Abstract
Objectives
To investigate the association between exposures to polycyclic aromatic hydrocarbons (PAH) that arises during asphalt paving, and risk of bladder cancer.
Methods
7298 men included in the historical cohort were first employed between 1913 and 1999 in companies applying asphalt in Denmark, Norway, Finland and Israel. The minimal duration of employment for inclusion in the cohort was two seasons of work. Occupational histories were extracted from personnel files. A follow‐up for cancer incidence was conducted through national cancer registries. The authors estimated exposures to benzo(a)pyrene as a marker for 4–6 ring PAH. Exposures were reconstructed by using information about changes in asphalt paving technology in each company over time, the modelled relation between production characteristics and exposure levels, and job histories. Relative risks and associated 95% confidence intervals were estimated using Poisson regression.
Results
48 bladder cancers among asphalt paving workers were detected; of these, 39 cases were exposed at least 15 years before the diagnosis. Cumulative exposure to PAH was not associated with the incidence of bladder cancer. The association with average exposure became stronger when 15‐year lag was considered, revealing a twofold increase in relative bladder cancer risk in the two higher exposure categories. There was an indication of exposure‐response association with lagged averaged exposure. Risk estimates were adjusted for age, country, duration of employment and calendar period, did not show heterogeneity among countries and did not materially change when re‐estimated after excluding non‐primary cancers from follow‐up. Previously conducted sensitivity analysis indicates that confounding by cigarette smoking is an unlikely explanation for the observed exposure‐response trends.
Conclusions
The authors were unable to control for all possible sources of confounding and bias. The results do not allow conclusion on the presence or absence of a causal link between exposures to PAH and risk of bladder cancer among asphalt workers.
Bladder cancer is currently the seventh most common cancer worldwide, with 273 000 new cases and more than 108 000 deaths estimated to have occurred in the year 2002.1 The most important risk factor is cigarette smoking, which accounts for approximately 65% of male and 30% of female cases in developed countries. Occupational exposures to polycyclic aromatic hydrocarbons (PAH), polychlorinated biphenyls, aromatic amines, formaldehyde, asbestos and solvents have been associated with increased bladder cancer risk.2 While high PAH exposure associated with these settings (in μg/m3 in air) has been linked to bladder cancer risk, it is unclear whether lower PAH exposures (in ng/m3 in air), such as those observed in the asphalt industry3,4 confer risk as well. A recent meta‐analysis of 11 case‐control studies, conducted from 1976–96 in six European countries and comprising 3346 incident bladder cancers and 6840 controls, attributed a 23% increase in bladder cancer risk among men to PAH exposure (odds ratio (OR) for the highest exposure tertile 1.23, 95% CI 1.07 to 1.4).5 It was estimated that 4.3% of bladder cancers in men can be attributed to occupational PAH exposure.5
The cohort of European asphalt workers, coordinated by the International Agency for Research on Cancer, was originally assembled to study the risk of lung cancer following exposure to bitumen fume. However, it evolved into a very rich resource for studying focused (bitumen and cancer) and broad aetiological questions (PAH and lung cancer, cardiorespiratory diseases). The key findings from the cohort study include observations of increased lung cancer mortality with increasing bitumen fume exposure among asphalt pavers (a subcohort for which the best exposure estimates were available),6 positive associations of exposure to PAH among asphalt pavers with the risk of death from obstructive lung7 and ischaemic heart disease,8 lack of increased risk for fatal occupational injuries among asphalt workers in the cohort9 and an indication that lung and bladder cancer incidence may be increased in the subcohorts from Nordic countries.10 More specifically, although no overall increased incidence of bladder cancer was observed (standardised incidence ratio on 112 cases of 0.91 (95% CI 0.75 to 1.10), there was a tendency for higher risk with longer time since first employment, with a relative risk (RR) of 1.85 (95% CI 0.90 to 3.78) for more than 30 years vs 1–14 years (p value for trend 0.1, 22 cases).10 The main limitations of these analyses were that (a) they did not include incident bladder cancer cases available across the national subcohorts, (b) data on cigarette smoking, a major potential confounder, was not available, and (c) lack of exposure‐response analysis that uses job‐exposure matrix that we constructed for the cohort.11 In this paper, we aim to overcome two of these limitations by investigate the association between PAH exposure and bladder cancer incidence among asphalt pavers using quantitative exposure indices and by including eligible incident bladder cancers from the entire international cohort.
Methods
Study population
People included in the historical cohort with incidence follow‐up were first employed between 1913 and 1999 in companies applying and mixing asphalt in Denmark, Norway, Finland and Israel. The requirement for the inclusion of a company in the study was the availability of a complete retrospective employee roster during the enrolment period. Once a company had been selected for the study, efforts were made to enrol all manual workers. Personal identifiers and employment histories of workers were abstracted from company records. The minimal duration of employment for inclusion in the cohort was two seasons of work, with season duration reported by companies in a questionnaire that described production characteristics (see below). The inclusion criterion was applied because short‐term workers may differ in their occupational and non‐occupational risk factors from long‐term employees.12 Occupational histories were coded on the basis of information from personnel records according to classifications of jobs constructed for the study. A “job” in this classification scheme represented the primary activity of a worker in a given time period (for example, asphalt paving), but does not reflect specific tasks performed. In the current analysis we included 7298 men who appeared to have been exclusively employed in paving with asphalt (table 1). The reasons for restricting the cohort were twofold. First, quantitative exposure estimates were only available for persons who only worked in asphalt paving. Second, we were more certain of the accuracy and completeness of employment histories for these subjects. At follow‐up, they had a median age of 54 years (interquartile range (IQR) 43–65) and were employed for a median of approximately 8 seasons (IQR 4–15) (season ⩽ one year of work). Cohort members were born between 1887 and 1979 (median 1947, IQR 1933–1957) and had median age of 29 years at hire (IQR 22–39).
Table 1 Description of the cohort.
| Country | Follow‐up start | Follow‐up end (year, all 31 December) | Persons only employed in asphalt paving | Person‐years | Bladder cancers* |
|---|---|---|---|---|---|
| Denmark | 1 April 1968 | 2003 | 2352 | 47202 | 27 (20) |
| Norway | 1 January 1953 | 2005 | 3193 | 74767 | 14 (12) |
| Finland | 1 January 1969 | 1994 | 1049 | 16302 | 1 (1) |
| Israel | 1 January 1968 | 1998 | 704 | 12887 | 6 (6) |
| Total | 7298 | 151158 | 48 (39) |
*Figures in brackets refer to number of bladder cancer diagnoses in subjects with at least 15 years after first polycyclic aromatic hydrocarbon exposure in asphalt paving.
Cancer incidence follow‐up
A follow‐up for cancer incidence was conducted in all participating countries through national cancer registries. Subjects were followed beyond the period of employment in companies studied. The follow‐up periods were country‐specific (table 1): the earliest started in 1953 and the latest ended in 2005. Follow‐up of study subjects ended when they died, were diagnosed with bladder cancer or emigrated. For subjects who emigrated or were lost to follow‐up, the last known date of employment was used as the end of follow‐up. Mortality follow‐up was conducted through national registries. The subjects started to contribute person‐years when they completed two seasons of employment and remained “at risk” until the end of follow‐up. Median duration of follow‐up was 21 years (IQR 15–29). These figures were similar for all countries: median follow‐up duration between 18 and 23 years. Cohort members accumulated 151 158 person‐years of observations (table 1). None of the subjects were lost to follow‐up and only 0.55% emigrated. Incident bladder cancers were coded according to the ninth revision of the International Classification of Diseases (ICD‐9) using code 188. In Denmark, 25 cases were verified by cystoscopy and/or operation and all 27 cases are verified by a histological examination. In other countries all cases were confirmed by a histological examination alone.
Follow‐up for primary tumours
Individuals diagnosed with cancer may be at an increased risk for developing a second cancer due to metastasis, side effects of the treatment of primary tumour, and shared susceptibility or casual exposure among the tumours. To evaluate potential bias from these sources, we performed analyses censoring follow‐up at the first non‐melanoma cancer diagnosis, thereby restricting definition of cases to people with bladder as the primary cancer site. We allowed the participants in the study to be diagnosed with a non‐melanoma skin cancer because (a) the natural history and treatment of this cancer type are relatively gentle, and (b) the capture of non‐melanoma cancers by the incidence registries is incomplete. Thus, follow‐up for these analyses ended at the first cancer diagnosis except non‐melanoma skin cancer, emigration, death or end of follow‐up period.
Exposure assessment
We estimated exposures to benzo(a)pyrene as a marker for 4–6 ring PAH.11 Exposures were reconstructed by using information about changes in asphalt paving technology in each company over time, the modelled relation between production characteristics and exposure levels, and job histories.11
We gathered information about companies enrolled in the study through a company questionnaire that ascertained temporal changes in production characteristics and work organisation. These included average time spent using a given paving method (for example, mastic laying, hot mix paving) and use of coal tar (an important source of exposure to PAH). The questionnaire was administered to knowledgeable company representatives, and reviewed for inconsistencies and errors.
Quantitative estimates of inhalation exposure to benzo(a)pyrene were obtained for paving operations on the basis of previously available personal exposure measurements from workers in the asphalt industry (but not necessarily from cohort members), which were assembled into a single database for the study.13 The contextual information coded for each exposure measurement included production characteristics within a job (using a classification similar to that used in the company questionnaire), repeated measurements within a worker, and country. We constructed4,14 and validated15 mixed‐effects exposure assessment models, with production characteristics as fixed effects and repeated measurements on the same worker as random effects, to predict quantitative exposures to benzo(a)pyrene among asphalt paving workers. Use of coal tar was the strongest determinant of exposure to benzo(a)pyrene, but time period, mastic laying, re‐paving, surface dressing and oil gravel paving were also important predictors.
All predicted exposure levels in the exposure matrix11 were standardised to an 8‐hour work‐shift, using information on average work‐shift duration reported in the company questionnaires. The exposure matrix was time period‐ and company‐specific.
For all subjects, we estimated cumulative exposure (product of exposure duration and intensity, integrated over work history) and average exposure over the work history (ratio of cumulative exposure and duration of exposure). The estimates of duration of exposure, but not duration of employment, were corrected for differences in duration of working season between companies and countries. More specifically, duration of exposure was defined as aggregated length of seasons (for example, two seasons of 8‐month duration contributed 16 months to the duration of exposure). Exposure indices were constructed with and without a 15‐year lag in order to reflect possible latency period between exposure and detection of bladder cancer. In the lagged analysis, person‐years accumulated during the last 15 years before end of follow‐up were ignored. This resulted in the exclusion of 1628 workers, whose exposure occurred exclusively during the last 15 years before end of follow‐up, leaving 5670 exposed workers in the lagged analysis (table 1). For each exposure index, the distribution of its values among bladder cancer cases was divided into quartiles and their boundaries used to define exposure groups in Poisson regression (see below). Thus, each exposure group included approximately one quarter of incident bladder cancers. Because all members of this cohort worked in paving with asphalt that entails some exposure to PAH, there were no subjects who were never exposed.
Exposure‐response analyses
Relative risks and associated 95% confidence intervals (CI) were estimated using Poisson regression.16 All Poisson regression models included an exposure index for proxy of 4–6 ring PAH exposure (benzo(a)pyrene), age at exit from cohort (⩽39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70+ years), calendar period of exit from cohort (pre‐1974, 1975–9, 1980–4, 1985–9, 1990 and later), total duration of employment (2–4, 5–9, 10–14, 15–19, 20+ years) and country (referent: Denmark), using the category at the lowest exposure as the reference. Duration of employment was included to partially control for the healthy worker survivor effect; it was not equal to the duration of exposure because of the seasonal nature of work that was considered in estimation of duration of exposure. Heterogeneity of exposure‐response associations among different countries in the study was tested though interaction terms. Linear trends in log(RR) were estimated assuming constant differences between exposure categories. Only the p values for these tests are reported. Poisson regression analyses were carried out in SAS 9.1: PROC GENMOD (SAS Institute, Cary, North Carolina, USA). Person‐years were allocated also in SAS 9.1, using a computer program developed at the International Agency for Research on Cancer. In allocating person‐years, exposure variables (cumulative and average) were treated as time‐dependent, because they changed throughout each individual's working history.
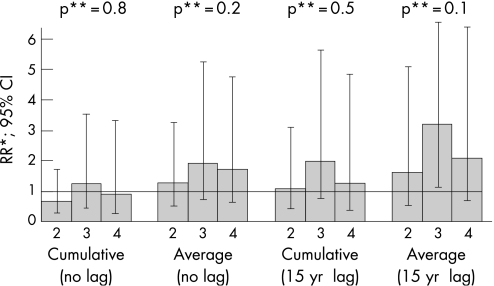
Figure 1 Relative risk (RR) of primary bladder cancer by exposure to polycyclic aromatic hydrocarbons (for actual boundaries of exposure groups, see tables; the lowest group (1) used as reference).
Results
During the follow‐up, 48 bladder cancers among asphalt paving workers were detected; of these 39 cases were exposed to PAH through employment in asphalt paving at least 15 years before the diagnosis. Cumulative exposure did not appear to be associated with risk of bladder cancer (tables 2 and 3). However, higher categories of average exposure indicated approximately 40% excess in bladder cancer risk, but there was no significant dose‐response trend (table 2). The association with average exposure became stronger when 15‐year lag was considered, revealing a twofold increase in bladder cancer risk in the two higher exposure categories, albeit not always statistically significant (table 3). There was an indication that exposure‐response association with lagged averaged exposure was not entirely due to chance: p value for trend 0.15. None of the interactions between country and exposure groups were significant. Data were too sparse to stratify analyses by country, yielding unreliable relative risk estimates in country‐specific Poisson regressions.
Table 2 Exposure to polycyclic aromatic hydrocarbons (no lag) among persons employed only in asphalt paving and incidence of bladder cancer; all Poisson regression models adjusted for age, calendar period, total duration of employment and country.
| Exposure metric (units) | Exposure group | n* | Person‐years† | RR estimates (95% CI) |
|---|---|---|---|---|
| Average | 0<–<65 | 12 | 79760 | 1.00 |
| (ng benzo(a)pyrene/m3) | 65–<126 | 11 | 37367 | 1.01 (0.43 to 2.39) |
| 126–<198 | 12 | 17742 | 1.41 (0.55 to 3.60) | |
| 198+ | 13 | 16290 | 1.36 (0.54 to 3.44) | |
| p trend = 0.4 | ||||
| Cumulative | 0<–<253 | 12 | 70658 | 1.00 |
| (ng benzo(a)pyrene/m3–years) | 253–<895 | 12 | 47851 | 0.69 (0.29 to 1.63) |
| 895–<1665 | 12 | 15828 | 1.21 (0.45 to 3.25) | |
| 1665+ | 12 | 16822 | 0.84 (0.24 to 2.94) | |
| p trend = 0.9 |
*Number of incident bladder cancers.
Table 3 Exposure to polycyclic aromatic hydrocarbons (15 year lag) among persons employed only in asphalt paving and incidence of bladder cancer; all Poisson regression models adjusted for age, calendar period, total duration of employment, and country.
| Exposure metric (units) | Exposure group | N | py | RR (95%CI) |
|---|---|---|---|---|
| Average (ng benzo(a)pyrene/m3) | 0<–<99 | 10 | 36948 | 1.00 (–) |
| 99–<139 | 9 | 14739 | 1.53 (0.54 to 4.38) | |
| 139–<204 | 10 | 7358 | 2.71 (1.01 to 7.27) | |
| 204+ | 10 | 9743 | 1.90 (0.66 to 5.47) | |
| p‐trend = 0.15 | ||||
| Cumulative (ng benzo (a)pyrene/m3‐years) | 0<–<385 | 10 | 36674 | 1.00 (–) |
| 385–<883 | 9 | 15634 | 1.13 (0.44 to 2.90) | |
| 883–<1755 | 12 | 9611 | 1.67 (0.62 to 4.48) | |
| 1755+ | 8 | 6869 | 1.09 (0.30 to 3.99) | |
| p‐trend = 0.63 |
N, number of incident bladder cancers; py, person‐years.
If durations of employment and exposure were strongly correlated, then adjusting the effect of cumulative exposure for the duration of employment would mask true association. This does not appear to be the case in this study, in part due to the fact that duration of employment was not corrected for duration of working season, which is typically much less than 12 months of employment (for example, 4–6 months in the Nordic countries and 10–11 months in the south of Europe). When analyses with cumulative exposure indices were not adjusted for duration of employment, the results were virtually identical to those presented in the tables. In the case of cumulative exposure, RRs (95% CI) for different exposure groups, in order of increasing exposure, were 0.66 (0.29 to 1.49), 1.17 (0.50 to 2.75), 0.89 (0.36 to 2.21); p trend = 0.8. In the case of cumulative exposure lagged by 15 years, RRs (95% CI) for different exposure groups, in order of increasing exposure, were 1.13 (0.45 to 2.83), 1.70 (0.70 to 4.14), 1.17 (0.41 to 3.31); p trend = 0.5.
Five bladder cancers in the cohort were diagnosed after another tumour was identified (3 from Norway and 2 from Denmark); four of these subjects were exposed 15 years before diagnosis of bladder cancer. Primary tumours in these subjects were carcinoma of the skin, malignant lymphoproliferative disease, as well as cancers of oesophagus, kidney and prostate. Censoring follow‐up at the first tumour did not alter the overall pattern of the results (fig).
Discussion
After allowing for a 15‐year lag, we observed approximately a twofold increase in risk of bladder cancer incidence between the highest and the lowest PAH exposure groups among people engaged in paving with asphalt. This suggests that exposure to PAH in the asphalt industry can lead to the development of bladder cancer. Increased strength of the association after allowing for a latency period is consistent with the aetiology of bladder cancer. This association can result from exposure to causal agents within the cohort, biases in exposure assessment, confounding by occupational exposures and lifestyle factors, and chance.
The plausibility of our findings is supported by epidemiological investigations in other PAH‐exposed occupations. Cohort studies of aluminium smelter workers in Norway and Canada revealed a significant excess in incidence of bladder cancer among PAH‐exposed workers, which increased with greater cumulative exposure.17,18 The association is typically stronger when latency is considered in constructing exposure index.17 A Canadian case‐control study nested in a cohort of aluminium production workers reported that cumulative exposure to benzo(a)pyrene lagged by 10 years was the best predictor of bladder cancer risk after adjustment for smoking.19 Some mortality studies of PAH‐exposed aluminium workers suggest an increased risk of bladder cancer risk,20 but not others;21 overall mortality studies of this effect are typically not statistically significant, due to good survival rates among bladder cancer patients. A population based case‐control study from Canada, which included 835 cases, showed an increased risk of bladder cancer for aluminium smelting workers exposed for more than 10 years (OR 5.9, 95% CI 1.0 to 7.32) and for workers exposed to “tar asphalt” (OR 3.1, 95% CI 1.2 to 9.7 in lagged analysis).22 It should be noted that mixture of PAH (and other co‐exposures) emitted in the aluminium industry is likely to be different from that of asphalt paving, making our results important in advancing the notion that PAH is a common agent in bladder cancer aetiology across different environments. A hospital‐based case‐control study in France, including 658 male cases, showed an increased risk (OR 1.3, 95% CI 1.2 to 9.7) for PAH exposure (adjusted for smoking, coffee drinking and occupational exposure to aromatic amines) and a significant trend for a dose‐response relation (p <0.05).23 A population‐based study in Italy, based on 150 cases, found a non‐significantly elevated risk (OR 2.1, 95% CI 0.8 to 5.6) for “definite PAH exposure”.24 However, there are examples of negative case‐control population‐based studies from the US (417 cases), which reported no association for either ever/never exposure to PAH, or duration,25 Canada (486 cases),26 and Norway (52 incident cases).27 Thus, increased risk of bladder cancer is mainly recognised in industries with high exposure to PAH from coal tars and pitches.28 This was confirmed in a recent meta‐analysis.5 Much less is known about risk of bladder cancer that may be conferred at PAH exposures that tend to be almost an order of magnitude lower, as seen in the asphalt paving industry, but it is commonly assumed that there is no threshold of action for carcinogens. It must be noted that exposures to PAH in ng/m3 are quite common in urban areas polluted by vehicle traffic and consequently our findings may have important implications for assessing bladder cancer risk due to air pollution in general. However, this generalisability is undermined by the possibilities that (a) dermal route of exposure among asphalt pavers (which is generally not believed to be present among the general public) confounds our risk estimates, and (b) composition of mixtures of PAH, and hence its toxicity profile, emitted from asphalt may be different from that present in polluted urban air.
We focused on airborne exposures because we lacked dermal exposure estimates to PAH. If exposure from the two routes were strongly correlated, the risk attributed to the airborne route would be overestimated (though not the risk due to PAH dose). If exposures from the two routes are not correlated, then our airborne exposure may be weakly related to the true dose of PAH, most likely resulting in attenuation of risk attributed to airborne exposures.
As we lacked complete occupational histories, it is possible that some cohort members were occupationally exposed to factors outside of the asphalt industry (such as PAH emitted during aluminium smelting29,30) that contributed to the development of bladder cancer in the studied population. If these exposures were correlated with exposures incurred during employment in the asphalt industry, our estimates of PAH‐attributable risk could be confounded. This issue can be resolved only by collecting full occupational histories, including information on jobs outside the paving industry, in a nested case‐referent design. Such data were available on the Danish subcohort, which has data from its pension fund covering the whole occupational history of its members.31 An analysis of this subset of workers does not indicate an increased prevalence of employment in PAH‐exposed occupations outside of the asphalt industry (data not shown).
We lack data on individual smoking habits and other lifestyle (for example, diet, physical activity) that may have contributed to the risk of bladder cancer. Therefore, confounding by these factors cannot be ruled out. In particular, we demonstrated that average exposure to PAH was higher in the past,4 as was the prevalence of cigarette smoking. Previously conducted sensitivity analyses8,32 indicate that it is very unlikely that differences in cigarette smoking explain even modest (RR∼1.6 in the highest exposure group) exposure‐response gradients observed in this cohort when a strong effect of smoking is assumed (RR 3–5 for smokers vs never smokers33). Typically, confounding by smoking and other lifestyle factors does not produce false‐positive results in industry‐based studies.34,35
Considerations of longer latency periods would have addressed the matter of the biological plausibility of our findings to a greater extent, but such analyses would have been uninformative in this relatively young cohort: too few cases remain in exposed rubrics if lags in the order of 30 years are considered.
Misclassification of exposure might have arisen during exposure assessment. For example, we assumed that the use of all products containing coal tar resulted in the same exposures to benzo(a)pyrene. We know from analysis of measurements gathered in the asphalt industry in France14 that this assumption is flawed, but we could not obtain more detailed data on past use of coal tar in the cohort. Exposure to diesel exhaust, a source of PAH exposure, was assessed with too much uncertainty to draw reliable inferences. In addition, all pavers in the study were exposed to diesel exhaust either from passing traffic or from diesel‐powered paving machines, resulting in limited variation in diesel‐exhaust exposure estimates. We were also not able to fully validate information that was gathered in company questionnaires, creating the possibility that not all cohort members were correctly classified with respect to determinants of exposure that were used in quantitative assessment of exposure to benzo(a)pyrene.
Occupational histories could have been extracted with errors for the cohort members, resulting in biases of unpredictable direction and magnitude. We attempted to mitigate this problem by selecting companies into the study that had precise personnel records,36 and limiting current analyses to those subjects who were employed only in paving, one of the most clearly identifiable group in the study. Through this restriction, however, those who switched jobs were excluded and may have gone on to more or less exposed jobs, but we lack good data on this. It is not possible to predict the extent of bias from this limitation of our study, but the findings should be internally valid for the selected group.
We observed associations only with average, but not cumulative, exposure indices—a pattern that is reminiscent of the results of the investigation of lung cancer mortality in the cohort.6 This detracts from causal interpretation of our findings, but it should be noted that neither the cumulative nor average exposure index represents an ideal proxy of biologically effective dose. Positive findings with average exposure can perhaps be attributed to the likelihood that average exposures were assessed with greater certainty than cumulative exposure: unlike most industries, duration of employment is very difficult to accurately estimate in asphalt paving, as it is affected by weather in a given month and year (for example, paving season ends with the first frost). On the other hand, exposure intensity models that drive average exposure estimates were considered to be reasonably valid and precise for the purposes of this epidemiological study,15 even though actual estimation of average exposure involved several assumptions that were impossible to verify.
Exposures were assessed without consideration of vital status of a cohort member. Therefore we expect exposure misclassification to be non‐differential, yielding attenuated risk estimates (at least in the highest exposure categories), rather than positively‐biased risk estimates.37 We observed a drop in the relative risk in the highest, relative to the second‐highest, average exposure categories in a manner that is consistent with such an effect of measurement error.38 Bias in risk due to heterogeneity of measurement error in group‐based exposure assessment39,40 and categorisation of continuous exposure variables41 in occupational cohorts could arise when misclassification of assessed exposures is non‐differential. However, such effects are small, depend of the choice of a disease model and are unlikely to affect the direction of observed associations.
Historically, coal tar content of asphalt was an important source of PAH exposure in the cohort,11,14 but coal tar use has been voluntarily discontinued by the asphalt industry in the studied countries. Due to long latency, the beneficial effect of this policy on health may not yet be apparent. However, some asphalt paving operations generate high PAH exposures even in the absence of coal tar in the asphalt (for example, indoor mastic paving and hot re‐paving).4,14 Consequently, vigilance is required to ensure that PAH exposure in the industry remain as low as possible.
We do not know whether secondary bladder cancers were metastatic, de novo tumours resulting from treatment of the primary cancer, or de novo tumours resulting from exposure to PAH. People surviving primary tumour may be at an increased risk for secondary tumour because of cancer treatment. If diagnosis and treatment resulted in termination of employment in asphalt paving, there would be a negative relation between exposures to PAH and cancer treatment, creating a situation in which confounding by previous cancer treatment may arise. This is unlikely to affect analyses in which exposure was lagged by 15 years, because the time between primary tumour and secondary bladder cancer seemed to be of the order of 1–4 years. If there is no association between cancer treatment and PAH exposure, there may still be an interaction between previous PAH exposure and cancer treatment, both contributing to risk of bladder (secondary) cancer and leading to modification of the effect of PAH in subjects with secondary bladder cancer. If exposure to PAH caused both the primary and secondary tumours, then cancer treatment following diagnosis of primary tumour would be in the causal pathway towards bladder cancer, making “adjustment” for it inappropriate. If bladder cancers were metastatic, we would want to exclude them because primary research question is whether exposure to PAH produces de novo bladder cancers. If appearance of secondary bladder tumours is the result of shared susceptibility with the primary tumour, the underlying susceptibility (genetic, metabolic, lifestyle) would act as an effect modifier, manifesting through treatment/primary tumour amplifying the influence of exposure to PAH on risk of bladder cancer (especially when non‐primary). Lastly, primary tumour independent of occupational exposure to PAH (for example, due to tobacco smoking) may be a competing cause with a secondary tumour, if a primary tumour is rapidly fatal and masks diagnosis of bladder cancer that would have otherwise been detected, leading to underestimation of PAH‐attributed risk. By modifying the population at risk to those without previous diagnosis of cancer (except for non‐melanoma skin cancer), we tested the extent to which these mechanists may have affected our findings. It is likely that a small number of non‐primary bladder cancers in our study is the main reason why the selection of the population at risk has no impact on the overall results.
One of the strengths of our study is that bladder cancer diagnoses were based on histological examinations, greatly limiting the possibility of outcome misclassification. The likelihood of completeness of bladder cancer ascertainment was maximised by the use of data from countries with excellent information on cancer incidence, rather than merely relying on mortality figures. However, we were not able to control for all possible sources of confounding and bias. The results do not allow us to conclude on the presence or absence of a causal link between exposures to PAH and risk of bladder cancer among asphalt workers.
Main messages
Work in asphalt paving may be associated with increased risk of bladder cancer.
Exposure to polycyclic aromatic hydrocarbons (PAH) may confer risk of bladder cancer at relatively “low” airborne exposure levels.
Interpretation of results is hampered by borderline statistical significance of findings and our inability to control for all suspected sources of confounding and bias, as well as absence of monotonic exposure‐response.
Policy implications
New specially‐designed studies should focus on characterising bladder cancer risk of PAH at exposure levels typical of polluted urban environments and “low exposed” occupational settings.
Accurate quantitative exposure assessment that considers all routes of exposure is essential in making policy recommendations aimed at reduction of bladder cancer risk from PAH exposure.
Acknowledgements
The international component of the study was supported by share cost contracts from the European Commission (grant no BMH4‐CT95‐1100), EAPA, Eurobitume and CONCAWE. The Israeli component of the study was funded by the Committee for Preventive Action and Research on Occupational Health, Ministry of Labor and Social Welfare. The Finnish component of the study was funded by the Finnish Work Environment Fund. The Norwegian component of the study was financed by the Norwegian Asphalt Entrepreneur Association, the Oslo Road Maintenance Service, the Public Roads Administration, the Working Environment Fund of the Confederation of Norwegian Business and Industry, and the Working Environment Fund of Statoil. Igor Burstyn worked on this study under the tenure of a Special Training Award from the International Agency for Research on Cancer.
The study sponsors did not contribute to study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication. However, the industry study sponsors were given an opportunity to comment on the final draft of the manuscript. In addition to the authors of this report, many people have contributed to the study. The following individuals contributed to data collection, processing, and analysis in the collaborating centres: Pekka Ylöstalo, Anneli Ojajärvi, Raija Vuorela, Anja Savela, Heikki Koskinen, Eero Pukkala, Paul Brennan, Timo Partanen, Ole Svane, Britt Randem, Pirjo Heikkilä. Collaboration with the European asphalt industry and trade union representatives was essential in the conduct of the study, in particular in the exposure assessment. The following people are especially acknowledged for their valuable contributions in this regard: H Fabiansen, C Bisgaard, (Denmark); S Lundgren, H Jämsä, T Salomaa, T Blomberg, M Ginman, L Forsten, M Marjasalo, M L Puranen, S Heikkinen, J Kylmänen, K Thomssen, L Koponen, S Kauppinen, P Tammi, A Taipale (Finland); T Jørgensen, E Lorentzen (Norway). The representatives of the European industrial association, as well as members of the Study Liaison Committee, were instrumental in the starting of the project and very supportive during the whole duration of the study: M von Devivere, P Marsal (European Asphalt Paving Association (EAPA)); D M Lyall (Eurobitume); B Simpson, J Urbanus (CONCAWE). Elsebeth Lynge (Copenhagen, Denmark) and Nils Plato (Stockholm, Sweden) served on the Study Advisory Committee.
Abbreviations
PAH - polycyclic aromatic hydrocarbons
References
- 1.Ferley J, Bray F, Pisani P.et alGlobocan 2002: Cancer incidence, mortality and prevalence worldwide. IARC Cancerbase. No 5, Version 2. 0. Lyon: IARC Press, 2004
- 2.Stewart B W, Kleihues P.World Cancer Report. IARC Press 2003
- 3.Burstyn I, Kromhout H, Boffetta P. Literature review of levels and determinants of exposure to potential carcinogens and other agents in road construction industry. Am Ind Hyg Assoc J 200061715–726. [DOI] [PubMed] [Google Scholar]
- 4.Burstyn I, Kromhout H, Kauppinen T.et al Statistical modeling of the determinants of historical exposure to bitumen and polycyclic aromatic hydrocarbons among paving workers. Ann Occup Hyg 20004443–56. [PubMed] [Google Scholar]
- 5.Kogevinas M, ‘t Mannetje A, Cordier S, Ranft U.et al Occupation and bladder cancer among men in Western Europe. Cancer Causes Control 200314907–914. [DOI] [PubMed] [Google Scholar]
- 6.Boffetta P, Burstyn I, Partanen T.et al Cancer mortality among European asphalt workers: An international epidemiological study. II. Exposure to bitumen fume and other agents. Am J Ind Med 20034328–39. [DOI] [PubMed] [Google Scholar]
- 7.Burstyn I, Boffetta P, Heederik D.et al Mortality from obstructive lung diseases and exposure to polycyclic aromatic hydrocarbons among asphalt workers. Am J Epidemiol 2003158468–478. [DOI] [PubMed] [Google Scholar]
- 8.Burstyn I, Kromhout H, Partanen T.et al Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology 200516744–750. [DOI] [PubMed] [Google Scholar]
- 9.Burstyn I, Boffetta P, Jarvholm B.et al Risk of fatal industrial accidents and death from other external causes among asphalt workers. Occup Environ Med 20046186–88. [PMC free article] [PubMed] [Google Scholar]
- 10.Randem B G, Burstyn I, Langard S.et al Cancer incidence of Nordic asphalt workers. Scand J Work Environ Health 200430350–355. [DOI] [PubMed] [Google Scholar]
- 11.Burstyn I, Boffetta P, Kauppinen T.et al Estimating exposures in the asphalt industry for an international epidemiological cohort study of cancer risk. Am J Ind Med 2003433–17. [DOI] [PubMed] [Google Scholar]
- 12.Boffetta P, Sali D, Kolstad H.et al Mortality of short‐term workers in two international cohorts. J Occup Environ Med 1998401120–1126. [DOI] [PubMed] [Google Scholar]
- 13.Burstyn I, Kromhout H, Cruise P J.et al Designing an international industrial hygiene database of exposures among workers in the asphalt industry. Ann Occup Hyg 20004457–66. [PubMed] [Google Scholar]
- 14.Burstyn I, Kromhout H. Are all the members of a paving crew uniformly exposed to bitumen fume, organic vapour and benzo(a)pyrene? Risk Analysis 200020653–664. [DOI] [PubMed] [Google Scholar]
- 15.Burstyn I, Boffetta P, Burr G A.et al Validity of empirical models of exposure in asphalt paving. Occup Environ Med 200259620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breslow N E, Day N E.Statistical methods in cancer research. Vol II: The design and analysis of cohort studies. Lyon: International Agency for Research on Cancer Scientific Publications, 1987 [PubMed]
- 17.Ronneberg A, Haldorsen T, Romundstad P.et al Occupational exposure and cancer incidence among workers from an aluminum smelter in western Norway. Scand J Work Environ Health 199925207–214. [DOI] [PubMed] [Google Scholar]
- 18.Spinelli J J, Band P R, Svirchev L M.et al Mortality and cancer incidence in aluminum reduction plant workers. J Occup Med 1991331150–1155. [DOI] [PubMed] [Google Scholar]
- 19.Tremblay C, Armstrong B, Theriault G.et al Estimation of risk of developing bladder cancer among workers exposed to coal tar pitch volatiles in the primary aluminum industry. Am J Ind Med 199527335–348. [DOI] [PubMed] [Google Scholar]
- 20.Moulin J J, Clavel T, Buclez B.et al A mortality study among workers in a French aluminium reduction plant. Int Arch Occup Environ Health 200073323–330. [DOI] [PubMed] [Google Scholar]
- 21.Carta P, Aru G, Cadeddu C.et al Mortality for pancreatic cancer among aluminium smelter workers in Sardinia, Italy. G Ital Med Lav Ergon 20042683–89. [PubMed] [Google Scholar]
- 22.Risch H A, Burch J D, Miller A B.et al Occupational factors and the incidence of cancer of the bladder in Canada. Br J Ind Med 198845361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clavel J, Mandereau L, Limasset J C.et al Occupational exposure to polycyclic aromatic hydrocarbons and the risk of bladder cancer: a French case‐control study. Int J Epidemiol 1994231145–1153. [DOI] [PubMed] [Google Scholar]
- 24.Bonassi S, Merlo F, Pearce N.et al Bladder cancer and occupational exposure to polycyclic aromatic hydrocarbons. Int J Cancer 198944648–651. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher M C, Slattery M L, West D W. Occupation and bladder cancer in Utah. Am J Ind Med 19891689–102. [DOI] [PubMed] [Google Scholar]
- 26.Siemiatycki J, Dewar R, Nadon L.et al Occupational risk factors for bladder cancer: results from a case‐control study in Montreal, Quebec, Canada. Am J Epidemiol 19941401061–1080. [DOI] [PubMed] [Google Scholar]
- 27.Grimsrud T K, Langseth H, Engeland A.et al Lung and bladder cancer in a Norwegian municipality with iron and steel producing industry: population based case‐control studies. Occup Environ Med 199855387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 19978444–472. [DOI] [PubMed] [Google Scholar]
- 29.Theriault G P, Tremblay C G, Armstrong B G. Risk of ischemic heart disease among primary aluminum production workers. Am J Ind Med 198813659–666. [DOI] [PubMed] [Google Scholar]
- 30.Ronneberg A. Mortality and cancer morbidity in workers from an aluminium smelter with prebaked carbon anodes—Part III: Mortality from circulatory and respiratory diseases. Occup Environ Med 199552255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen J H, Jensen O M. Occupation and risk of cancer in Denmark. An analysis of 93,810 cancer cases, 1970–1979. Scand J Work Environ Health 198713(Suppl 1)1–91. [PubMed] [Google Scholar]
- 32.Hooiveld M, Spee T, Burstyn I.et al Lung cancer mortality in a Dutch cohort of asphalt workers: evaluation of possible confounding by smoking. Am J Ind Med 20034379–87. [DOI] [PubMed] [Google Scholar]
- 33.Boyle P. Cancer, cigarette smoking and premature death in Europe: a review including the recommendations of European Cancer Experts Consensus Meeting, Helsinki, October 1996. Lung Cancer 1997171–60. [DOI] [PubMed] [Google Scholar]
- 34.Spinelli J J, Band P R, Gallagher R P. Adjustment for confounding in occupational cancer epidemiology. Recent Results Cancer Res 199012064–77. [DOI] [PubMed] [Google Scholar]
- 35.Kriebel D, Zeka A, Eisen E A.et al Quantitative evaluation of the effects of uncontrolled confounding by alcohol and tobacco in occupational cancer studies. Int J Epidemiol 2004331040–1045. [DOI] [PubMed] [Google Scholar]
- 36.Partanen T, Boffetta P, Heikkilä P R.et al Cancer risk for European asphalt workers. Scand J Work Environ Health 199521252–258. [DOI] [PubMed] [Google Scholar]
- 37.Dosemeci M, Wacholder S, Lubin J H. Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol 1990132746–748. [DOI] [PubMed] [Google Scholar]
- 38.Stayner L, Steenland K, Dosemeci M.et al Attenuation of exposure‐response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health 200329317–324. [DOI] [PubMed] [Google Scholar]
- 39.Steenland K, Deddens J, Zhao S. Biases in estimating the effect of cumulative exposure in log‐linear models when estimated exposure levels are assigned. Scand J Work Environ Health 20002637–43. [DOI] [PubMed] [Google Scholar]
- 40.Burstyn I, Kim H M, Cherry N.et al Metamodels of bias in Cox proportional‐hazards and logistic regressions with heteroscedastic measurement error under group‐level exposure assessment. Ann Occup Hyg 200650271–279. [DOI] [PubMed] [Google Scholar]
- 41.Richardson D B, Loomis D. The impact of exposure categorisation for grouped analyses of cohort data. Occup Environ Med 200461930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]