 200026461–469. [DOI] [PubMed] [Google Scholar]
- 5.Romundstad P, Haldorsen T, Andersen A. Cancer incidence and cause specific mortality among workers in two Norwegian aluminum reduction plants. Am J Ind Med 200037175–183. [DOI] [PubMed] [Google Scholar]
- 6.Ronneberg A, Andersen A. Mortality and cancer morbidity in workers from an aluminium smelter with prebaked carbon anodes‐‐Part II: cancer morbidity. Occup Environ Med 199552250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronneberg A, Haldorsen T, Romundstad P.et al Occupational exposure and cancer incidence among workers from an aluminum smelter in western Norway. Scand J Work Environ Health 199925207–214. [DOI] [PubMed] [Google Scholar]
- 8.Spinelli J J, Band P R, Svirchev L M.et al Mortality and cancer incidence in aluminum reduction plant workers. J Occup Med 1991331150–1155. [DOI] [PubMed] [Google Scholar]
- 9.National Toxicology Program Polycyclic aromatic hydrocarbons. Report on carcinogens, 11th edn. National Institute of Environmental Health Services, North Carolina, USA 2005
- 10.Skogland M. A survey of the PAH problem in the aluminum industry. Light Metals 199149797–98. [Google Scholar]
- 11.Farant J P, Ogilvie D. Investigation of the presence of amino and nitro polycyclic aromatic hydrocarbons in a Soderberg primary aluminum smelter. Am Ind Hyg Assoc J 200263721–725. [DOI] [PubMed] [Google Scholar]
- 12.Roussel R, Gaboury A, Lariviere C. Aromatic amines in the workplace atmosphere of an aluminum smelter. Light Metals 1991497503–507. [Google Scholar]
- 13.Theriault G P, Tremblay C G, Armstrong B G. Risk of ischemic heart disease among primary aluminum production workers. Am J Ind Med 198813659–666. [DOI] [PubMed] [Google Scholar]
- 14.Burstyn I, Kromhout H, Partanen T.et al Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology 200516744–750. [DOI] [PubMed] [Google Scholar]
- 15.Straif K, Baan R, Grosse Y.et al Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet 20056931–932. [DOI] [PubMed] [Google Scholar]
- 16.Farant J P, Gariepy M. Relationship between benzo(a)pyrene and individual polycyclic aromatic hydrocarbons in a Soderberg primary aluminum smelter. Am Ind Hyg Assoc J 199859758–765. [Google Scholar]
- 17.Tremblay C, Armstrong B, Theriault G.et al Estimation of risk of developing bladder cancer among workers exposed to coal tar pitch volatiles in the primary aluminum industry. Am J Ind Med 199527335–348. [DOI] [PubMed] [Google Scholar]
- 18.Benke G, Abramson M, Sim M. Exposures in the alumina and primary aluminium industry: an historical review. Ann Occup Hyg 199842173–189. [DOI] [PubMed] [Google Scholar]
- 19.Friesen M C, Demers P A, Spinelli J J.et al From expert‐based to quantitative retrospective exposure assessment at a Söderberg aluminum smelter. Ann Occup Hyg 200650359–370. [DOI] [PubMed] [Google Scholar]
- 20.Sanderson E, Kelly P, Farant J P. Effect of Soderberg smelting technology, anode paste composition, and work shift on the relationship between benzo[a]pyrene and individual polycyclic aromatic hydrocarbons. J Occup and Environ Hyg 2005265–72. [DOI] [PubMed] [Google Scholar]
- 21.Ronneberg A. Mortality and cancer morbidity in workers from an aluminium smelter with prebaked carbon anodes‐‐Part I: Exposure assessment. Occup Environ Med 199552242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong B K, White E, Saracci R.Principles of exposure assessment in epidemiology. Oxford: Oxford University Press, 1994
- 23.Richardson D B, Loomis D. The impact of exposure categorisation for grouped analyses of cohort data. Occup Environ Med 200461930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steenland K, Deddens J A. A practical guide to dose‐response analyses and risk assessment in occupational epidemiology. Epidemiology 20041563–70. [DOI] [PubMed] [Google Scholar]
- 25.Kromhout H, Loomis D P, Kleckner R C.et al Sensitivity of the relation between cumulative magnetic field exposure and brain cancer mortality to choice of monitoring data grouping scheme. Epidemiology 19978442–445. [DOI] [PubMed] [Google Scholar]
- 26.Friesen M C, Demers P A, Spinelli J J.et alSensitivity of exposure‐response relationships to exposure assessment strategies in retrospective cohort studies. Vancouver, University of British Columbia, [PhD dissertation] 2006
- 27.Dosemeci M, Wacholder S, Lubin J H. Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol 1990132746–748. [DOI] [PubMed] [Google Scholar]
- 28.Loomis A, Kromhout H, Kleckner R C.et al Effects of the analytical treatment of exposure data on associations of cancer and occupational magnetic field exposure. Am J Ind Med 19983449–56. [DOI] [PubMed] [Google Scholar]
- 29.Kromhout H, Loomis D P, Kleckner R C. Uncertainty in the relation between exposure to magnetic fields and brain cancer due to assessment and assignment of exposure and analytical methods in dose‐response modeling. Ann NY Acad Sci 1999895141–155. [DOI] [PubMed] [Google Scholar]
- 30.Stayner L, Steenland K, Dosemeci M.et al Attenuation of exposure‐response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health 200329317–324. [DOI] [PubMed] [Google Scholar]
- 31.Aubin S, Farant J P. Benzo[b]fluoranthene, a potential alternative to benzo[a]pyrene as an indicator of exposure to airborne PAHs in the vicinity of Soderberg aluminum smelters. J Air Waste Manage Assoc 2000502093–2101. [DOI] [PubMed] [Google Scholar]
- 32.Rappaport S M, Waidyanatha S, Serdar B. Naphthalene and its biomarkers as measures of occupational exposure to polycyclic aromatic hydrocarbons. J Environ Monit 20046413–416. [DOI] [PubMed] [Google Scholar]