Abstract
Background
Family members of laboratory animal workers are at risk of developing allergy to laboratory animals. Little is known about the spreading of laboratory animal allergens outside the animal facilities.
Objective
To assess the presence of laboratory animal allergens in dust collected from mattresses of laboratory animal workers and unexposed controls.
Methods
Mouse and rat urinary proteins were measured in samples of mattress dust collected by laboratory animal workers and unexposed controls. In addition, rat and mouse allergens were determined in extracts of hair‐covering caps, used during laboratory animal work, to estimate spreading of allergen through dust captured on hair. Allergen concentrations on hair caps were compared with exposure measured by personal airborne dust sampling.
Results
Levels of rat urinary allergens (RUA) and mouse urinary allergens (MUA) and mouse urinary protein (MUP) 8, a specific pheromone‐binding mouse allergen, were significantly higher in mattress samples of laboratory animal workers than in those of controls. Hair‐covering caps used in animal facilities harboured large amounts of RUA and MUA, which correlated significantly with exposure measured by the personal sampling technique in the animal facility.
Conclusions
Occupational laboratory animal allergens are detectable in mattress dust of laboratory animal workers. Transfer of allergens via uncovered hair of animal workers is likely contributing to this phenomenon. This study stresses the importance of using hair caps to prevent spreading of occupational allergens.
Occupational allergy against laboratory animals is a common problem among laboratory animal workers. The prevalence of laboratory animal allergy is reported to be 10–25%.1 Allergens of laboratory animals are potent sensitisers and small amounts can elicit symptoms in sensitised individuals. Moreover, there are also indications that reduction of exposure may lead to a decreased incidence of laboratory animal allergy.2,3,4 Methods for controlling exposure to laboratory animal allergens include the choice of bedding materials and adjustment of cage‐changing processes, and the use of personal protective equipment.5 Despite the fact that the risk of developing laboratory animal allergy is high and personal protective equipment is widely available, respiratory protection is not routinely used. Laboratory coats and protective gloves are widely used, but the use of hair‐covering caps and facemasks is mostly restricted to already sensitised individuals to prevent symptoms.
Although direct contact with animals probably accounts for most of the airway exposure, a possibly underestimated route of exposure may be subsequent exposure to allergens transferred from the animal facility through hair, clothing and documents.5 It was shown for cat allergen that transfer can lead to exposure of individuals without direct contact with animals.6,7 Moreover, children of laboratory animal workers were shown to have an increased risk of developing laboratory animal allergy,8 suggesting that subsequent exposure also influences allergen loads in houses of laboratory animal workers and may sensitise family members.
It has been suggested that allergens captured in human hair can play an important role in exposure to laboratory animal allergens outside the animal facility. So far, evidence supporting the relevance of this route of exposure is scarce, but animal workers are generally advised to wash their hair after work to prevent contamination of the home environment with occupational aeroallergens.5 The use of hair‐covering caps is another method to prevent allergen transfer through human hair. Despite this advice, regular use of hair caps or washing hair after finishing work was a standard procedure in <20% of the laboratory animal facilities we studied in The Netherlands. By contrast, special clothing was used in all facilities.
We measured the levels of laboratory animal allergens in the mattress dust of laboratory animal workers and compared it with allergen concentrations in mattresses of controls who are not occupationally exposed. The allergen load on hair‐covering caps used by laboratory animal workers was measured to assess whether carry‐over through the hair of workers may be a relevant route of allergen transfer. In addition, the allergen load on hair‐covering caps was compared with the level of airborne exposure as determined by the personal airborne‐dust sampling technique.
Methods
Mattress samples
Fifteen laboratory animal workers and 15 controls were asked to collect dust samples from their mattresses and pillows. The controls and their partners had never worked with laboratory animals or in animal facilities, and never had mice or rats as pets. They included medical physicians, medical laboratory staff and office employees.
The 15 laboratory animal workers were employed in five different research facilities. All laboratory animal workers worked with rats and six of them also worked with mice, with the mean (range) time of laboratory animal work estimated at 19 (2–45) h/week. All participating laboratory animal workers wore special, protective clothing during animal work, but no hair‐covering caps.
Participants received a sampling sock (Allied Filter Fabrics, Hornsby, Australia) and illustrated instructions for sampling. Using sampling socks in a vacuum cleaner tube, participants collected dust from their pillow and mattress (without blankets) by vacuuming for 30 s and 2 min, respectively.9 The sampling sock was attached into the vacuum's extension tube and sealed with tape. Before turning the vacuum cleaner off, the extension tube was held vertically to keep the dust in the sock. The sock was removed and placed in a sealed bag and mailed to the laboratory. Information about sampled area size, mattress age, pets at home and details on laboratory animal work were collected through a questionnaire. Dust samples were weighed and dust was extracted in 25 ml phosphate‐buffered saline (PBS)/tween (0.05% tween) by end‐over‐end rolling for 1 h followed by centrifugation at 2000 g for 15 min. Supernatants were stored in small aliquots at −20°C until analysis. Allergens found in mattress samples were expressed as total amount recovered and as amount of allergen per gram of dust (allergen/gram dust).
Hair‐covering caps
Laboratory animal workers from six large animal facilities of universities and research institutes in The Netherlands were asked to wear surgical hair‐covering caps (Klinedrape basic, Mölnlycke Health Care, Götenborg, Sweden) during their laboratory animal work. We collected 45 caps worn in different rooms, for different times (mean (range) duration 84 (15–200) min) while performing different tasks. Unused caps served as a control. After use, caps were stored at −20°C in sealed bags. For allergen extraction, caps were defrosted and washed with 10 ml PBS/tween (0.05% tween) and after centrifugation at 700 g, supernatant was stored at −20°C until use in allergen assays. Allergen levels on hair caps were expressed as the amount of allergen per hour the cap was worn.
Personal sampling
At the time that hair‐covering caps were collected for this study, personal airborne‐dust sampling was performed (n = 20). Hair‐covering caps and personal samplers were used simultaneously by the same person. PAS‐6 sampling heads10 were equipped with glass micro‐fibre filters (Ø 25 mm, Fischer Scientific, 's‐Hertogenbosch, The Netherlands) and were driven by Gillian air pumps operating at a flow rate of 2 l/min. The mean (range) sampling time was 94 (39–380) min. Filters were extracted as described previously and supernatants were tested in the MUA and RUA enzyme immunoassay.10 Airborne samples were expressed as nanogram rat or mouse urinary protein equivalent/m3 (ng eq/m3).
Determination of RUA, MUA and MUP8
For enzyme immunoassay, polystyrene high‐capacity microtitre plates were coated with ammonium sulphate‐precipitated rabbit anti‐rat urine antibodies or ammonium sulphate‐precipitated rabbit anti‐mouse urine antibodies (2 μg/ml in PBS) at 4°C overnight. Specifications of the antibodies have been previously described.10 After incubation, the plates were washed and blocked with PBS/0.05% Tween‐20 and 0.2% gelatine, followed by incubation of 100 μl of the samples for 60 min at 37°C. After washing, bound rat and mouse allergen was detected using biotinylated rabbit anti‐RUA or anti‐MUA antibodies, respectively, followed by avidin peroxidase conjugate and o‐pheylenediamine. The reaction was stopped with 2M hydrochloric acid and the absorbance of the samples was measured at 492 nm.10 Concentrations were read from a standard dose–response callibration curve obtained with rat and mouse urinary proteins. Concentrations were expressed in nanograms of animal urinary protein equivalent (ng eq) per millilitre in which 1 ng eq was defined as the amount giving the same assay result as 1 ng of the standard, ranging from 0.03 to 4 ng eq/ml.
For detection of MUP8, one of the lipocalin isoforms from the major urinary protein complex known as the major mouse allergen Mus m 1,11,12 we used a competitive radioimmunoassay with recombinant MUP8.13 Expression vector for MUP8 was expressed in Escherichia coli and purified as described elsewhere.11 MUP8 was labelled with 125I. Ammonium sulphate‐precipitated rabbit anti‐mouse urine antibodies10 were coupled to Protein G (CNBr‐activated Sepharose 4B; Pharmacia, Uppsala, Sweden) and preincubated with 200 µl supernatant of the mattress samples for 1 h at room temperature before adding radiolabelled recombinant MUP8. After overnight incubation, samples were washed and the amount of bound radioactivity was measured. Allergen concentrations were read from a standard dilution curve of unlabelled recombinant MUP8. Due to high‐sequence homology between different major urinary proteins of the mouse and possible cross‐reactivity of MUPs, results were expressed in nanograms of MUP8 protein equivalent (ng eq) per millilitre in which 1 ng eq was defined as the amount giving the same assay result as 1 ng recombinant MUP8.
Statistical analysis
Statistical analysis was performed using the SPSS V.10.0. Because of a skewed distribution of data, all values were log‐transformed for parametric statistical analyses, while non‐parametric analysis was performed when normal distributions were not obtained after log transformation. Differences in allergen concentrations between different tasks and between mattress samples were tested for significance using Student's t test and Mann–Whitney U test. Pearson correlation coefficients (r) and Spearman correlation coefficients (r) were calculated to study relationships between parameters. We considered p<0.05 to represent a significant difference.
Results
Allergens in mattress samples
To estimate indirect exposure to laboratory animal allergens during the night, samples of pillow and mattress dust from 15 participants working with laboratory animals (mice and/or rats) were compared with samples collected by 15 volunteers without contact with laboratory animals. Table 1 summarises the characteristics of the groups.
Table 1 Characteristics of the groups.
| Animal workers | Controls | |
|---|---|---|
| Number (n) | 15 | 15 |
| Sex (male/female) | 7/8 | 7/8 |
| Pets at home (n) | 8 | 7 |
| Pets | ||
| Cat (n) | 4 | 7 |
| Dog (n) | 4 | 1 |
| Age of mattress (years: median, range) | 5 (1–20) | 6 (1–20) |
| Mattress size (m2: mean, range) | 2.8 (1.9–4.5) | 2.5 (1.5–3.9) |
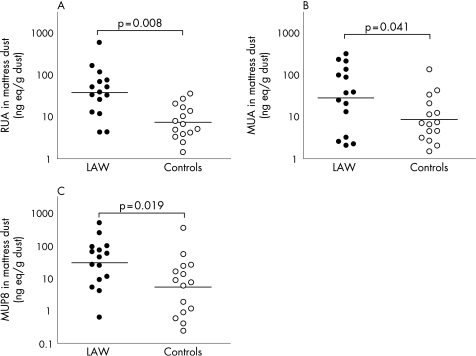
Although laboratory animal workers vacuumed lower amounts of dust from their mattresses, their total amount of RUA, MUA and MUP8 were higher than in samples of controls (table 2, fig 1). The difference in amounts per sample was significant for RUA only, whereas the difference in ng eq/g dust was significant for both RUA and MUA. We found a trend for an association between the total amount of RUA per mattress and the duration of laboratory animal exposure (hours per week) at work within the group of laboratory animal workers (r = 0.504, p = 0.054). The amount of dust collected was related to the age of the mattress (r = 0.390, p = 0.037). The amount of MUA was related to the age of the mattress (r = 0.452, p = 0.014) and there was a trend between the amount of MUA/g dust and the age of the mattress (r = 0.350, p = 0.062). No such relationship between RUA and the age of the mattress was found.
Table 2 Laboratory animal allergens in mattress dust.
| Animal workers | Controls | p Value | |
|---|---|---|---|
| Collected dust (g, median, range) | 0.5 (0.2–1.5) | 0.9 (0.3–2.0) | 0.033 |
| Total collected RUA (ng eq) | 18.9 (9.6 to 37.2) | 5.9 (3.8 to 9.2) | 0.008 |
| RUA/g dust (ng eq/g) | 39.3 (19.8 to 78.0) | 7.6 (4.7 to 12.2) | <0.001 |
| Total collected MUA (ng eq) | 14.2 (5.3 to 37.7) | 6.9 (3.2 to 14.7) | 0.251 |
| MUA/g dust (ng eq/g) | 29.5 (11.7 to 74.6) | 8.8 (4.6 to 16.8) | 0.041 |
| Total collected MUP8 (ng eq) | 14.9 (5.8 to 38.3) | 4.4 (1.4 to 14.1) | 0.116 |
| MUP8/g dust (ng eq/g) | 30.9 (12.8 to 74.8) | 5.6 (2.0 to 16.0) | 0.019 |
MUA, mouse urinary allergen; MUP, mouse urinary protein; RUA, rat urinary allergen.
All values are expressed in nanograms of animal urinary protien equivalent per hour and allergen values are represented by their geometric means and 95% CIs, unless otherwise specified.
Comparison was carried out with Student's t test.
Figure 1 Laboratory animal allergens in mattress dust. The amounts of (A) rat urinary allergen (RUA), (B) mouse urinary allergen (MUA) and (C) mouse urinary protein (MUP) 8 were measured in the mattress dust of laboratory animal workers (LAW) and controls. The amount of allergen is expressed in nanograms of animal urinary protein equivalent (ng eq) per gram (g) dust collected. There is a significant difference in all allergens between LAW and controls.
There was no significant difference between laboratory animal workers and controls with respect to the number of pets that were kept. When comparing the mattress dust of participants with or without pets, pet owners had overall more RUA and MUA in their mattress dust, both in laboratory animal workers and in controls. We therefore investigated the cross‐reactivity of cat and dog proteins in the RUA and MUA assay. Neither urine nor dander extracts of cats or dogs showed any binding in the RUA and MUA assay and neither did house dust mite or human urine, serum and dander (data not shown).
In addition to the assay with polyclonal reagents directed against rodent urinary proteins, the concentration of the major MUA was estimated with a competitive immunoassay for MUP8. The amounts of MUP8 per gram dust found in mattresses of laboratory animal workers were significantly higher than in samples of controls (p = 0.019, fig 1). There was no relationship between the levels of MUP8 and presence of pets, the age of the mattress, the amount of dust collected or the size of the mattress. The amounts of MUA and MUP8 showed a significant association (total amount per sample r = 0.706, p<0.001; amount per gram dust r = 0.682, p<0.001).
Hair‐covering caps
Laboratory animal allergens were detected on all 45 hair caps used during laboratory animal work, while no allergen was found on an unused control cap. Allergen levels were clearly associated with the animal species handled. RUA levels on the 15 caps used in rat rooms varied from 88 to 3661 ng eq and MUA levels on the 30 caps used while working with mice varied from 6 to 14 586 ng eq (table 3).
Table 3 Hair‐covering caps used during laboratory animal work.
| Caps used in: | RUA | MUA |
|---|---|---|
| Rat rooms (n = 15) | 318 (155 to 654) | 11 (4 to 29) |
| Mouse rooms (n = 30) | 4 (2 to 9) | 363 (169 to 777) |
| Rat rooms during care‐taking tasks (n = 7) | 537 (199 to 1449) | 25 (5 to 121) |
| Rat rooms during biotechnical tasks (n = 8) | 201 (76 to 533) | 5 (2 to 15) |
| Mouse rooms during care‐taking tasks (n = 19) | 4 (2 to 12) | 1051* (467 to 2365) |
| Mouse rooms during biotechnical tasks (n = 11) | 5 (1 to 15) | 58 (30 to 110) |
MUA, mouse urinary allergen; RUA, rat urinary allergen.
All values are expressed in nanograms of animal urinary protien equivalent per hour and represent geometric means and 95% CIs.
*Significantly different from MUA measured during biotechnical tasks (p<0.001).
Although mice and rats were kept in separate rooms in the participating laboratory animal facilities, we also found low levels of MUA on caps used only in rat rooms and RUA on caps used in mouse rooms (table 3).
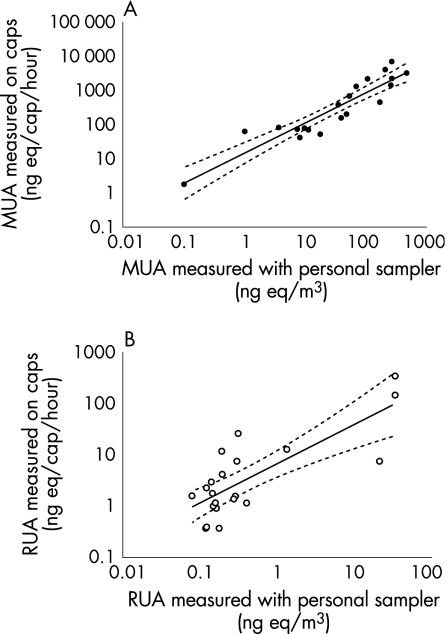
Allergen levels on hair caps used during care‐taking tasks (cleaning, feeding and handling) were higher than during biotechnical tasks (operating, injection and blood sampling). This difference was more pronounced for MUA than for RUA (table 3). Airborne allergen exposure levels were estimated, using the PAS technique, concurrently with the use of 18 hair‐covering caps in mouse rooms and two in rat rooms (fig 2). Allergen levels measured on caps and allergens measured by personal airborne dust sampling were highly correlated (MUA r = 0.908, p<0.001, fig 2A; RUA r = 0.608, p = 0.004, fig 2B). The results of allergen measurements on caps and allergen levels detected with personal samplers showed an association with the number of animals present in the rooms (MUA: caps r = 0.794, p<0.001; personal sampling r = 0.81, p<0.001; RUA: caps r = 0.549, p = 0.012; personal sampling r = 0.587, p = 0.007).
Figure 2 Exposure measurements of mouse urinary allergen (MUA) and rat urinary allergen (RUA). Exposure to MUA (A) and RUA (B) was measured using the personal sampling technique and using hair‐covering caps. Results from 20 measurements, 18 in mouse rooms and two in rat rooms, are expressed in both graphs. There was a significant correlation between the results of the two assays (MUA, r = 0.908, p<0.001; RUA, r = 0.608, p = 0.004). ng eq, nanograms of animal urinary protien equivalent.
Discussion
In this study, we showed that mattresses of laboratory animal workers contain significantly higher amounts of RUA and MUA than mattresses of controls. In addition, substantial amounts of rat and mouse urinary proteins were recovered from hair caps worn during laboratory animal work and levels were significantly associated with airborne allergen concentrations determined by the PAS technique.
As we found higher levels of rodent allergens in mattress dust from homes with pets, we reassessed the specificity of the assays for rodent urinary allergens, but found no cross‐reactivity when testing cat allergens, dog allergens, house dust mite and human serum, dander and urine. Moreover, it was shown previously that there was no cross‐reactivity with other rodent allergens and rodent food.10 A possible explanation for higher levels of rodent allergens in the mattress dust of pet owners could be pets transferring rodent allergens through their fur. Especially, allergens transferred by pets that are allowed in bedrooms could influence rodent allergen levels in mattress dust.
Differences between levels of rodent allergens in mattress dust from laboratory animal workers and controls were less pronounced for MUA than for RUA. This is probably due to the lower number of employees working with mice in our study. Moreover, MUA is reported to be found in settled dust in most houses, indicating that it should be considered a common environmental allergen as well as an occupational allergen.14,15
Detection of MUA is generally carried out with polyclonal antibodies raised against urinary proteins. The major MUA is Mus m 1, consisting of a complex of lipocalin isoforms, but mouse urine also contains minor allergens like prealbumin. MUP8, one of the lipocalin isoforms of Mus m 1, is a pheromone‐binding urinary protein excreted mainly by adult male mice and has been shown to remain airborne for long periods of time.5,11 Availability of recombinant MUP8 made it possible to make an assay specific for Mus m 1. The agreement between the results of the MUA assay and the assay for MUP8 confirms that mouse‐specific allergens were detected in mattresses.
The levels of rat and mouse allergens in the mattress dust of occupationally exposed workers are significantly lower than those of well‐known common environmental allergens like house dust mite (Der p 1, Der f 1) or cat (Fel d 1).16 Nevertheless, such low amounts of rodent allergens were associated with an enhanced risk of sensitisation in children living in inner cities and suburban areas and in children of laboratory animal workers.8,14,17
The laboratory animal allergens found in mattresses from laboratory animal workers are likely to be carried from the laboratory to their homes. Human hair is often considered a reservoir for allergen and may serve as a secondary source of allergen.5 It was shown before that the hair of cat owners is a reservoir for cat allergens and also clothing has been reported as a carrier for allergens.18,19,20,21 By using hair‐covering caps, we showed that high amounts of laboratory animal allergens settle on the heads of laboratory animal workers. We choose to measure allergen in extracts of hair caps instead of direct measurements in hair wash fluid as the latter fluid is influenced by type and length of the hair and requires larger amounts of fluid and, as a consequence, leads to a lower detection threshold. We assume that, due to the larger total surface area and the electrostatic properties of uncovered hair, the results of allergen on hair caps will underestimate allergen load on uncovered hair.
Main messages
Occupational allergens can be carried home and contaminate the mattresses of laboratory animal workers.
High amounts of occupational allergens can be recovered from hair‐covering caps used by laboratory animal workers and these caps can be used as a tool to estimate occupational exposure.
Policy implications
Employees exposed to occupational allergens should wear hair‐covering caps or wash their hair before going home to prevent contamination of the home environment with occupational allergens.
Used hair‐covering caps, supplied with additional information concerning the time used and tasks performed, provide a simple and inexpensive technique for estimating occupational exposure.
All participating laboratory animal workers wore special clothing in the animal facility and changed their clothes before going home. However, wearing hair caps or washing the uncovered hair before leaving work was not a standard procedure in most facilities. Therefore, human hair is probably the main carrier for animal allergens. Although none of the participants in our study had beards or moustaches, facial hair might also be considered as a transfer route for occupational allergens.
As previous studies showed exposure–response relationships for laboratory animal workers to develop laboratory animal allergy,2,3 the use of caps can reduce secondary exposure and may reduce the incidence of laboratory animal allergy. As family members of laboratory animal workers have been shown to have an enhanced risk for developing rodent allergy,8 prevention of carrying home allergens is also expected to decrease development of allergy in this group.
Like Hollander et al,22 we found that care‐taking tasks are associated with higher levels of exposure to laboratory animal allergens. When measuring exposure with PAS, we found airborne allergen levels to correlate significantly with allergen levels found on hair‐covering caps. There is no established gold standard for collecting dust samples for measuring occupational allergen levels.23 Compared with PAS, the use of hair caps for estimating allergen‐exposure levels is simple and inexpensive. Therefore, this method should be considered as an alternative to more sophisticated tools in the field of exposure measurements.
In conclusion, we showed that laboratory animal allergens can be carried home and contaminate the bedding of laboratory animal workers. Considering the high amounts of allergens recovered from hair caps, human hair is probably one of the main routes of transportation. The amount of allergens found on hair‐covering caps correlated well with the exposure measured by PAS techniques. Therefore, measuring allergen on hair caps may be a simple and inexpensive alternative for estimation of occupational airway exposure to laboratory animal allergens. It should be advised that laboratory animal workers, especially those employees with care‐taking tasks, wear hair‐covering caps or wash their hair before leaving the animal facility. This may reduce their own exposure to laboratory animal allergens, and also decrease exposure of family members.
Acknowledgement
We acknowledge Dr Scott Sharrow (Bloomington, Indiana, USA) for the preparation of recombinant MUP8 protein.
Abbreviations
MUA - mouse urinary allergen
MUP - mouse urinary protein
ng eq - nanograms of animal urinary protein equivalent
PAS - personal air sampling
PBS - phosphate‐buffered saline
RUA - rat urinary allergen
RUP - rat urinary protein
Footnotes
Funding: This study was supported by a research grant from the Netherlands Asthma Foundation.
Competing interests: None declared.
References
- 1.Aoyama K, Ueda A, Manda F.et al Allergy to Laboratory‐Animals—An Epidemiologic‐Study. Br J Ind Med 19924941–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollander A, Heederik D, Doekes G. Respiratory allergy to rats: exposure‐response relationships in laboratory animal workers. Am J Respir Crit Care Med 1997155562–567. [DOI] [PubMed] [Google Scholar]
- 3.Matsui E C, Krop E J, Diette G B.et al Mouse allergen exposure and immunologic responses: IgE‐mediated mouse sensitization and mouse specific IgG and IgG4 levels. Ann Allergy Asthma Immunol 200493171–178. [DOI] [PubMed] [Google Scholar]
- 4.Bush R K. Mechanism and epidemiology of laboratory animal allergy. ILAR J 2001424–11. [DOI] [PubMed] [Google Scholar]
- 5.Harrison D J. Controlling exposure to laboratory animal allergens. ILAR J 20014217–36. [DOI] [PubMed] [Google Scholar]
- 6.D'Amato G, Liccardi G, Russo M.et al Clothing is a carrier of cat allergens. J Allergy Clin Immunol 199799577–578. [DOI] [PubMed] [Google Scholar]
- 7.De Lucca S D, O'meara T J, Tovey E R. Exposure to mite and cat allergens on a range of clothing items at home and the transfer of cat allergen in the workplace. J Allergy Clin Immunol 2000106874–879. [DOI] [PubMed] [Google Scholar]
- 8.Krakowiak A, Szulc B, Gorski P. Allergy to laboratory animals in children of parents occupationally exposed to mice, rats and hamsters. Eur Respir J 199914352–356. [DOI] [PubMed] [Google Scholar]
- 9.Schram‐Bijkerk D, Doekes G, Boeve M.et al Nonlinear relations between house dust mite allergen levels and mite sensitization in farm and nonfarm children. Allergy 200661640–647. [DOI] [PubMed] [Google Scholar]
- 10.Hollander A, Van Run P, Spithoven J.et al Exposure of laboratory animal workers to airborne rat and mouse urinary allergens. Clin Exp Allergy 199727617–626. [PubMed] [Google Scholar]
- 11.Sharrow S D, Vaughn J L, Zidek L.et al Pheromone binding by polymorphic mouse major urinary proteins. Protein Sci 2002112247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price J A, Longbottom J L. Allergy to mice. II. Further characterization of two major mouse allergens (AG 1 and AG 3) and immunohistochemical investigations of their sources. Clin Exp Allergy 19902071–77. [DOI] [PubMed] [Google Scholar]
- 13.Van Ree R, van Leeuwen W A, Bulder I.et al Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J Allergy Clin Immunol 19991041223–1230. [DOI] [PubMed] [Google Scholar]
- 14.Matsui E C, Simons E, Rand C.et al Airborne mouse allergen in the homes of inner‐city children with asthma. J Allergy Clin Immunol 2005115358–363. [DOI] [PubMed] [Google Scholar]
- 15.Phipatanakul W, Eggleston P A, Wright E C.et al Risk factors for sensitization to mouse allergen in inner‐city children with asthma. J Allergy Clin Immunol 2000105S79. [DOI] [PubMed] [Google Scholar]
- 16.van Strien R T, Koopman L P, Kerkhof M.et al Mattress encasings and mite allergen levels in the Prevention and Incidence of Asthma and Mite Allergy study. Clin Exp Allergy 200333490–495. [DOI] [PubMed] [Google Scholar]
- 17.Chew G L, Perzanowski M S, Miller R L.et al Distribution and determinants of mouse allergen exposure in low‐income New York City apartments. Environ Health Perspect 20031111348–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amato G, Liccardi G, Russo M.et al Clothing is a carrier of cat allergens. J Allergy Clin Immunol 199799577–578. [DOI] [PubMed] [Google Scholar]
- 19.De Lucca S D, O'meara T J, Tovey E R. Exposure to mite and cat allergens on a range of clothing items at home and the transfer of cat allergen in the workplace. J Allergy Clin Immunol 2000106874–879. [DOI] [PubMed] [Google Scholar]
- 20.Tovey E R, Mahmic A, McDonald L G. Clothing—an important source of mite allergen exposure. J Allergy Clin Immunol 199596(Pt 1)999–1001. [DOI] [PubMed] [Google Scholar]
- 21.Liccardi G, Barber D, Russo M.et al Human hair: an unexpected source of cat allergen exposure. Int Arch Allergy Immunol 2005137141–144. [DOI] [PubMed] [Google Scholar]
- 22.Hollander A, Heederik D, Doekes G.et al Determinants of airborne rat and mouse urinary allergen exposure. Scand J Work Environ Health 199824228–235. [DOI] [PubMed] [Google Scholar]
- 23.Hollander A, Gordon S, Renstrom A.et al Comparison of methods to assess airborne rat and mouse allergen levels. I. Analysis of air samples. Allergy 199954142–149. [DOI] [PubMed] [Google Scholar]