Abstract
Aim
To investigate the risk of death associated with selected cut‐off points for rate of decline of forced expiratory volume in one second (FEV1).
Methods
Mortality rates of a cohort of 1730 coal miners who had performed two pulmonary function tests 12.8 years apart were followed up for an additional 12 years. Based on previous studies, cut‐off points for FEV1 rate of decline (ml/year) were selected as 30, 60 and 90 ml/year. Cox proportional hazard regression was used to estimate multivariate risk ratio of death in each category.
Results
The risk ratios (compared to “below 30 ml/year”) were 1.39 (95% CI 0.99 to 1.97) in the “60 to less than 90 ml/year” category and 1.90 (95% CI 1.32 to 2.76) in the “90 ml/year and above” category. Rates of decline above 90 ml/year were consistently related to excess mortality. In non‐smokers and those with neither restrictive nor obstructive patterns at the first survey, rates of decline above 60 ml/year were significantly associated with increased mortality.
Conclusions
Risk of death increases in individuals with rates of decline above about 60 ml/year and is statistically significant with declines of 90 ml/year or more. These results should be useful to healthcare providers in assessing lung function declines observed in individuals.
Pulmonary function has been recognised as an important predictor of mortality.1,2,3,4,5,6,7,8,9,10 Studies have conclusively demonstrated that the level of pulmonary function, measured using various functional parameters, is inversely associated with subsequent mortality from all causes, lung cancer and cardiovascular disease.1,2,3,4,5,6,7,8,9,10 Additionally, an association between the rate of decline in lung function and both cardiovascular and all‐cause mortality has been observed in several studies.11,12,13,14,15 Although previous studies have shown an association between rate of decline and mortality,12,13,14,15 they did not define the degree of mortality risk associated with categories of rates of decline that may be useful in screening for respiratory impairment and preventing lung disease. Such risk information may be helpful in guiding the interpretation of lung function decline in relation to the risk of impairment, disability and, ultimately, death.
When monitoring lung health, the rate of pulmonary function decline in an individual provides information that can be used for triggering interventions before an individual develops irreversible respiratory impairment and disability. The question addressed in this study is: what rate of sustained forced expiratory volume in one second (FEV1) decline indicates a significant risk for death?
In healthy adults, FEV1 declines on average between 25 and 30 ml/year as part of the normal aging process.16 Mean FEV1 declines of 60 ml/year, double the rate in healthy individuals, have often been observed in cigarette smokers,17,18 while declines greater than 90 ml/year, or triple what is expected, may be seen in susceptible individuals who progress to chronic obstructive pulmonary disease (COPD).19 Rate of FEV1 decline cut‐off points, such as 60 ml/year and 90 ml/year could be considered clinical benchmarks.
The aim of this study is to further investigate in a male working population the relation between rates of decline in pulmonary function and subsequent mortality, and to quantify the excessive mortality risk associated with different categories of decline considered important in clinical practice and research.
Materials and methods
Study population
The cohort examined in this study was selected from participants in the National Study of Coal Workers' Pneumoconiosis (NSCWP). The NSCWP consisted of three serial cross‐sectional and one follow‐up survey over a period of about 18 years (1969–88) in US underground coal mines.20,21 At each survey, participants completed spirometry testing and a questionnaire regarding their symptoms, smoking status, medical history and demographics. In addition, participants in the first survey were followed up for mortality to 30 June 1997.22 Pulmonary function testing and quality control for the first survey is published elsewhere.23,24 In the third and fourth survey, testing was done according to the then available American Thoracic Society (ATS) criteria.25 Vital status was determined through national databases and individual follow‐up conducted by mailings to family members of the deceased.22 Death certificate information was acquired from the National Death Index. The underlying cause of death was coded by a certified nosologist according to the ninth revision of the International Classification of Diseases (ICD‐9).
The study mortality cohort consisted of participants from the first survey (1969–71, n = 9076) who had valid pulmonary function tests (PFT) during the first survey and also during either the third (1978–80) or fourth survey (1985–8). Participation rates were 90% and 52% in the first and third surveys respectively. For the fourth survey—a follow‐up of participants of the first and second surveys—the participation rate was approximately 70%.26 Participants who had their last PFT at the second survey (1972–3) were not included in this study because there was a maximum of four years between the surveys; it has been shown that a five year minimum follow‐up interval is necessary for relatively precise estimation of the FEV1 rate of decline.27 Participants who were younger than 30 years at the first survey (n = 735) were excluded in order to reduce the effect of continued lung growth in the analysis. There were 1730 participants who fulfilled the study eligibility criteria.
Statistical methods
FEV1 rate of decline (ml/year) was calculated for each person in the study as the difference between the first and last FEV1 measurements divided by the time between the measurements. ATS criteria28 were used to define obstructive and restrictive patterns (see table 1) where cross‐sectional lower limits of normal were determined using published prediction equations.29 Presence of obstructive and/or restrictive patterns was determined in order to identify individuals with pre‐existing lung‐related disease.
Table 1 Patterns of lung abnormalities.
| Pattern | Definition |
|---|---|
| Restrictive | FVC<LLN |
| Obstructive | FEV1/FVC<LLN and FEV1% Pred<100% |
| Mixed | FVC<LLN and FEV1/FVC<LLN and FEV1% Pred<100% |
| Normal | Does not fall into any of the above categories |
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; LLN, lower limits of normal.
Three statistical methods were used: (1) crude and age‐adjusted mortality rates were calculated by categories of FEV1 rate of decline to determine trends in mortality; (2) smoothed plots were constructed to graphically illustrate the relation between mortality and FEV1 rate of decline and (3) a Cox proportional hazard model was used to estimate crude and adjusted risk ratios (RR) of death for selected categories of FEV1 rate of decline. SAS/STAT version 9.1 (SAS Institute Inc, Cary, NC, USA) was used for most of the analysis. S‐Plus version 6.2 (Insightful Corp, Seattle, WA, USA) was used to construct the smoothed plots.
Crude and age‐adjusted mortality rates (per 1000 person‐years) were calculated in the study cohort for the following four categories of FEV1 rate of decline: “below 30 ml/year”, “30 to less than 60 ml/year”, “60 to less than 90 ml/year” and “90 ml/year and above”. Rates were adjusted by direct standardisation using the age distribution of the entire study cohort.30 These calculations were completed for both (1) all‐cause and (2) cardiovascular mortality (ICD‐9 code: 390–459) and non‐malignant respiratory disease mortality (ICD‐9 code: 490–518). Rates were also calculated for the non‐study cohort members (NSCWP participants not included in our mortality cohort) who participated in the first survey, were age 30 or older and survived until 1 January 1980 (the end of the third survey). In this analysis, the rates were standardised using the age distribution of all participants in the first survey.
Penalised splines31 were used to derive smoothed plots of the shape of the relation between the log hazard mortality ratio and FEV1 rate of decline. Splines were incorporated into a Cox regression model that adjusted for confounders described below. Smoothing parameters were chosen by various criteria, including Akaike's Information Criterion.32 Separate shape analyses were completed in the overall cohort and three subgroups: (1) never smokers, (2) current and former smokers and (3) those with neither airway obstructive nor restrictive patterns at the first survey.
Kaplan–Meier survival curves were used to test for proportionality in the hazard function. After proportionality was demonstrated, Cox proportional hazard regression (PROC PHREG in SAS) was used to estimate the risk ratio for the association between FEV1 rate of decline categories and all‐cause mortality while adjusting for the confounders described below. The risk ratio was calculated using the category of “below 30 ml/year” as the reference. Person‐years were calculated from the date of last PFT to the date of death or 30 June 1997. A level of significance of 0.05 was applied. Adjusted and unadjusted models were fitted for the overall cohort, for never smokers, for current and former smokers and for those with neither obstructive nor restrictive patterns at the first survey. Smoking status was determined using responses at both surveys, as follows: a never smoker stated on both surveys that they never smoked; current smokers and former smokers reported themselves as such on their last survey. Other permutations of smoking status were considered inconsistent.
Possible confounders of the relation of FEV1 rate of decline and mortality were added to the multivariate regression. These variables included initial age, initial FEV1, height and rate of change in weight. Rate of change in weight was calculated as the difference in weight at the initial and follow‐up surveys divided by time between the two measurements (kg/year).
Results
Table 2 describes the basic demographics of the study cohort stratified by mortality status, and the non‐study cohort. At the first survey, the cohort was relatively young, 42 years old on average, ranging from 31 to 61 years. There were 285 deaths during an average of 12.4 years (range 0.3–19.7).
Table 2 Basic demographic characteristics of the study cohort (n = 1730) and of non‐study cohort who were in survey 1 (n = 7309), US, National Study of Coal Workers' Pneumoconiosis, 1969–97.
| Characteristic | Cohort | Non‐study cohort | |||
|---|---|---|---|---|---|
| Alive | Dead | n (%) | |||
| n, % | 1,445 | 83.5 | 285 | 16.5 | 7309* (100) |
| Initial age (years) (mean, SD) | 41.6 | 6.6 | 46.1 | 6.4 | 45.5 (12.9) |
| Person‐years of mortality follow‐up (mean, SD) | 13.5 | 3.7 | 7.14 | 4.3 | 21.2† (7.7)† |
| Initial BMI (mean, SD) | 25.9 | 3.4 | 26.2 | 3.8 | 25.9 (3.8) |
| Change in weight (kg/year) (mean, SD) | 0.36 | 0.61 | 0.19 | 0.6 | NA |
| Pack‐years (mean, SD) | 19.2 | 18.9 | 22.6 | 18.9 | 17.8 (18.1) |
| Initial FEV1 (l) (mean, SD) | 3.8 | 0.6 | 3.5 | 0.6 | 3.5 (0.8) |
| Obstructive/restrictive patterns at first survey | |||||
| Obstructive pattern only (n, %) | 239 | 16.5 | 51 | 17.9 | 1267 (17.3) |
| Restrictive pattern only (n, %) | 150 | 10.3 | 48 | 16.8 | 765 (10.5) |
| Both obstructive and restrictive pattern (n, %) | 31 | 2.1 | 13 | 4.6 | 1124 (15.4) |
| FEV1 rate of decline (ml/year) (mean, SD) | 43.5 | 31.5 | 53.6 | 39.0 | NA |
| Years between PFT (mean, SD) | 13.2 | 3.6 | 10.9 | 3.6 | NA |
| Smoking status | |||||
| Current smoker (n, %) | 487 | 33.7 | 145 | 50.9 | 3966 (54.3) |
| Never smoked (n, %) | 284 | 19.7 | 43 | 15.1 | 1470 (20.1) |
| Former smoker (n, %) | 607 | 42.0 | 82 | 28.8 | 1873 (25.6) |
| Indeterminate smoker (n, %) | 67 | 4.6 | 15 | 5.2 | NA |
BMI, body mass index; FEV1, forced expiratory volume in one second; PFT, NA, not available; PFT, pulmonary function test.
*Dead (2971, 40.7%).
†Calculated from survey 1.
At the initial survey, the 1730 participants had an average FEV1 of 3.7 l. Spirometry at the first survey was entire normal in 69%, while 17% showed an obstructive pattern, 11% showed a restrictive pattern, and 3% showed both obstructive and restrictive patterns. The mean FEV1 rate of decline was 45.1 ml/year. The interval between testing averaged 12.8 years, ranging from 7.2 to 18.4. Among never, former, current and inconsistent smokers the mean FEV1 rates of decline were 37.6 ml/year, 42.5 ml/year, 51.6 ml/year and 47.9 ml/year respectively. The average body mass index (BMI) was 26.0.
Table 3 shows the crude and age‐adjusted all‐cause mortality rate by FEV1 rate of decline categories for the whole cohort (table 3A) and for cardiovascular and non‐malignant respiratory disease crude (table 3B). Both the crude and age‐adjusted mortality rate rises with increasing FEV1 rate of decline category, although there are slight differences in trend. The age‐adjusted mortality rate in the non‐study cohort participants, 16.2 per 1000 person years (95% CI 16.1 to 16.2), was higher than that for the study cohort at 12.8 per 1000 person‐years (95% CI 12.7 to 12.8) suggesting those who participated in one of the follow‐up surveys were healthier than those who did not.
Table 3 Vital status and crude and age‐adjusted death rate, by FEV1 rate of decline categories, US, National Study of Coal Workers' Pneumoconiosis, 1969–97.
| FEV1 rate of decline category (ml/year) | ||||
|---|---|---|---|---|
| <30 | 30 to <60 | 60 to <90 | ⩾90 | |
| (A) Death from all‐cause (n = 1730) | ||||
| n = 580 | n = 688 | n = 300 | n = 162 | |
| Dead (n, %) | 78 (3.4) | 99 (14.3) | 61 (20.4) | 47 (29.0) |
| Crude mortality rate per 1000 person‐years | 11.0 (8.5 to 13.4) | 11.8 (9.5 to 14.2) | 15.5 (11.6 to 19.3) | 22.6 (16.1 to 29.0) |
| Age adjusted mortality rate per 1000 person‐years | 11.4 (11.3 to 11.5) | 11.8 (11.7 to 11.8) | 13.2 (13.0 to 13.3) | 24.0 (23.8 to 24.3) |
| (B) Death from cardiovascular disease and non‐malignant respiratory disease (n = 1584) | ||||
| n = 542 | n = 631 | n = 266 | n = 145 | |
| Dead (n, %) | 40 (7.3) | 42 (5.3) | 27 (7.3) | 30 (15.2) |
| Crude mortality rate per 1000 person‐years | 5.8 (4.0 to 7.6) | 5.3 (3.7 to 6.9) | 7.3 (4.6 to 10.1) | 15.2 (9.8 to 20.6) |
| Age adjusted mortality rate per 1000 person‐years | 6.1 (6.0 to 6.1) | 5.2 (5.2 to 5.3) | 5.5 (5.4 to 5.5) | 16.1 (15.9 to 16.3) |
FEV1, forced expiratory volume in one second.
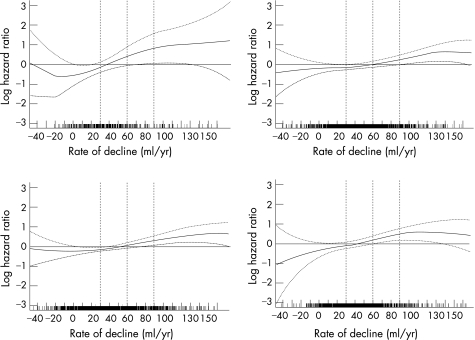
Figure 1A displays the smoothed plot of log hazard ratio and 95% confidence interval for the multivariate Cox regression with penalised spline for the study cohort. The tick marks on the x axis demonstrate the frequency of the observed FEV1 rates of decline and indicated that the rates below −40 ml/year and above 160 ml/year were rare (not shown). FEV1 declines between zero and around 50 ml/year appear to have little impact on risk for mortality, while declines greater than about 50 ml/year are associated with increased risk for mortality. Figure 1B presents the smoothed plots in never smokers where the increase in log hazard ratio is more pronounced. Given the small sample size (n = 327, deaths = 43), the confidence intervals are wider. Nevertheless, the risk is significantly increased at the 60 ml/year decline level. Current and former smokers showed a relation similar to the overall cohort (fig 1C). Figure 1D illustrates the relation between FEV1 rate of decline and log hazard ratio among participants who had neither obstructive nor restrictive patterns at the first survey (n = 1198, deaths = 173).
Figure 1 (A–D) Cox multivariate regression model using penalised splines, log hazard ratio of death and 95% pointwise confidence interval by FEV1 rate of decline (ml/year): (A) for the entire cohort (n = 1730); (B) for never smokers (n = 327); (C) for current or former smokers (n = 1403); and (D) in those with neither restrictive nor obstructive patterns at first survey (n = 1198). 30, 60, and 90 ml/year rate of decline are marked by US, NSCWP, 1969–97.
Tables 4A–D show the adjusted and unadjusted RR of death by categories of FEV1 rate of decline in the entire study cohort, in never smokers, in current and former smokers, and among those participants with neither obstructive nor restrictive patterns at the first survey. In the entire cohort (table 4A), the adjusted RR increases progressively by FEV1 decline category and is statistically significant in the “90 ml/year and above” category, 1.78 (1.22 to 2.59). In the 327 never smokers, the adjusted mortality risk is significant in both the “60 to less than 90 ml/year” and the “90 ml/year and above” categories (table 4B). In current and former smokers, the adjusted mortality risk is statistically significant in the “90 ml/year and above” category (table 4C). Among the 1198 participants who had neither restrictive nor obstructive patterns at the initial survey, the adjusted RR is significantly increased in both the “60 to less than 90 ml/year” and the “90 ml/year and above” categories (table 4D). An additional multivariate Cox regression analysis was preformed to examine possible interactions between smoking and rate of decline category (not shown). The interaction term is not statistically significant, indicating that the relation between rate of decline and mortality is not modified by smoking status.
Table 4 Adjusted and unadjusted Cox proportional hazard model risk ratio for death with 95% confidence intervals. Adjusted for initial FEV1, change in weight, initial age, height.
| n | Not adjusted | Adjusted | ||
|---|---|---|---|---|
| Deaths, n | RR (95% CI) | RR (95% CI) | ||
| (A) Entire cohort (n = 1730) | ||||
| <30 ml/year | 580 | 78 | 1.00 | 1.00 |
| 30 to <60 ml/year | 688 | 99 | 1.07 (0.80 to 1.44) | 1.13 (0.84 to 1.52) |
| 60 to <90 ml/year | 300 | 61 | 1.40 (1.00 to 1.96) | 1.39 (0.99 to 1.97) |
| ⩾90 ml/year | 162 | 47 | 2.05 (1.42 to 2.94) | 1.90 (1.32 to 2.76) |
| (B) Never smokers (n = 327) | ||||
| <30 ml/year | 151 | 17 | 1.00 | 1.00 |
| 30 to <60 ml/year | 116 | 14 | 1.08 (0.53 to 2.19) | 1.45 (0.70 to 3.04) |
| 60 to <90 ml/year | 37 | 7 | 1.56 (0.64 to 3.78) | 3.31 (1.16 to 8.31) |
| ⩾90 ml/year | 23 | 5 | 1.72 (0.63 to 4.69) | 3.34 (1.12 to 9.98) |
| (C) Current and former smokers (n = 1403) | ||||
| <30 ml/year | 429 | 61 | 1.00 | 1.00 |
| 30 to <60 ml/year | 572 | 85 | 1.04 (0.75 to 1.45) | 1.11 (0.80 to 1.55) |
| 60 to <90 ml/year | 263 | 54 | 1.33 (0.92 to 1.92) | 1.28 (0.88 to 1.87) |
| ⩾90 ml/year | 139 | 42 | 2.03 (1.37 to 3.00) | 1.82 (1.22 to 2.71) |
| (D) No obstructive or restrictive pattern at first survey (n = 1198) | ||||
| <30 ml/year | 386 | 42 | 1.00 | 1.00 |
| 30 to <60 ml/year | 492 | 62 | 1.17 (0.79 to 1.74) | 1.24 (0.83 to 1.84) |
| 60 to <90 ml/year | 216 | 42 | 1.64 (1.07 to 2.52) | 1.64 (1.05 to 2.56) |
| ⩾90 ml/year | 104 | 27 | 2.16 (1.33 to 3.51) | 2.15 (1.31 to 3.54) |
US, National Study of Coal Workers' Pneumoconiosis, 1969–97.
Changing procedures, spirometers and technicians between surveys may lead to misclassification, often called survey bias. To test for the effect of survey bias on the risk ratio, a dummy variable is added to the model representing the third versus the fourth survey. When added to our model, the dummy variable was neither statistically significant nor did it notably change the risk ratio estimates, suggesting that the survey bias is likely to be small (not shown). Calendar year was added to the model, to test for cohort effect, and was shown to be non‐significant.
Discussion
This study substantiates the importance of FEV1 rate of decline as a predictor of all‐cause mortality. The mortality trends were similar regardless of smoking status, as well as among participants with normal lung function at the first survey. The trend based on the spline analysis is as follows: below 30 ml/year the mortality risk is not significant (that is, the lower 95% CI is below the null value). Between 30 and 60 ml/year, the log hazard ratio crosses the null value as it ascends, but is not statistically significant. The risk continues to increase between 60 and 90 ml/year and becomes statistically significant around 60 ml/year in never smokers and in those with neither restrictive nor obstructive patterns at first survey and around 75 ml/year in all other cohorts. The multivariate Cox regression model without splines quantifies the risk of the FEV1 rates of decline shown in the smoothing curves. For example, in the overall cohort, the risk in the “90 ml/year and above” category is nearly double compared to FEV1 rates of decline below 30 ml/year.
Overall, the study's findings are consistent with published literature. The Honolulu Heart Program12 showed an association between FEV1 rate of decline and mortality in smokers. The Busselton Health Study13 observed a risk of all‐cause mortality for FEV1 rate of decline that was statistically non‐significant in males but significant in females, after adjusting for risk factors. The Baltimore Study on Aging14 compared individuals in the first quintile of decline to those in the second quintile, the adjusted relative mortality risk was not significant while those in the third and fourth quintiles had statistically significant risks. The fifth quintile risk was elevated but did not reach statistical significance. In Finnish cohorts of the Seven Countries Study,11 the adjusted relative mortality risk was significant for “intermediate” decliners and “rapid” decliners, compared to “slow” FEV0.75 decliners (tertile of lowest decline). Another study of coal miners, using a matched case control study design, found that rapid decliners (having an average FEV1 decline around 90 ml/year) had significantly increased mortality compared to those with a low rate of decline.33 Lastly, in the US Six Cities Study, rapid decliners had an increased mortality compared to slow decliners among males.15
Our study extends the findings from prior research by investigating in greater detail the mortality risk in relation to specific cut‐off points for the FEV1 rate of decline. In past studies, rates of decline were analysed as a continuous variable,13 divided into categories11,14,15 or defined into groups not relevant to clinical practice and research. For example, the Honolulu Heart Program12 split slopes of FEV1 into tertiles, slopes “between +15 to −12 ml/year”, “between −13 to −38 ml/year” and “between −39 to −232 ml/year”. Thus, declines of 60 and 90 ml/year would have both fallen into the latter category. Our analysis was able to demonstrate a difference in the mortality risk ratio in those with declines “60 to less than 90 ml/year” and “90 ml/year and above”, categories which may be important to research and clinical practice. A better understanding of mortality risks of these cut‐off points should be helpful in designing preventive programmes to preserve lung health and to prevent premature death.
Several factors with recognised potential to influence the results were taken into account in this study. Level of FEV1 is a well‐established predictor of mortality.1,2,3,4,5,6,7,8,9,10 Additionally, an interaction may be observed between initial FEV1 level and rate of decline, attributed either to regression to the mean or a so‐called “horseracing effect”. To address these concerns, initial FEV1 was added to the multivariate model. Pre‐existing medical conditions related to lung function may act as a confounder in the relation between FEV1 rate of decline and mortality rate. Working miners are generally quite healthy, but the cohort may have included individuals with pre‐existing disease. To reduce this effect, a separate analysis was completed excluding those with restrictive or obstructive patterns that have already had a measurable effect on pulmonary function (table 3D and fig 1D). Extremes of weight may also affect mortality risk and the impact of lung function and weight together may be synergistic.34 Association between FEV1 rate of decline and both BMI and weight gain have been observed.35 The physiological reasons are thought to be multifactorial and complex.36,37 Our study cohort consisted of working miners who were generally not obese. Change in weight was added to the multivariate model to account for its influence on FEV1 rate of decline. In addition, those who survived gained more weight than those who died. Therefore, neither initial BMI nor weight gain was thought to have had an important impact on the association between mortality and rate of decline.
Smoking and dust are causes of rapid FEV1 decline. Smoking, the most common cause of rapid lung function decline in the general population, is also a confounder. In addition to accelerating FEV1 declines with resulting mortality from non‐malignant respiratory disease, smoking leads to death through other biological pathways, such as malignancy. Similarly, dust, an occupational cause of rapid lung function decline, is also a confounder as it leads to death from pneumoconiosis. These variables, although in part confounders, were not added to the overall model because the true risk of FEV1 decline would be obscured and the risk ratio may be shifted towards the null. However, we investigated this issue by comparing multivariate risk ratios in the overall cohort with estimated total dust at first survey, smoking status and pack‐years included the model to multivariate risk ratios estimated without these factors. The rate ratios with these three variables in the model were as follows: 1.09, 1.41 and 1.83 in the “30 to less than 60 ml/year”, “60 to less than 90 ml/year” and “90 ml/year and above” rate of decline categories respectively where only the “90 ml/year and above” was statistically significant. The results of this analysis suggest that exclusion of the smoking and dust effect did not markedly influence the mortality risk.
Importantly, a significant increased risk of mortality in relation to the FEV1 rate of decline was observed in the subgroup of never smokers, a finding not identified in many studies. For example, the Honolulu Heart Program observed the association in current smokers but not in never smokers.12 Several studies such as, the US Six Cities Study15 and the Baltimore Longitudinal Study of Aging,14 did not stratify by smoking status. The Busselton Health Study13 observed a statistically significant relative risk in female, but not male, never smokers. In miners, coal dust exposure has been shown to increase the rate of decline in lung function and the effect is independent of smoking.20,38,39 It is also possible that in our study we may have been able to detect an effect of FEV1 decline on mortality in never smokers because of the influence of dust exposure on the relationship. Classification of smoking status in the study should have been reliable. Study participants generally reported their smoking status accurately, and in this study had to characterise their smoking status at two surveys performed more than seven years apart, thus reducing the chance of misclassification.40,41
A small number of participants showed an increase in FEV1 during follow‐up, and this increase appeared to be associated with increased mortality risk (fig 1A), although not significantly. The increased mortality in this group may be explained by baseline lung function impairment; whereas 31% of the entire cohort had obstructive and/or restrictive patterns at the first survey, that proportion was 50% (n = 49/98) among participants whose FEV1 increased during follow‐up. Longitudinal FEV1 increases also may indicate excessive spirometry variability which has been associated with poorer heath.36,37 When those with restrictive or obstructive patterns at first survey are removed, increases in FEV1 were not associated with increased mortality.
There are several potential limitations in this study. The participation rates decreased between the first survey and the third and fourth surveys. Although there are several possible reasons for this decrease, a healthy worker survival effect is suggested, in which ill participants fail to have lung function follow‐up. Workers with excessive FEV1 declines are more likely to leave and thus not participate in follow‐up pulmonary function testing.33 Consequently, age‐adjusted mortality rates were higher, compared to the study cohort, among those who participated in the first survey but did not perform follow‐up testing in the third or fourth surveys and thus were not in the study cohort. In addition, table 2 shows that, at the first survey, obstructive and/or restrictive patterns were more prevalent in the non‐study cohort (43%) compared to the study cohort (31%), and there were more current smokers in the non‐study cohort (54.3%) than in the study cohort (36.5%). If the rate of decline was, on average, higher in those who were not included in the study cohort, then the reported mortality risks would be biased towards the null and the actual mortality risk due to decline in lung function would be higher than reported. We also have no information on lifestyle changes in the period between the last PFT and date of death. During that time period, participants may have made substantial modifications to their lifestyle known to affect FEV1 rate of decline and the risk of mortality, for example, quitting smoking or losing weight (if overweight or obese).
In conclusion, the results of this study help to quantify the relation between FEV1 rate of decline and mortality. The findings indicate that risk of mortality starts increasing for FEV1 rates of decline between 30 and 60 ml/year and is significant for declines above 90 ml/year. Statistically significant mortality risk was observed in non‐smokers with occupational dust exposure, suggesting that monitoring lung function is also important in this group. These results should provide information useful to healthcare providers in evaluating the importance of longitudinal changes in lung function observed in individuals.
Abbreviations
ATS - American Thoracic Society
BMI - body mass index
FEV1 - forced expiratory volume in one second
ICD‐9 - International Classification of Diseases Ninth Revision
NSCWP - National Study of Coal Workers' Pneumoconiosis
PFT - pulmonary function test
Footnotes
Funding: National Institute for Occupational Safety and Health.
Competing interests: None.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.Bang K M, Gergen P J, Kramer R.et al The effect of pulmonary impairment on all‐cause mortality in a national cohort. Chest 1993103536–540. [DOI] [PubMed] [Google Scholar]
- 2.Beaty T H, Cohen B H, Newill C A.et al Impaired pulmonary function as a risk factor for mortality. Am J Epidemiol 1982116102–113. [DOI] [PubMed] [Google Scholar]
- 3.Beaty T H, Newill C A, Cohen B H.et al Effects of pulmonary function on mortality. J Chronic Dis 198538703–710. [DOI] [PubMed] [Google Scholar]
- 4.Hole D J, Watt G C, Davey‐Smith G.et al Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313: 711–5; discussion 5–6, [DOI] [PMC free article] [PubMed]
- 5.Krzyzanowski M, Wysocki M. The relation of thirteen‐year mortality to ventilatory impairment and other respiratory symptoms: the Cracow Study. Int J Epidemiol 19861556–64. [DOI] [PubMed] [Google Scholar]
- 6.Mannino D M, Buist A S, Petty T L.et al Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 200358388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meijers J M, Swaen G M, Slangen J J. Mortality of Dutch coal miners in relation to pneumoconiosis, chronic obstructive pulmonary disease, and lung function. Occup Environ Med 199754708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neas L M, Schwartz J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol 19981471011–1018. [DOI] [PubMed] [Google Scholar]
- 9.Persson C, Bengtsson C, Lapidus L.et al Peak expiratory flow and risk of cardiovascular disease and death. A 12‐year follow‐up of participants in the population study of women in Gothenburg, Sweden. Am J Epidemiol 1986124942–948. [DOI] [PubMed] [Google Scholar]
- 10.Sin D D, Wu L, Man S F. The relationship between reduced lung function and cardiovascular mortality: a population‐based study and a systematic review of the literature. Chest 20051271952–1959. [DOI] [PubMed] [Google Scholar]
- 11.Pelkonen M, Notkola I L, Tukiainen H.et al Smoking cessation, decline in pulmonary function and total mortality: a 30 year follow up study among the Finnish cohorts of the Seven Countries Study. Thorax 200156703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez B L, Masaki K, Burchfiel C.et al Pulmonary function decline and 17‐year total mortality: the Honolulu Heart Program. Am J Epidemiol 1994140398–408. [DOI] [PubMed] [Google Scholar]
- 13.Ryan G, Knuiman M W, Divitini M L.et al Decline in lung function and mortality: the Busselton Health Study. J Epidemiol Community Health 199953230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tockman M S, Pearson J D, Fleg J L.et al Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med 1995151390–398. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Dockery D W, Ware J H.et al Decline of pulmonary function as a predictor of mortality in adults [abstract]. Am Rev Repir Dis 1992145A198 [Google Scholar]
- 16.Sherrill D L, Lebowitz M D, Knudson R J.et al Continuous longitudinal regression equations for pulmonary function measures. Eur Respir J 19925452–462. [PubMed] [Google Scholar]
- 17.Bloom J W, Sugihara S, Garfield M D.et al Characteristics of individuals with accelerated declines in lung function. Chest 19848518S–9S. [Google Scholar]
- 18.Burchfiel C M, Marcus E B, Sharp D S.et al Characteristics associated with rapid decline in forced expiratory volume. Ann Epidemiol 19966217–227. [DOI] [PubMed] [Google Scholar]
- 19.Buist A S, Vollmer W M. The use of lung function tests in identifying factors that affect lung growth and aging. Stat Med 1988711–18. [DOI] [PubMed] [Google Scholar]
- 20.Attfield M D. Longitudinal decline in FEV1 in United States coalminers. Thorax 198540132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seixas N S, Robins T G, Attfield M D.et al Longitudinal and cross sectional analyses of exposure to coal mine dust and pulmonary function in new miners. Br J Ind Med 199350929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuempel E D, Stayner L T, Attfield M D.et al Exposure‐response analysis of mortality among coal miners in the United States. Am J Ind Med 199528167–184. [DOI] [PubMed] [Google Scholar]
- 23.Morgan W K, Handelsman L, Kibelstis J.et al Ventilatory capacity and lung volumes of US coal miners. Arch Environ Health 197428182–189. [DOI] [PubMed] [Google Scholar]
- 24.Wang M L, Petsonk E L, Attfield M D.et al Miners with clinically important declines in FEV1: analysis of data from the U.S. National Coal Study. Appl Occup Environ Hyg 199611989–995. [Google Scholar]
- 25.ATS statement‐‐Snowbird workshop on standardization of spirometry Am Rev Respir Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 26.Attfield M D, Castellan R M. Epidemiological data on US coal miners' pneumoconiosis, 1960 to 1988. Am J Public Health 199282964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrows B, Lebowitz M D, Camilli A E.et al Longitudinal changes in forced expiratory volume in one second in adults. Methodologic considerations and findings in healthy nonsmokers. Am Rev Respir Dis 1986133974–980. [DOI] [PubMed] [Google Scholar]
- 28.Lung function testing: selection of reference values and interpretative strategies American Thoracic Society. Am Rev Respir Dis 19911441202–1218. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson J L, Odencrantz J R, Fedan K B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999159179–187. [DOI] [PubMed] [Google Scholar]
- 30.Breslow N E, Day N E. Statistical methods in cancer research: Lyon: The Agency, 1980–1987 1980
- 31.Eisen E A, Agalliu I, Thurston S W.et al Smoothing in occupational cohort studies: an illustration based on penalised splines. Occup Environ Med 200461854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastie T J, Tibshirani R J.Generalized additive models. Boca Raton: Chapman & Hall/CRC, 1990
- 33.Beeckman L A, Wang M L, Petsonk E L.et al Rapid declines in FEV1 and subsequent respiratory symptoms, illnesses, and mortality in coal miners in the United States. Am J Respir Crit Care Med 2001163633–639. [DOI] [PubMed] [Google Scholar]
- 34.Sharp D S, Masaki K, Burchfiel C M.et al Prolonged QTc interval, impaired pulmonary function, and a very lean body mass jointly predict all‐cause mortality in elderly men. Ann Epidemiol 1998899–106. [DOI] [PubMed] [Google Scholar]
- 35.Wang M L, McCabe L, Petsonk E L.et al Weight gain and longitudinal changes in lung function in steel workers. Chest 19971111526–1532. [DOI] [PubMed] [Google Scholar]
- 36.Bedell G N, Wilson W R, Seebohm P M. Pulmonary function in obese persons. J Clin Invest 1958371049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemery B, Moavero N E, Brasseur L.et al Smoking, lung function, and body weight. BMJ (Clin Res Ed) 1983286249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attfield M D, Hodous T K. Pulmonary function of U.S. coal miners related to dust exposure estimates. Am Rev Respir Dis 1992145605–609. [DOI] [PubMed] [Google Scholar]
- 39.Love R G, Miller B G. Longitudinal study of lung function in coal‐miners. Thorax 198237193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ismail A A, Gill G V, Lawton K.et al Comparison of questionnaire, breath carbon monoxide and urine cotinine in assessing the smoking habits of Type 2 diabetic patients. Diabet Med 200017119–123. [DOI] [PubMed] [Google Scholar]
- 41.Patrick D L, Cheadle A, Thompson D C.et al The validity of self‐reported smoking: a review and meta‐analysis. Am J Public Health 1994841086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]