Abstract
Background
Rectal cancer has been previously associated with exposure to metalworking fluids in a cohort mortality study of autoworkers.
Objective
To better specify the exposure–response relationship with straight metalworking fluids (mineral oils) by applying non‐parametric regression methods that avoid linearity constraints and arbitrary exposure cut points and by lagging exposure to account for cancer latency, in a nested case–control analysis.
Methods
In addition to the classical Poisson regression with categorical exposure, survival models with penalised splines were used to estimate the exposure–response relationship between cumulative exposure to straight metalworking fluid and mortality from rectal cancer. Exposures to water‐based metalworking fluids were treated as potential confounders, and all exposures were lagged by 5, 10, 15 and 20 years to account for cancer latency. The influence of the highest exposures was dealt with by a log transformation and outlier removal. The sensitivity of the penalised splines to alternative criteria for model selection and to the placement of knots was also examined.
Results
The hazard ratio for mortality from rectal cancer increased essentially linearly with cumulative exposure to straight metalworking fluid (with narrow confidence bands) up to a maximum of 2.2 at the 99th centile of exposure and then decreased (with wide confidence bands). Lagging exposure up to 15 years increased the initial steepness of the curve and raised the maximum hazard ratio to 3.2.
Conclusions
Non‐parametric smoothing of lagged exposures has shown stronger evidence for a causal association between straight metalworking fluid and rectal cancer than was previously described using standard analytical methods. This analysis suggests an exposure–response trend that is close to linear and statistically significant over most of the exposure range and that increases further with lagged exposures. Smoothing should be regularly applied to environmental studies with quantitative exposure estimates to refine characterisation of the dose–response relationship.
Metalworking fluids are complex mixtures of oils and chemicals used to cool and lubricate metal machining operations in many industrial settings. Automobile manufacturing is the largest sector with widespread metalworking fluid exposure. More than 1.2 million workers are exposed to metalworking fluids in the US,1 and their use is increasing internationally as developing countries industrialise. This growth is occurring despite the presence of known carcinogens, such as chlorinated compounds and polycyclic aromatic hydrocarbons in oil‐based fluids, and ethanolamines and nitrosamines in water‐based fluids.2
Metalworking fluids are generally classified into four types in two overlapping groups, according to base composition as oil based (straight and soluble mineral oil) or water based (soluble, semisynthetic and synthetic). All types include some oil (except for synthetics or “chemical fluids”), as well as a variety of additional compounds. The earliest report of cancer among workers exposed specifically to oils used in metalworking appeared in 1950.3 Between 1950 and 1967, two thirds of the 187 cases of scrotal cancer in the West Midlands area of England were attributed to exposure to machining fluid.4 With the introduction of high‐speed machining and grinding, concern has shifted from dermal exposures to inhalation of aerosolised metalworking fluids and possible respiratory and gastrointestinal cancers.
The cancer mortality study of a United Autoworkers‐General Motors cohort is the largest study with quantitative estimates of lifetime exposure to specific types of metalworking fluid.5 In the extended follow‐up of this cohort, results were presented from exposure–response models for 12 cancers, including colon and rectal cancers, using a uniform set of exposure categories.6 Although most epidemiological studies examine rectal and colon cancer as a single outcome, they were considered separately because there is growing evidence that they are distinct diseases.7 Results of that study suggested the increasing risk of rectal cancer, but not of colon cancer, in relation to increasing exposure to straight metalworking fluids. In light of widespread industrial exposure and poor survival rates for rectal cancer, we have taken this opportunity to re‐examine the data more closely by refining the measures of exposure to account for latency and applying more informative non‐parametric exposure–response models with fewer statistical assumptions.
Methods
Cohort data
Descriptions of the autoworkers cohort and exposure data have been discussed in detail previously.5,6,8,9 We present a brief outline of the study. The cohort consists of 46 399 autoworkers from three manufacturing plants in Michigan, USA. All employees who worked for at least 3 years before 1 January 1985 were included in the cohort and followed from 1941 to 1985. The end of the follow‐up was later extended to 1995.6 Demographic information was collected from work records. Vital status was obtained from the Social Security Administration and the National Death Index. Plant, union and state vital statistics office records were used along with the National Death Index to determine the underlying cause of death as recorded on the death certificate. The original study was approved by the internal review board of Harvard School of Public Health and the extended follow‐up by internal review boards of University of Massachusetts and General Motors Corporation.
Exposure
An extensive exposure assessment was conducted to retrospectively estimate past exposure levels to specific types of metalworking fluids.8 All three major types of fluid had been used at each of the three study sites, and each plant provided past industrial hygiene records for exposure reconstruction. Plant II was the newest of the three and used the most water‐based fluids. Based on sampling data collected by the research team and plant records, the amount of exposure to each of three types of metalworking fluids, straight mineral oils, soluble (mineral oils emulsified in water) and synthetic (chemical fluids without oil), was estimated for homogeneous exposure categories in each calendar year of the study. (Semisynthetics were combined with soluble metalworking fluids in all analyses.) Combining this exposure matrix with employment records, cumulative exposure to each type of metalworking fluid was estimated for each participant in the cohort. Participants with entirely missing work histories were excluded. For each fluid, cumulative exposure was measured in mg/m3‐years and lagged by 5, 10, 15 and 20 years to account for disease latency.10 Previous findings have indicated an association with straight mineral oils, therefore we focused primarily on straight oils, and included exposure to soluble and synthetic fluids as potential confounders.
Statistical methods
We began by fitting a Poisson regression model to the full dataset with a categorical variable defined for exposure to straight metalworking fluids, controlling for cumulative exposure to each of the other fluid types. We repeated this for synthetic and soluble exposures. Along with a non‐exposed reference group, cut points for the exposure levels were chosen as the quartiles of the case distribution and exposures were lagged up to 20 years. Relative risks (RRs) were estimated in these models as adjusted mortality ratios.
We then fit Cox regression models with continuous exposure variables and adjusted hazard ratios (HRs) as estimates of RR. We used a flexible smoothing approach to estimate the exposure–response curve by fitting penalised splines11 of exposure to straight metalworking fluids. Soluble and synthetic fluids were included as linear terms in the models. The full dataset consists of approximately 1.5 million person‐years of data, and to make the analysis more efficient, we created a nested case–control sample by randomly selecting 20 controls for each case from the cohort who were alive at the time of the case's death. Values of the time‐dependent cumulative exposure variables were determined in the year the control reached the age of the case death.
Splines were used to model exposure–response to avoid parametric assumptions about the shape of the curve. The exposure for subject i, denoted xi(t), is first transformed using a power basis expansion.
 |
where Kk are known knots, x+ = max(x,0) and the dependency on time is suppressed. The unknown parameters, β1, … βp, b1, …, bK, are estimated in the Cox regression model by maximising the penalised partial log–likelihood,12 where the bk coefficients are constrained to control the amount of smoothness in the fitted curve. This smoothness can be described in terms of the degrees of freedom (df), the effective number of parameters estimated after constraining the bk coefficients. We initially applied the standard method for selecting the df to optimise the smoothing, Akaike's Information Criteria (AIC).13 Calculated as AIC = –2logL+2df, where L denotes the likelihood evaluated at the maximum likelihood estimates of the parameters in the model, the optimal model minimises the AIC with respect to df, so that models with similar fits based on the log likelihood will be penalised for having more df—that is, less smoothness. This balances good fit to the data with smoothness of the spline. One drawback of AIC is that it is known to under‐penalise,14 and so we also applied corrected AIC, AICc, which adjusts the AIC formula by substituting r(df+1)/(r−(df+2)), where r is the number of events, for degrees of freedom.15 Finally, we considered cross validation12,16 as a third alternative for selecting the optimal amount of smoothness. Cross validation involves the sequential removal of each observation, the prediction of this deleted observation using the remaining data and the partial log likelihood as a measure of how well the predicted values fit the observed data.
We also used three approaches to deal with the influence of the highest exposures in the tail of the distribution on the shape of the exposure–response curve:
We took a natural logarithm transformation of the exposure variable.
We used alternative criteria for the number of knots and placement, locating knots at fixed centiles of the exposure distribution rather than equally spaced.
We examined models with outliers held out of the analysis, defined as those participants with exposures larger than the highest exposed case.
Results provide a sensitivity analysis for the final exposure–response model.
Results
There were 90 deaths due to rectal cancer in the cohort (table 1). Most of the cases were white and male, and had worked in plant I, the oldest of the three plants. The oldest case was 94 years of age and the youngest 32 years. The maximum cumulative exposure to straight metalworking fluid was 108.6 mg/m3‐years among cases and 188.2 mg/m3‐years among the non‐cases selected for the nested case–control sample. All models included the three types of metalworking fluids, as well as race, sex, year of hire, calendar year and manufacturing plant at which the participant worked as potential confounders.
Table 1 Demographics for cases of mortality from rectal cancer and non‐cases in the United Autoworkers‐General Motors autoworkers cohort.
| Cases | Non‐cases | |
|---|---|---|
| Participants, n | 90 | 1707* |
| Race, n (%) | ||
| African American | 9 (10) | 199 (11.7) |
| White | 59 (56.6) | 1144 (67) |
| Unknown | 22 (24.4) | 364 (22.3) |
| Males, n (%) | 86 (95.6) | 1576 (92.3) |
| Calendar year of death, mean (SD) | 1975 (13) | |
| Year of birth, mean (SD) | 1910 (15.5) | 1913 (15.3) |
| Duration of follow‐up, mean (SD), (years) | 32.9 (13.4) | 41.6 (12.6) |
| Plant, n (%) | ||
| 1 | 52 (57.8) | 853 (50) |
| 2 | 23 (25.5) | 570 (33.4) |
| 3 | 15 (16.7) | 284 (16.6) |
| Cumulative straight exposure (mg/m3‐year)† | ||
| Mean (SD) | 7.1 (17.7) | 4 (14.6) |
| 25th centile | 0 | 0 |
| 50th centile | 0.5 | 0.004 |
| 90th centile | 19.2 | 7.3 |
| 99th centile | 108.6 | 73.8 |
| Maximum | 108.6 | 188.2 |
*Distinct participants; 83 individuals appeared in >1 risk set.
†Computed at the end of follow‐up.
Poisson models
Table 2 shows the results for the Poisson regression models with unlagged cumulative exposure to each type of metalworking fluid. Cut points for the categories were selected separately for each fluid type by the exposure quartiles for the exposed cases, using participants unexposed to that particular fluid as the reference group. Models with lags of 5, 10, 15 and 20 years were also considered (data not shown). Raised RRs were found for exposures to both oil‐based (straight and soluble) as well as synthetic metalworking fluid; however, the strongest associations were seen for straight metalworking fluid, with a significant RR of 2.7 in the highest exposure category. The adjusted RR for this category increased up to 3.6 for a lag of 20 years (with a 95% confidence interval (CI) that excluded 1). Mortality ratios for synthetic and soluble fluids were raised, with wide 95% CIs and without trends for all lagged and unlagged exposures.
Table 2 Adjusted RRs* (95% CI) for mortality from rectal cancer and exposure to cumulative metalworking fluid (by type of fluid) estimated in Poisson regression.
| Straight | Soluble | Synthetic | |||
|---|---|---|---|---|---|
| Cut‐offs | RR (95% CI) | Cut‐offs | RR (95% CI) | Cut‐offs | RR (95% CI) |
| 0 | 1 | 0 | 1 | 0 | 1 |
| >0–0.68 | 1.2 (0.6 to 2.2) | >0–6.51 | 1.2 (0.5 to 3) | >0–0.52 | 0.6 (0.2 to 1.9) |
| >0.68–1.93 | 1.6 (0.9 to 3.2) | >6.51–15.55 | 1.9 (0.8 to 4.6) | >0.52–1.34 | 1.3 (0.4 to 4) |
| >1.93–10.09 | 1.5 (0.8 to 2.8) | >15.55–48.7 | 1.4 (0.6 to 3.6) | >1.34–2.54 | 1.8 (0.6 to 5.7) |
| >10.09 | 2.7 (1.4 to 5.3) | >48.7 | 1.9 (0.6 to 6.4) | >2.54 | 1.5 (0.4 to 6.3) |
*Models adjusted for age, sex, race, plant, start year, calendar year and all types of fluid exposure.
Cox models with penalised splines
Figure 1 presents the results of the penalised spline model with unlagged exposure to straight metalworking fluid. The R software package (R Development core team, Vienna, Austria) provides automatic selection of the smoothing parameter by minimising AIC or AICc using a default of cubic splines with 17 terms in the power basis expansion. In this application, the optimal smoothing parameter using AIC was df = 1.86, whereas the AICc criteria selected a slightly lower df = 1.67. Cross validation gave an identical model to AIC. The resulting log RR of rectal cancer death for the two selected models illustrates the smoothness of each model fit (fig 1). Both exposure–response curves increase to a maximum RR of 2.2. The AIC selected curve reaches the maximum earlier, at a cumulative exposure of 69.4 v 83.8 mg/m3‐years for the AICc curve. The point‐wise confidence bands (similar for both curves, shown for AIC) support an increasing exposure–response curve up to approximately the 99th centile of exposure, after which they widen dramatically. The shape of the confidence bands reflects the sparseness of data beyond the 99th centile, illustrated by the vertical line in fig 1.
Figure 1 Log hazard ratio for mortality from rectal cancer versus cumulative exposure to straight metalworking fluid (MWF; mg/m3‐year) with 95% point‐wise confidence curves using the R default of 17 terms in the spline expansion. Optimal curves were found by minimising Akaike's Information Criteria (AIC) and (AICc) criteria using equally spaced knots in the spline expansion. Models adjusted for age, sex, race, plant, start year, calendar year, soluble and synthetic MWF exposures as linear terms. The rug plot along the horizontal axes gives the distribution of the exposure data. The vertical line indicates the 99th centile of the exposures.
Lagged exposures
We then fit a series of Cox models with penalised splines for the exposure variable with increasing lags, from 0 to 20 years. AIC and AICc were used to determine the optimal degrees of freedom. Figure 2 gives the results for the 15‐year and 20‐year lags along with the unlagged model, focusing on the exposures up to 57 mg/m3‐years, the 99th centile of 15‐year lagged exposures. Beyond that point, the confidence curves widened and the plot resembles fig 1. The lag 5 and lag 10 models had overall shapes similar to the unlagged model up to 40 mg/m3‐years, and then decreased at slower rates than the 15‐year and 20‐year lag models (data not shown). Up to the 15‐year lag, the curves increased in initial steepness and maximum RR. The RR reached 3.2 at 40 mg/m3‐years when exposure was lagged by 15 years. The estimated RRs at fixed cumulative exposure values for the unlagged, 15‐year lagged and 20‐year lagged models (table 3) indicate that the exposure–response was stronger as the lag increased up to 15 years. Although the intent of the lagging was to account for latency, it also had the unintended consequence of reducing exposures.
Figure 2 Log hazard ratio for mortality from rectal cancer versus cumulative exposure to straight metalworking fluid (MWF; mg/m3‐year) for lagged models. Degrees of freedom selected by Akaike's Information Criteria (AIC) using seven terms in the expansion. Models adjusted for age, sex, race, plant, start year, calendar year, soluble and synthetic MWF exposures as linear terms. The data rug is for the lag‐15 exposure distribution. Fitted curves were shifted to go through (0,0).
Table 3 RRs for mortality from rectal cancer predicted from Cox models in this study* and a past analysis†.
| Straight exposure (mg/m3‐year) | Penalised spline* | Linear† | ||
|---|---|---|---|---|
| No lag | 15‐year lag | 20‐year lag | No lag | |
| 0.5 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1 | 1.0 | 1.0 | 1.1‡ | 1.0 |
| 3 | 1.1‡ | 1.1‡ | 1.2‡ | 1.0 |
| 5 | 1.1‡ | 1.2‡ | 1.2‡ | 1.0 |
| 10 | 1.3‡ | 1.5‡ | 1.6‡ | 1.0 |
| 25 | 1.7‡ | 2.5‡ | 2.6‡ | 1.1 |
| 40 | 2.1‡ | 3.2‡ | 2.9‡ | 1.2 |
*Corresponds to results in fig 3.
†Past model is from Eisen et al.6
‡Significant result at the 0.05 level.
Influence
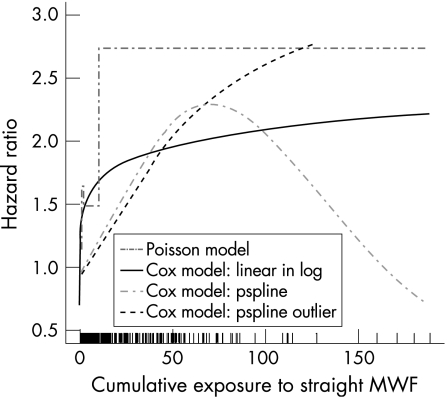
As typical of environmental data and seen in the rug plot on the horizontal axis of fig 1, the exposure distribution is highly right skewed. We used three approaches to deal with the influence of the highest exposures in the tail of the distribution. Results of alternative models are shown in fig 3.
Figure 3 Estimated hazard ratios for mortality from rectal cancer predicted from a (A) Poisson model based on the full cohort with exposure categorised into four levels. (B) Cox model based on the case–control sample with the logarithm of exposure entered as a linear term. (C) Cox model based on the case–control sample with a penalised spline for exposure (seven terms, df = 1.84). (D) Cox model based on the case–control sample with a penalised spline for exposure (seven terms, df = 1.46) with the four participants with highest exposure held out. All models adjusted for age, sex, race, plant, start year, calendar year, soluble and synthetic metalworking fluid (MWF) exposures as linear terms.
Firstly, we examined the natural logarithm of the exposure variable. AIC, AICc and cross‐validation model‐fit criteria selected a Cox model with penalised splines having df = 1, suggesting that the optimal model was linear with respect to the logarithm of the exposure data. The solid curve in fig 3 gives the RR for this model. The RR reaches a maximum value of 2.22, and is significant beyond a cumulative exposure of 0.04 mg/m3‐years (as reflected by the lower point‐wise confidence curve, output omitted).
In another approach to deal with the skewed distribution, we considered alternative numbers and placement of the knots. With knots placed at centiles of exposure on the original scale, instead of equally spaced (R default), the three model‐fit criteria were all optimised at df = 1. With just a linear term for the untransformed exposure variable, the RR increased with cumulative straight exposure across the range. Thus, the data are consistent with models for RR that are linear on both the raw and log transformed straight metalworking fluid exposure scale. We also used 5, 8, 12 and 25 equally spaced knots. The number of knots did not affect the overall shape and size of the exposure–response curve (fig 3). This is consistent with the findings of Ruppert17 who suggested that number of knots typically has a small effect on the smoothness provided a sufficient number has been chosen.
Finally, we examined models with outliers held out of the analysis. Of the 10 most extreme observations (down to the exposure of the case with the highest exposure), all but one represented distinct non‐cases. Sequentially removing the three most extreme participants had only a local effect on the curves, where with each successive deletion the end of the truncated curves rose (data not shown). Holding out ⩾4 of the outliers resulted in models close to linear with a maximum RR of 3.5 at the highest cumulative exposure with a slower rate of initial increase.
Discussion
Straight metalworking fluid contains a number of known or suspected carcinogens, including polycyclic aromatic hydrocarbons, chlorinated paraffins and sulphur compounds. Epidemiological analysis of metalworking fluids and cancer is further complicated by changes in the composition of these complex mixtures over time. For example, as mineral oils have become more highly refined, the amount of polycyclic aromatic hydrocarbons has decreased, presumably reducing carcinogenic risk.18,19,20 The composition of metalworking fluid, however, is also a function of the industrial application itself, as well as the recycling of used fluids, whereby polycyclic aromatic hydrocarbons can be reintroduced in response to heat, and metals can accumulate. In addition to changes in composition, the route and nature of exposure have also changed over calendar time as the industrial operations have evolved. Originally dermal exposures were the primary concern, but as machine speeds have increased, metalworking fluid particulates have become aerosolised, resulting in exposure via inhalation and ingestion. The effect of past and present changes in industrial processes and machinery on the particle size distribution of aerosolised metalworking fluid has not been fully explored, leaving open the question of greater deposition and dose to some target tissues in the modern workplace.21 Thus, even with the benefit of an extensive exposure assessment, we need to interpret results assuming that the presence of non‐differential exposure misclassification has produced some bias towards the null.
Most epidemiological studies of rectal cancer have considered this disease in combination with colon cancer; however, there is growing evidence that these are two distinct diseases with different aetiologies and risk factors.7,22,23 There are several differences between the two organs that might explain why an environmental exposure might cause cancer at one site and not at the other. The rectum transports less water and electrolytes than the proximal colon, is more acidic and is exposed longer to waste.7,24 In addition, molecular pathways leading to cancer of the large bowel are thought to differ by subsite. For example, people with the inherited syndrome familial adenomatous polyposis, characterised by mutations in the APC gene, are more likely to develop polyps first in the rectum, whereas those with hereditary non‐polyposis colorectal cancer, arising from mutations in mismatch repair genes, are more likely to develop cancer in the proximal colon.25 These differences between the colon and rectum underscore the need to consider their aetiology separately.
Eisen et al6 reported a marginally significant linear trend in rate ratios for rectal cancer with cumulative exposure to straight metalworking fluid in a Poisson regression and a non‐significant slope in a standard Cox model with a linear exposure term. (No associations between colon cancer and type of metalworking fluid were observed.) The present results based on penalised splines suggest a much stronger exposure–response trend for rectal cancer that is close to linear and statistically significant over most of the exposure range before lagging. For example, at 10 mg/m3‐years (20 years at the current recommended exposure limit1 of 0.5 mg/m3), the previous linear model predicted an RR of 1, in contrast with a significantly increased RR of 1.3 based on penalised splines. When exposure to straight metalworking fluids was lagged by 20 years, the smoothed models predicted a significant RR of 1.6 at this cumulative exposure. Penalised spline models for colon cancer were consistent with past null results6 for all fluid types, with straight exposure lagged up to 20 years (data not presented). Thus, applying the more flexible penalised splines provided a clearer picture of the exposure–response relationship and showed a strong near‐linear trend over the denser region of the exposure distribution.
A few other studies show relevant findings specifically regarding rectal cancer and exposure to metalworking fluids, and most are limited in size, study design and/or exposure assessment. Increased RR has been reported for rectal cancer in several proportionate mortality ratio (PMR) studies. Silverstein et al26 found a PMR of 1.4 (95% CI 0.8 to 2.3) in white ball‐bearing plant workers potentially exposed to oil‐based metalworking fluid and Vena et al27 reported a PMR of 2.8 (based on four observed deaths) among engine plant workers employed for >20 years. In an extended follow‐up of an automobile manufacturing plant, Kazerouni et al28 found no association with rectal cancer among those with heavy exposure to metalworking fluid. There was an association in a study of magazine production workers, with a standardised mortality ratio of 1.5 among a small number of unskilled machine assistants with a wide CI.29 Overall, these studies contribute little additional evidence to support the findings reported here because they lack an adequate quantitative exposure assessment for metalworking fluid.
There have also been several relevant studies that report results for exposure to metalworking fluids and colon and rectal cancer combined into a single outcome. (The American Cancer Society presents national incidence and mortality statistics only for the two cancers combined because of potential misclassification.32) Cohort studies of autoworkers31 and aerospace workers32 reported an RR close to 1, whereas an RR of 1.6 was reported for unskilled rotary press operators in a study of magazine production workers.29 In a population‐based case–control study of colorectal cancer in Sweden, Gerhardsson de Verdier et al33 found a significant increased RR of 2.1 for workers ever exposed to cutting fluids. The relative mix of colon and rectal cancers in any combination will presumably be dominated by colon cancers, although poorer survival rates for rectal cancer will reduce the imbalance among deceased cases. In this autoworkers cohort study, 90 of the 374 colorectal cancer deaths were due to cancer of the rectum, and in a recent prospective study the incidence of colon cancer was close to four times that of rectal cancer.7 With this uncertainty, the contribution of studies of colon and rectal cancer combined to assessing the role of metalworking fluids in the aetiology of rectal cancer alone is limited.
In the context of existing studies, the United Autoworkers‐General Motors study thus provides a unique opportunity to quantify the relationship between mortality from rectal cancer and exposure to straight metalworking fluids. The use of Cox regression serves as an improvement over other techniques, such as standardised mortality ratio and Poisson regression analyses, which ignore the continuity of the exposure variable, in favour of a dichotomous or categorical variable, and cannot handle time‐varying covariates well. In categorical analyses, the cut points between exposure categories are arbitrarily selected and can influence the shape of the dose–response curve.34,35,36 Penalised splines allow more flexibility in analysing the shape of exposure–response curves than models with a linear term for exposure by avoiding parametric constraints.
All the models considered here suggest increasing risk with increasing cumulative exposure up to about the 99th centile of exposure. The model of most interest was semiparametric and based on penalised splines with equally spaced knots throughout the upper region of sparser data. These better reflect the observed data in both the denser and sparser regions of the exposure distribution. The initial rise in HR was essentially linear for lagged and unlagged data, and the confidence curves widened where the data were sparse. These models also allowed us to observe the decrease in risk at the highest exposure levels that may reflect the common bias due to healthy worker survivor effect.37,38,39 Once seen, we can then choose between lagged or truncated exposures as a method to downweigh the tail. Although truncating graphs or deleting outliers may, in the end, be the best summary of the exposure–response, fitting the curve over the full range of exposure provides the information necessary to select the best representation of the data. This added flexibility makes penalised splines an attractive method.
Conclusions
The HR increased essentially linearly with cumulative exposure to straight metalworking fluids lagged 15 years, to a maximum of 3.2 at 40 mg/m3‐years and then decreased (with wide confidence bands). All three model‐fit criteria selected roughly the same degrees of freedom, resulting in similar curves over the entire exposure range. Where data were sparse in the tail, confidence curves widened, and the shape of the curves depended on the knot location. However, results over the relevant range of human exposures suggest a moderately strong and essentially linear association, and further clarify the evidence that exposure to straight metalworking fluid is causally associated with rectal cancer.
Abbreviations
AIC - Akaike's Information Criteria
PMR - proportionate mortality ratio
Footnotes
Funding: This study was supported by Grant CA74386 from the National Cancer Institute.
Competing interests: None.
The original study was approved by the internal review board of Harvard School of Public Health and the extended follow‐up was approved by internal review boards of University of Massachusetts and General Motors Corporation.
References
- 1.NIOSH Criteria for Recommended Standard—occupational exposure to metalworking fluid. DHHS (NIOSH) Publication Number 98–102. Cincinnati, OH: NIOSH, 1998
- 2.Mirer F. Updated epidemiology of workers exposed to metalworking fluids provides sufficient evidence for carcinogenicity. Appl Occup Environ Hyg 200318902–912. [DOI] [PubMed] [Google Scholar]
- 3.Cruickshank C N D, Squire J R. Skin cancer in the engineering industry from the use of mineral oil. Br J Ind Med 195071–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterhouse J A. Lung cancer and gastro‐intestinal cancer in mineral oil workers. Ann Occup Hyg 19721543–45. [DOI] [PubMed] [Google Scholar]
- 5.Eisen E A, Tolbert P E, Monson R R.et al Mortality studies of machining fluid exposure in the automobile industry, I: a standardized mortality ratio analysis. Am J Ind Med 199222809–824. [DOI] [PubMed] [Google Scholar]
- 6.Eisen E A, Bardin J, Gore R.et al Exposure‐response models based on extended follow‐up of a cohort mortality study in the automobile industry. Scand J Work Environ Health 200127240–249. [DOI] [PubMed] [Google Scholar]
- 7.Wei E K, Giovannucci E, Wu K.et al Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004108433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallock M F, Smith T J, Woskie S R.et al Estimation of historical exposures to machining fluids in the automotive industry. Am J Ind Med 199426621–634. [DOI] [PubMed] [Google Scholar]
- 9.Woskie S R, Smith T J, Hallock M F.et al Size‐selective pulmonary dose indexes for metal‐working fluid aerosols in machining and grinding operations in the automobile manufacturing‐industry. Am Ind Hyg Assoc J 19945520–29. [DOI] [PubMed] [Google Scholar]
- 10.Checkoway H, Pearce N, Hickey J L S. Latency analysis in occupational epidemiology. Arch Environ Health 19904595–100. [DOI] [PubMed] [Google Scholar]
- 11.Thurston S W, Eisen E A, Schwartz J. Smoothing in survival models: an application to workers exposed to metalworking fluids. Epidemiology 200213685–692. [DOI] [PubMed] [Google Scholar]
- 12.Verweij P J M, Van Houwelingen H C. Penalized likelihood in Cox regression. Stat Med 1994132427–2436. [DOI] [PubMed] [Google Scholar]
- 13.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, eds. 2nd International symposium on information theory. Budapest: Akademiai Kiado, 1973267–281.
- 14.Hurvich C M, Simonoff J S, Tsai C L. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J R Statist Soc B 199860271–293. [Google Scholar]
- 15.Therneau T M, Grambsch P M.Penalized Cox models and frailty. Technical report. Rochester, MN: Division of Biostatistics, Mayo Clinic, 1998
- 16.Verweij P J M, Van Houwelingen H C. Cross‐validation in survival analysis. Stat Med 1993122305–2314. [DOI] [PubMed] [Google Scholar]
- 17.Ruppert D. Selecting the number of knots for penalized splines. J Comput Graph Stat 200211735–757. [Google Scholar]
- 18.Tolbert P E, Eisen E A, Pothier L J.et al Mortality studies of machining‐fluid exposure in the automobile industry, II: risks associated with specific fluid types. Scand J Work Environ Health 199218351–360. [DOI] [PubMed] [Google Scholar]
- 19.IARC Polynuclear aromatic hydrocarbons, part II: carbon blacks, mineral oils (lubricant‐based oils and derived products), and some nitroarenes. IARC Monogr Eval Carcinog Risks Hum 19843387–168. [PubMed] [Google Scholar]
- 20.IARC Mineral oils. IARC Monogr Eval Carcinog Risks Hum Suppl 19877252–254. [Google Scholar]
- 21.Stear M. Metalworking fluids—clearing away the mist? Ann Occup Hyg 200549279–281. [DOI] [PubMed] [Google Scholar]
- 22.Colditz G A, Cannuscio C C, Frazier A L. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control 19778649–667. [DOI] [PubMed] [Google Scholar]
- 23.Young T B, Wolf D A. Case‐control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer 198842167–175. [DOI] [PubMed] [Google Scholar]
- 24.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer 2002101403–408. [DOI] [PubMed] [Google Scholar]
- 25.Lynch H T, Watson P, Lanspa S J.et al Natural history of colorectal cancer in hereditary nonpolyposis colorectal cancer (Lynch syndromes I and II). Dis Colon Rectum 198831439–444. [DOI] [PubMed] [Google Scholar]
- 26.Silverstein M, Park R, Marmor M.et al Mortality among bearing plant workers exposed to metalworking fluids and abrasives. J Occup Environ Med 198830706–714. [PubMed] [Google Scholar]
- 27.Vena J E, Sultz H A, Fiedler R C.et al Mortality of workers in an automobile engine and parts manufacturing complex. Br J Ind Med 19854285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazerouni N, Thomas T L, Petralia S A.et al Mortality among workers exposed to cutting oil mist: update of previous reports. Am J Ind Med 200038410–416. [DOI] [PubMed] [Google Scholar]
- 29.Leon D A, Thomas P, Hitchings S. Lung cancer among newspaper printers exposed to ink mist: a study of trade union members in Manchester, England. Occup Environ Med 19945187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Percy C, Stanek E, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health 198171242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delzell E, Brown D A, Matthews R. Mortality among hourly motor vehicle manufacturing workers. J Occup Environ Med 200345813–830. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y X, Krishnadasan A, Kennedy N.et al Estimated effects of solvents and mineral oils on cancer incidence and mortality in a cohort of aerospace workers. Am J Ind Med 200548249–258. [DOI] [PubMed] [Google Scholar]
- 33.Gerhardsson de Verdier M, Plato N, Steineck G.et al Occupational exposures and cancer of the colon and rectum. Am J Ind Med 199222291–303. [DOI] [PubMed] [Google Scholar]
- 34.Wartenberg D, Northridge M. Defining exposure in case‐control studies: a new approach. Am J Epidemiol 19911331058–1071. [DOI] [PubMed] [Google Scholar]
- 35.Wartenberg D, Savitz D A. Evaluating exposure cut‐point bias in epidemiologic studies of electric and magnetic‐fields. Bioelectromagnetics 199314237–245. [DOI] [PubMed] [Google Scholar]
- 36.Steenland K, Deddens J A. A practical guide to dose‐response analyses and risk assessment in occupational epidemiology. Epidemiology 20041563–70. [DOI] [PubMed] [Google Scholar]
- 37.Arrighi H M, Hertz‐Picciotto I. Controlling healthy worker survivor effect: an example of arsenic exposure and respiratory cancer. Occup Environ Med 199653455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox A J, Collier P F. Low mortality rates in industrial cohort studies due to selection for work and survival in the industry. Br J Prev Soc Med 197630225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monson R R. Observations on the healthy worker effect. J Occup Med 198628425. [DOI] [PubMed] [Google Scholar]