Abstract
Background
The Runcorn area, north‐west England, contains many pollution sources, the health effects of which have been under discussion for over 100 years. Preliminary investigations revealed an excess risk of mortality from kidney disease in people living nearest to several point sources of pollution, using distance as a proxy for exposure. Ongoing epidemiological investigations into the effect of ambient mercury exposure on dose and renal effect required a more refined assessment of exposure.
Methods
Atmospheric dispersion modelling was used to assess mercury dispersion from three mercury‐emitting sources (including a large chlor alkali plant), based on knowledge of emissions, local meteorology and topography.
Results
The model was sensitive to various input parameters, with different dispersion patterns and ground‐level concentrations, and therefore different exposed populations identified when different input parameters were defined. The different approaches to exposure assessment also had an impact on the epidemiological findings. The model output correlated well with weekly monitoring data collected in the local area, although the model underestimated concentrations in close proximity to the chlor alkali plant. The model identified that one point source did not contribute significantly to ground‐level mercury concentrations, so that inclusion of this source when using the “distance as a proxy” approach led to significant exposure misclassification.
Conclusions
The model output indicates that assessment of ambient exposure should give consideration to the magnitude of emissions, point source characteristics, local meteorology and topography to ensure that the most appropriate exposure classification is reached. Even if dispersion modelling cannot be undertaken, these data can be used to inform and improve the distance as a proxy approach, and improve the interpretability of the epidemiological findings.
Runcorn, an industrial town in the north‐west of England, contains many pollution sources, the health effects of which have been the subject of discussion for over 100 years.1 Although emissions of many substances are now greatly reduced compared with historical levels,2 local industries released over a ton of mercury per year during the period 1998–2002 (based on emissions data from the Environment Agency (EA) Pollution Inventory).3
Identifying populations at risk from exposure to hazardous substances can be a complex task, and may involve significant data input, expense and time.4 More simple approaches using a proxy measure of exposure, for instance, using distance of residence from a point source as an estimate of exposure,5 can be implemented relatively easily; however, they are limited in what they can reveal about any associations found.
Distance as a proxy for exposure was used in a preliminary investigation into the possible health effects associated with industrial activity in the Runcorn area.6 Exposure to pollutants from local industry was hypothesised to be associated with excess risks of specified diseases, and risks in populations living within 0–2 and 2–7.5 km of several major point sources were investigated. These distances were arbitrarily selected a priori to minimise the effect of boundary shrinkage. “Boundary shrinkage” refers to an investigation that focuses tightly on an apparent cluster of events, minimising the underlying population, and therefore the number of expected cases, thus maximising the excess risk. These arbitrary distances have been used in previous Small Area Health Statistics Unit studies to achieve a compromise between population size and proximity to the point source,5 although little has been done to assess the validity of these distances as an exposure measure. The main finding of this preliminary work was an excess mortality from renal disease in people living nearest to the point sources, a pattern that was also evident in renal hospital admissions investigated by the former North Cheshire Health Authority.6
Using distance as a proxy for exposure is rarely an accurate way of identifying exposed populations, as no consideration is given to point source characteristics (emissions, stack height and plume properties), to local meteorological conditions or to topographical features, all of which play a significant role in determining dispersion and pollutant concentration.7 By using mathematical representations of these factors, air dispersion models can—if sufficient data are available to describe these parameters—provide a more accurate assessment of potential exposure.8 Although air dispersion modelling has been used extensively for air quality management and regulatory purposes, this approach has rarely been applied to exposure assessment for epidemiological studies, despite proving to be a useful tool in the few studies where modelling has been used.9,10,11,12,13
Following the findings of the preliminary investigations in Runcorn, a decision was made to further investigate renal effects in this population. Mercury was of particular concern, because of its documented toxicity at low exposures, and because of concern over the release of this substance in Europe (eg, the European Mercury Emissions from Chlor Alkali Plants project).14 In this paper, we describe how dispersion modelling has been used to estimate ambient concentrations of mercury in the vicinity of several point sources, based on knowledge of emissions, local meteorology and topography, for use in an epidemiological study.
We compare the modelled exposure assessment with the crude estimate based on distance as a proxy for exposure, and provide some discussion of how the crude measure might be improved upon, based on limited knowledge of emissions, point source characteristics and local meteorology.
The term “exposure” has been used throughout to mean “ambient mercury concentrations”, as in our epidemiological study we were interested in possible health effects of any additional exposure due to living in the vicinity of a mercury‐emitting industry. We do, however, appreciate that ambient mercury exposure is not the same as personal mercury exposure. Ambient outdoor levels of mercury are only one of many sources of exposure to this substance, and exposure to inorganic mercury from dental amalgam (∼700 ng/day/filling) and diet (∼400 ng/day) usually far exceeds the exposure from non‐contaminated air (∼40 ng/day).15,16,17 It should also be noted that outdoor mercury levels are not necessarily a reflection of indoor concentrations,18 and that people do not spend all their time in the vicinity of their homes. Here we considered only inhalation exposure, as this route is considered to be most important in adult exposure to inorganic mercury (with ∼80% of inhaled inorganic mercury being retained in the body compared with ∼10% ingested inorganic mercury)17,19; furthermore, the industrial processes investigated emit mercury mainly to air (∼84% of total mercury emissions were to air over the period 1998–2002).
Methods
The modelling package used was the Atmospheric Dispersion Modelling System (ADMS) Urban V.2.0 (Cambridge Environmental Research Consultants, Cambridge, UK).20 This personal‐computer‐based model of atmospheric dispersion of pollutants from industrial, domestic and road sources is well established in the UK for investigating air pollution in cities and towns.
Model input data
There are three mercury‐emitting sites in the area. Quarterly/annual mercury emissions data and details of point source characteristics (grid reference, height, diameter and volume flow rate/exit velocity) were obtained from Integrated Pollution Control applications and emissions data held at the EA Public Registry, Warrington, UK, and are listed in table 1; the locations of these sites are indicated in fig 1A. The three sites consist of
Table 1 Point source characteristics.
| Site/point source | Height (m) | Diameter (m) | Flow rate (m3/s) | Vertical velocity (m/s) | Release temperature (°C) | Emissions (kg/hour) average, 1998–2001 |
|---|---|---|---|---|---|---|
| AL7294 Chlor alkali plant | ||||||
| 4—Cell room | 16.0 | 7.75 | 370.37 | — | 15 | 0.0417 |
| 5—Cell room | 16.0 | 8.49 | 370.37 | — | 15 | 0.0536 |
| 6—Cell room | 16.0 | 6.84 | 370.37 | — | 15 | 0.0274 |
| 100—Emergency vent | 25.0 | 0.40 | 4.17 | — | 15 | 0.0567 |
| 1 | 25.0 | 0.10 | 0.14 | — | 15 | 0.0003 |
| 2 | 25.0 | 0.10 | 2.46 | — | 15 | 0.0004 |
| 3 | 25.0 | 0.10 | 0.65 | — | 15 | 0.0002 |
| 7 | 9.9 | 0.35 | 46.30 | — | 15 | 0.0084 |
| 9 | 27.0 | 0.40 | 4.63 | — | 15 | 0.0014 |
| 10 | 24.4 | 0.80 | 5.00 | — | 15 | 0.0004 |
| AA3123 Multifuel power station stack | 106.8 | 5.40 | — | 8.55 | 110* | 0.0026 |
| AA3301 Coal‐fired power station stack | 199.0 | 3.91 | — | 31.1† | 134* | 0.0126 |
– Parameter not used.
*After the modelling was completed, additional data on temperature of release and exit velocity were made known to us. For the multifuel power station an exit temperature of 91°C was reported, and for the coal‐fired power station an exit temperature of 125°C–130°C and an exit velocity of 19 m/s were found. Incorporating these parameters into the model instead of the assumed values indicated in the table made no difference to the average ground‐level concentration, and made very little difference to the pattern of dispersion/exposed population (an additional 2 (0.04%) persons were identified as exposed using these recorded parameters compared with that using the assumed values presented above).
†For the coal‐fired power station, an exit velocity of 31.1 m/s was assumed, on the basis of model parameters for a large coal‐fired boiler detailed elsewhere.21
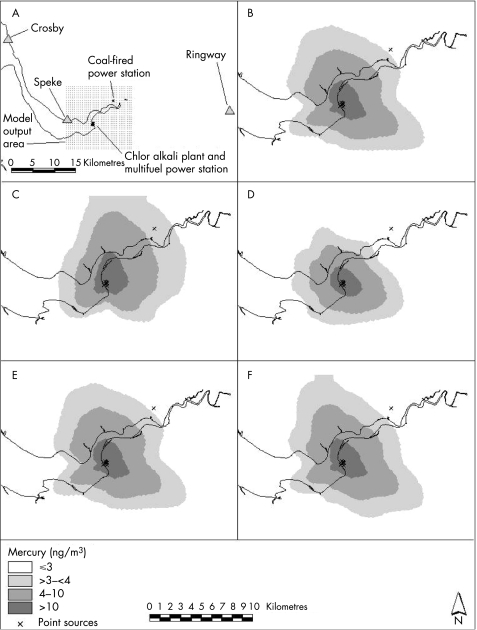
Figure 1 Sensitivity of the model to various input parameters. Models B–F use the same emissions data from 2000, and use meteorological data for 2000 from the specified meteorological station. (A) Map showing point sources, meteorological stations and model output area; (B) best‐estimate model output using meteorological data from Speke, with Ringway total cloud amount (TCA) data; (C) model output using meteorological data from Ringway; (D) model output using meteorological data from Crosby; (E) model output using meteorological data from Speke, with Crosby TCA data; and (F) model output using meteorological data from Speke, with Ringway TCA data but no topography data.
a large chlor alkali plant based at this site since the end of the 19th century (Mercury is reported to be released from 10 vents, mainly the cell rooms, and during some quarters from an emergency vent.)
a multifuel power station burning mercury‐saturated hydrogen from the nearby chlor alkali plant and emitting from a single tall stack
a large coal‐fired power station releasing lesser quantities of mercury from a single tall stack
Emissions data were available from 1995 for the chlor alkali plant, from 1996 for the multifuel power station and from 1998 for the coal‐fired power station.
Temperature‐of‐release data were not provided in the Integrated Pollution Control applications, so assumptions were made as detailed below. For the chlor alkali plant, emissions were assumed to be at ambient temperature,21 which was recorded to be 10.2°C during 2000. However, the temperatures of releases from the chlor alkali plant cell rooms are likely to be higher, as the electrolysis process generates heat. As such, a temperature of release of 15°C was modelled as the best estimate. The sensitivity of the model to this assumption was assessed. For the multifuel power station, flue gas temperatures exiting the boilers were reported to be typically 110°C on gas firing and 190°C on oil firing; however, details of fuel usage were not known. The lower temperature of 110°C was used throughout to represent the worst‐case temperature scenario. For the coal‐fired power station, an exit temperature of 134°C and an exit velocity of 31.1 m/s were assumed on the basis of model parameters for a large coal‐fired boiler detailed elsewhere.21
Background levels have been measured to be around 1.68–1.75 ng/m3 in the UK,22,23 and a background concentration of 1.75 ng/m3 has been added to the model outputs presented.
A terrain file of surface elevation was prepared from the appropriate Ordnance Survey Landform Panorama digital terrain model, incorporating a 32×32 grid of surface elevation into the model output area, allowing ADMS to adjust plume height and spread parameters according to local terrain characteristics.20 A surface roughness of 0.5 m was used (representative of parkland/open suburbia), as this was considered to be the most appropriate roughness length for the area being modelled.
Mercury speciation and deposition
In the environment, inorganic mercury exists as three main species—Hg0(g) (elemental (unreactive) gaseous mercury); Hg2+(g) (divalent (reactive) gaseous mercury); and Hg(p) (particulate mercury). These species have different chemical reactivities and deposition velocities.24 The relative speciation of mercury released from the processes in Runcorn is not known, so ratios of elemental:divalent:particulate mercury were assumed to be 50:30:20 for combustion processes and 70:30:0 for chlor alkali factories, after Bullock25 and the US Environmental Protection Agency.21
An average dry deposition velocity of 0.15 cm/s for total mercury released from these three plants was calculated on the basis of the assumed speciation (above) and the dry deposition values presented in other works (zero deposition for Hg0(g), 0.47 cm/s for Hg2+(g) and 0.2 cm/s for Hg(p))26 weighted by the relative emissions from each plant over the period 1998–2004. Wet deposition was not modelled in this exercise, as it was considered unlikely to affect on output at such a local scale.
Meteorological data
ADMS requires wind speed/direction, total cloud amount (TCA) and air temperature to calculate atmospheric boundary layer parameters. Hourly land surface meteorological observations from the Met Office station network were acquired for 1995–2004 from the British Atmospheric Data Centre.27 The nearest weather stations to Runcorn that provide these data are Crosby in Merseyside and Ringway in Greater Manchester, both approximately 30 km from the site of interest (fig 1A). Data from Speke in Merseyside, approximately 6 km away from the pollution sources, were also available, but lacked data on TCA.
A previous modelling exercise assessed the validity of using Speke meteorological data for dispersion of mercury at the site of the chlor alkali plant. Meteorological data for November 1975 were recorded at the plant, and, although hourly measurements cannot be directly compared at geographically separated sites owing to the progression of weather across a region, the overall agreement between the data sets was good.28
A best‐estimate meteorological data set was constructed using wind and temperature data from Speke and TCA data from Ringway (Ringway data were more complete than Crosby data). Although the cloud amount between these stations will not be the same in all weather conditions, local experience suggests that they will be similar (Met Office, personal communication, 2003), and the sensitivity of the model to this parameter was assessed using a similar data set constructed using Crosby TCA data.
Model output
For the sensitivity analysis, model outputs for 2000 were compared. Annual ground‐level mercury concentrations were also calculated for 1998–2001 and were averaged to provide an estimate of longer‐term exposure. Model outputs were mapped using ArcView GIS 3.2 (ESRI, Redlands, California, USA), on a 32×32 grid (the ADMS output), over an area covering 14 000×13 500 m (grid coordinates x axis 344 000 and y axis 375 500, to x axis 358 000 and y axis 389 000 (shaded area in fig 1A)), resulting in a mapping resolution of approximately 440×420 m.
Evaluation of model quality
Weekly active sampling of ambient vapour phase mercury (Hg0(g) and Hg2+(g)) onto gold‐coated silica adsorption tubes (flow rate 100 ml/min) was undertaken at nine sites (fig 2) over a 14‐week period commencing from 1 September 2004,29 to allow validation of the modelled output. Adsorption tubes were analysed at Casella laboratories, on a Sir Galahad II (PS Analytical, Orpington, Kent, UK), using amalgamation in conjunction with atomic fluorescence detection, with a detection limit of <34 pg per tube. The mercury levels recorded over this 14‐week period were compared with the modelled output over the same 14‐week period, and were not taken to be representative of the annual average.
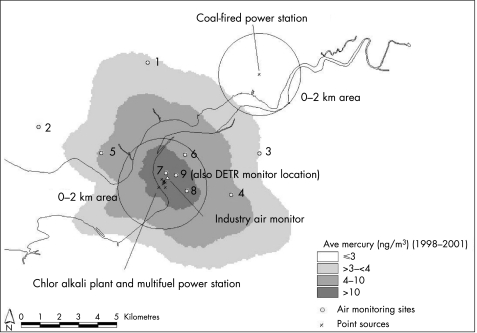
Figure 2 Best‐estimate model output (average 1998–2001), with the 0–2 km distance as a proxy area overlaid, and air monitoring sites. DETR, Department of the Environment, Transport and the Regions.
Weekly measurements of ambient mercury levels were available for 2000 from the Weston County Primary School (grid coordinates x axis 350 300 and y axis 381 300 (the same location as site 9, labelled site 9i)), collected for the Department of the Environment, Transport and the Regions (DETR (now DEFRA)) programme for monitoring heavy metal around industrial sites using the techniques described above.29 Quarterly ambient air monitoring data collected by the local industry in the vicinity of the chlor alkali plant were also available for 1995–2003 (monitor grid coordinates x axis 349 920 and y axis 381 180). The locations of the DETR and industry monitoring sites are indicated in fig 2.
Weekly or quarterly average mercury concentrations at each monitoring site were modelled using ADMS (using the relevant quarterly emissions and quarterly/weekly meteorological data) to allow comparison with these air monitoring data.
Identification of exposed population
The towns of Runcorn and Widnes make up the borough of Halton, with a population of 118 208.30
A level of ambient mercury of >10 ng/m3 has been used to define the exposed population. This ambient mercury level was considered high enough to be detected as being above the background level using biological markers (mean urinary mercury levels).31
The populations presented in this report are based on postcode‐level population counts for 2001 (derived from 2001 census data). Each postcode contains on average 15 households and approximately 35 people.32 The postcode code‐points (the x and y coordinates of the nearest delivery point to the calculated mean position of all the delivery points in the postcode) falling within the >10 ng/m3 contour (or within 0–2 km distance as proxy areas) were identified and populations summed to give the estimates of exposed populations presented. The postcode code‐points were also attributed modelled exposures, which were used to calculate the population‐weighted average exposures. Postcode populations of 2001 were used because estimates for years before this were based on populations extrapolated from the 1991 census, and are not available at the postcode level.
Results
Model sensitivity
Figure 1 and table 2 show the sensitivity of the model to the various meteorological data and less‐well‐characterised input data; all models used emissions and meteorological data for 2000. Figure 1B–F shows the dispersion of mercury for some of the model variations assessed. Table 2 shows the average concentrations of mercury predicted over the modelled output area, the percentage change in this average concentration and the Pearson correlation coefficient of each output compared with the best‐estimate output (model output B). The table also shows the different populations identified as being exposed to >10 ng/m3 mercury, and indicates the population‐weighted average exposure for this population.
Table 2 Sensitivity of the model to changes in model input parameters: average mercury concentration over the whole modelled area, percentage change in concentration (compared with best estimate (model output B)), correlation between modelled mercury concentrations (compared with best estimate (model output B)), estimate of the population exposed to >10 ng/m3 mercury, and population‐weighted average exposure for those classified as exposed to >10 ng/m3 mercury.
| Model parameters* | Average mercury (ng/m3) | Percentage change from model B | Correlation with model B† | Population exposed >10 ng/m3 | Population‐weighted exposure‡ (ng/m3) |
|---|---|---|---|---|---|
| (B) Speke meteorological data (using Ringway TCA data) | 4.29 | Reference | Reference | 5490 | 22.75 |
| (C) Ringway meteorological data | 4.31 | +0.5 | 0.940 | 10 190 | 19.48 |
| (D) Crosby meteorological data | 3.65 | −14.9 | 0.986 | 3152 | 26.29 |
| (E) Speke meteorological data (using Crosby TCA data) | 4.26 | −0.7 | 1.000 | 5544 | 22.56 |
| (F) Speke meteorological data; no topography data | 4.29 | +/−0.0 | 0.996 | 4773 | 24.07 |
| (G) Speke meteorological data; release temperature for chlor alkali plant 10.2°C | 4.53 | +5.9 | 0.998 | 6329 | 23.81 |
| (H) Speke meteorological data; release temperature for chlor alkali plant 25°C | 3.98 | −7.2 | 0.999 | 4052 | 23.07 |
| (I) Speke meteorological data; surface roughness 0.2 m | 4.64 | +8.2 | 0.995 | 7472 | 20.22 |
| (J) Speke meteorological data; surface roughness 1.0 m | 3.97 | −7.5 | 0.995 | 4152 | 25.33 |
| (K) Speke meteorological data; deposition velocity 0.47 cm/s | 4.29 | +/−0.0 | 1.000 | 5556 | 22.60 |
TCA, total cloud amount.
Model outputs B–F are also shown in fig 1.
*All models used emissions data and meteorological data for 2000.
†Two‐tailed p values all <0.01.
‡Population‐weighted average exposure for those classified as exposed to >10 ng/m3 mercury.
The best‐estimate model output (fig 1B and table 2(B)) uses meteorological data from the nearest meteorological station, Speke (with Ringway TCA data), incorporates topographical data, assumes a mercury release temperature of 15°C from the chlor alkali plant cell rooms and uses the default surface roughness of 0.5 m (representative of parkland/open suburbia).
All model outputs predict a similar average mercury concentration across the output area (within 15% of the best estimate), and correlate well with the best‐estimate output (correlations all ⩾0.94, two‐tailed p values <0.01). However, there were differences between the modelled outputs.
The model was particularly sensitive to the meteorological data used (fig 1B–D; table 2(B–D), but was not sensitive to changes in TCA data (fig 1E and table 2(E)), strengthening the hypothesis that it is sufficient to assume that cloud conditions in Speke are similar to cloud conditions in the greater surrounding area.
The sensitivity of the model to the topography data was investigated by removing this parameter from the model, which resulted in a slightly smaller exposed population (with a higher population‐weighted exposure) being identified (fig 1F and table 2(F)).
Plume buoyancy and effective stack height are influenced by the temperature of release.7 Assuming a lower temperature of mercury release from the chlor alkali plant (ambient temperature (10.2°C) versus best estimate (15°C)) revealed a slightly extended dispersion pattern and a larger exposed population (table 2(G)). Assuming a higher temperature of release (25°C vs 15°C) resulted in a smaller exposed population (table 2(H)).
Mechanical turbulence created by the flow of the wind over obstacles on the ground can influence dispersion, with the intensity of the mechanical turbulence increasing with increasing surface roughness.7 Decreasing the surface roughness to 0.2 m (representing agricultural areas) increased the exposed population (table 2(I)); increasing the surface roughness to 1 m (representing cities/woodland) decreased the exposed population (table 2(J)), compared with a best‐estimate surface roughness of 0.5 m (parkland/open suburbia).
The sensitivity of the model to the deposition parameter was found to be small when the deposition value was changed from the best estimate (0.15 cm/s) to a higher rate based on the assumption that all emissions were of divalent reactive gas mercury (0.47 cm/s) (table 2(K)).
Main messages
Crude, proxy measures of exposure are used in many point source epidemiological investigations, often resulting in misclassification of exposure and biased risk estimates.
We demonstrate how a proxy measure can be greatly improved upon by using atmospheric dispersion modelling, an approach that can easily incorporate the factors that drive ambient exposure (source characteristics, emissions, local meteorology and topography).
Where modelling is not possible, modification to the “distance as a proxy” approach based on even limited knowledge of the factors that drive exposure can also greatly improve the crude method and add to the interpretability of the resulting epidemiological findings.
Policy implications
Appropriate assessment of exposure is vital to any epidemiological study, and yet proxy measures continue to be used.
Dispersion modelling, which can reduce misclassification of exposure, should be considered a valuable tool for assessing exposure in epidemiological studies.
Evaluation of the model quality
The correlation between the mean measured value and the mean modelled value (using the best‐estimate model parameters defined above) at the nine monitoring sites was good (Pearson's correlation coefficient = 0.93, two‐tailed p value <0.01). The correlations week by week at each site were >0.75 (p<0.02) at seven of the nine monitoring sites, and at the two remaining sites (correlations 0.59 (p = 0.04) at site 3, and 0.24 (p = 0.45) at site 5) there were persistent battery and flow rate problems (table 3). The mean modelled values by site tended to underestimate ambient levels, especially at sites nearest to the chlor alkali plant (sites 7, 8 and 9), where modelled values were approximately half the measured values.
Table 3 Measured ambient mercury concentrations, modelled mercury concentrations and Pearson's correlation coefficients between the two at each air monitoring station.
| Site | No of weeks sampled | Measured [Hg] (ng/m3) | Modelled [Hg] (ng/m3) | Correlation (p value) |
|---|---|---|---|---|
| Mean (range) | Mean (range) | |||
| 1 | 14 | 3.38 (1.49 to 7.78) | 3.27 (2.10 to 5.86) | 0.84 (<0.01) |
| 2 | 14 | 2.12 (0.92 to 4.47) | 2.35 (1.75 to 3.63) | 0.83 (<0.01) |
| 3 | 12 | 4.03 (1.59 to 11.68) | 2.91 (1.81 to 6.57) | 0.59 (0.04) |
| 4 | 14 | 8.28 (1.86 to 21.98) | 4.36 (2.20 to 7.91) | 0.79 (<0.01) |
| 5 | 12 | 2.88 (1.13 to 10.64) | 3.38 (1.75 to 6.96) | 0.24 (0.45) |
| 6 | 13 | 11.79 (2.11 to 35.92) | 6.44 (1.87 to 15.17) | 0.75 (<0.01) |
| 7 | 12 | 26.59 (5.54 to 73.13) | 13.38 (2.61 to 33.50) | 0.78 (<0.01) |
| 8 | 9 | 21.72 (4.16 to 43.92) | 10.88 (3.51 to 23.76) | 0.76 (0.02) |
| 9 | 10 | 23.17 (3.40 to 54.00) | 18.63 (3.74 to 48.71) | 0.78 (0.01) |
| 9i* | 29 | 24.11 (2.27 to 95.40) | 36.45 (2.80 to 69.20) | 0.64 (<0.01) |
*Department of the Environment, Transport and the Regions monitoring site located at the same site as site 9, but monitoring carried out during 2000 (compared with modelled output during 2000).
The mean modelled value at the location of the DETR monitor (table 3, site 9i) for 2000 (36 ng/m3) also compared well with the mean measured value (24 ng/m3), Pearson's correlation coefficient = 0.64, p<0.01.
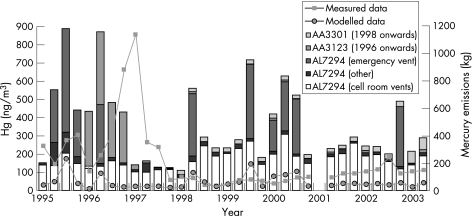
When the modelled output was compared with the quarterly industry‐measured data (1995–2003; fig 3), no correlation was seen. Again, the model underestimated concentrations; the mean modelled value over the period 1995–2003 was 43 ng/m3 compared with the mean measured value of 155 ng/m3.
Figure 3 Quarterly modelled concentrations of mercury and background concentrations of 1.75 ng/m3 compared with quarterly ambient air monitoring data for 1995–2003; bars represent the mercury emissions (kg) as a total by quarter and source.
Comparison of modelled output with distance as proxy
In the preliminary investigation, distance from point source was used as a proxy for exposure. This simple approach assumes, in this case, that the areas within a 2 km radius of the point sources are exposed similarly, irrespective of quantity of emissions, effective stack height (determined by the temperature and velocity of release) or local meteorological/topographical conditions. The modelled exposure output (average 1998–2001) and 0–2 km areas around the same 12 points of release from the three mercury‐emitting plants are overlaid in fig 2.
Obviously, the populations classified as exposed will vary enormously depending on which modelled contours and proxy distances are chosen to represent exposure. In this comparison, high exposure refers to people living within 2 km of the point sources using the distance as a proxy approach, and to the population exposed to >10 ng/m3 when using the modelling design. It is important to note, as indicated previously, that both the radii and concentration levels are set arbitrarily, but with reasons for the choice of values. Populations estimated at postcode level were 15 885 in the 0–2 km buffer, and 3378 with modelled exposure to >10 ng/m3 mercury (average 1998–2001).
The population‐weighted average mercury exposure in the 0–2 km area around all point sources based on the model output averaged over 1998–2001 was 8.5 ng/m3 (range 2.4–53.7 ng/m3). This elevation above the background level was mainly due to the higher mercury concentration in the 0–2 km area around the chlor alkali plant/multifuel power station, where the population‐weighted average was 14.5 ng/m3 (range 6.8–53.7 ng/m3), compared with the average around the coal‐fired power station of 2.7 ng/m3 (range 2.4–3.0 ng/m3).
The differing approaches to exposure assessment also have an impact on the epidemiological results. Table 4 shows the standardised mortality ratios adjusted for age, gender and socioeconomic status (Carstairs quintiles) for mortality from nephritis, nephritic syndrome and nephrosis (International Classification of Diseases, revision 9, codes 580–589; International Classification of Diseases, revision 10, codes N00–N06, N10–N12, N14–N15, N17–N19, N25–N27) calculated for the period 1981–2001 for populations living within 0–2 (high exposure) and 2–7.5 km (medium exposure) of the three mercury‐emitting industries (12 points of emissions), or populations with modelled ambient mercury levels of >10 ng/m3 (high exposure) or 4–10 ng/m3 (medium exposure) from the same points of emission. The reference population was the population of the north‐west government region.
Table 4 Comparison between renal disease standardised mortality ratios (adjusted for age, gender and socioeconomic status), 1981–2001, calculated using either distance as a proxy or modelled exposure contours to assess mercury exposure.
| Distance as proxy | Modelled exposure contours | |||||||
|---|---|---|---|---|---|---|---|---|
| Person years | Obs | Exp | SMR (95% CI) | Person years | Obs | Exp | SMR (95% CI) | |
| Males | ||||||||
| Reference | 65 394 296 | — | — | 100 (—) | 65 394 296 | — | — | 100 (—) |
| Medium exposure* | 2 669 850 | 148 | 120.04 | 123 (104 to 145) | 361 245 | 27 | 18.94 | 143 (94 to 207) |
| High exposure† | 174 006 | 18 | 9.68 | 186 (110 to 294) | 41 982 | 6 | 3.26 | 184 (68 to 400) |
| Females | ||||||||
| Reference | 68 870 244 | — | — | 100 (—) | 68 870 244 | — | — | 100 (—) |
| Medium exposure* | 2 755 171 | 194 | 148.70 | 131 (113 to 150) | ‡419 192 | ‡30 | ‡25.62 | ‡117 (79 to 167) |
| High exposure† | 179 417 | 23 | 11.49 | 200 (126 to 300) | ||||
Exp, expected; obs, observed; SMR, standardised mortality ratio.
*Medium exposure = 2–7.5 km (distance as proxy) or 4–10 ng/m3 (modelled exposure contours).
†High exposure = 0–2 km (distance as proxy) or >10 ng/m3 (modelled exposure contours).
– No data provided.
‡Due to small cell counts, the high and medium mercury exposure groups were combined to make one mercury‐exposed group (with ambient levels of >4 ng/m3).
Discussion
Appropriate assessment of exposure is vital to any epidemiological study, and, although many authors discuss the potential impact of exposure misclassification on study findings, very few attempt to quantify the extent of misclassification. The use of detailed, validated exposure modelling to reduce misclassification is still rather uncommon.
We used modelling to assess the dispersion of mercury around three mercury‐emitting plants, to improve an exposure assessment estimate for an epidemiological study. The exposure measure obtained relates only to ambient exposure, which is of specific interest in our epidemiological study, but it should be remembered that these modelled ambient levels are not the same as personal mercury exposure, which in the general population will be determined largely by amalgam fillings and diet.
Model sensitivity
The modelled pattern of mercury dispersion, predicted ground‐level concentrations, populations identified as being exposed and population‐weighted average exposure estimates were dependent on the model input parameters. The output quality will reflect any limitations in these data. This model was based on reported emissions data; however, we have still had to assume that emissions were constant within the quarter/year being modelled and have had to estimate the temperatures of release, as well as the exit velocity for the coal‐fired power station.
Model sensitivity was assessed for less‐well‐characterised parameters. The model was sensitive to the meteorological data used; however, having access to local meteorological data (Speke) that has previously been shown to correlate well with on‐site meteorological measurements should mean these data are representative. If this is the case, then the population exposed to mercury levels >10 ng/m3 ranged from 4052 to 7472 when the other input data (TCA, local topography, temperature of release, surface roughness and deposition/speciation parameters) were varied. This variation is much smaller than the effect of changes in emissions data (eg, year on year) or choice of cut‐off concentration for the exposed population, and the relative insensitivity of the model output to these parameters allows us to conclude that the dispersion model provides a greatly improved estimate of ambient mercury exposure compared with the distance as a proxy approach.
Evaluation of the model quality
Air monitoring indicated that the best‐estimate model output was a good reflection of current ambient mercury levels across the area, although the model did tend to underestimate concentrations at sites where high mercury levels were measured. None the less, there was a significant difference in means between measurements made at monitoring sites in the high exposure contour (>10 ng/m3) (sites 7–9 mean 24.06 ng/m3), those in the medium exposure contour (sites 4–6 mean 7.79 ng/m3) and those in the lower exposure contours (sites 1–3, mean 3.14 ng/m3 p values <0.01), indicating that average ambient exposures between the exposed and non‐exposed populations do differ.
The quarterly‐modelled output did not show good agreement with industry‐measured data collected in close proximity to the chlor alkali plant over the period 1995–2003. There are several possible reasons for these differences, which are discussed below.
Contractor work by Mercury Recovery Services to decontaminate waste at the chlor alkali plant site reportedly led to release of mercury to air of <0.25 kg between November 1996 and January 1998, which would be unlikely to be detected above the hundreds of kilograms released from the chlor alkali plant over this period. However, fugitive emissions could have contributed significantly to ambient contamination. A prohibition notice (EP/P 1352 for Authorisation AV6027, dated 20 March 1997) served by the EA Public Registry (Warrington, UK) reported that “Mercury emissions from the process are causing unacceptable levels of atmospheric mercury beyond the site boundary”. Fugitive releases are not recorded in the emissions inventory and so could not be modelled.
Mercury flux from environmental compartments (vegetation, soil and water) may affect ambient levels. Measurements of ambient mercury around a heavily contaminated former chlor alkali plant site in Germany showed that, 2 years after production had stopped, mercury levels of >100 μg/m3 could still be detected in air close to heavily contaminated soil surfaces, and that levels >500 ng/m3 were measured outside the factory premises.33 Mercury flux from contaminated soils at the chlor alkali plant in Runcorn was not accounted for in the model.
The European Mercury Emissions from Chlor Alkali Plants study used a range of techniques to measure mercury emissions and ambient concentrations around a chlor alkali plant in Sweden. One of these techniques, light detection and ranging, was used to measure mercury releases from the plant, and showed that measured emissions were up to twice those reported to be released by the plant.34 Whether this under‐reporting applies to other chlor alkali plants is not known; however, this potential bias for under‐reported emissions is acknowledged.
Nearby buildings and downwash effects can deflect the flow of the wind and plume.7 Emissions of mercury from the cell room vents are subject to significant downwash effects, and, as releases are at a height of only 16 m, nearby buildings could well have an entrainment effect; however, these effects have not been incorporated into the model.
In calm meteorological conditions, the wind speed and direction are very variable, and a well‐defined plume may not form. In these conditions, ADMS may underestimate ground‐level concentrations.35,36 However, the assessment of dispersion at low wind speeds is generally unimportant in the calculation of long‐term average concentrations for risk assessment.37 In this modelling exercise, 5.8% of the meteorological data described calm conditions, suggesting that any underestimation of exposure due to calm conditions should be minor. None the less, calm conditions can lead to some of the highest ground‐level concentrations in close proximity to a stack, as there is no wind to disperse the pollutants.
The reasons for the greater discrepancy between the modelled and measured data at the industry monitor (located 250–375 m from the chlor alkali plant cell rooms) than at sites further from the chlor alkali plant are probably a combination of these factors. The impact of remediation work, flux from heavily contaminated locations on site, underestimation of emissions, local‐scale building effects and underestimation in calm conditions would all be expected to influence ambient levels very close to the plant; by contrast, the monitors located further afield would be less affected by these factors.
Comparison of modelled output with distance as proxy
The distance as a proxy approach gives no consideration to the quantity of release or point source characteristics, and so will not discriminate between sources that are likely to contribute to local ground‐level mercury concentrations and those that are not. In this example, one of the point sources, the coal‐fired power station, does not seem to affect the local ambient mercury levels; people living within several kilometres of this plant are not being exposed to mercury from this source, suggesting a significant exposure misclassification in the proxy measure if this source is included. Dispersion modelling can help to tell what distances most appropriately represent exposure and reduce misclassification. In this instance, an approximately 2 km radius circle around the chlor alkali plant/multifuel power station and excluding the coal‐fired power station altogether approximates the >10 ng/m3 exposure contour, and might have provided a more useful proxy of exposure.
With respect to the epidemiological findings, the overall trend of an increased risk of mortality from renal disease with increasing exposure is revealed using either approach. However, the modelled exposure assessment adds considerably to the interpretability of the epidemiological results. Furthermore, the results from the distance as proxy approach are highly dependent on the chosen radii, which were chosen arbitrarily in the preliminary study. Knowledge of the actual ambient levels allows researchers to make a more informed choice of low versus high exposure categories.
Improving a proxy measure
In situations where input data or resources are lacking for detailed modelling, even relatively limited information on emissions, point source characteristics and/or local meteorology could be used to improve the distance as a proxy approach.
With knowledge of the relative emissions from the three mercury‐emitting industries in Runcorn, it became clear that the local impact of the chlor alkali plant (emitting approximately 93% of the mercury over the period 1998–2001) was much greater than that of the coal‐fired power station (emitting approximately 6% of the mercury over this period).
Dispersion models work on the principle that the higher the effective stack height, the lower the maximum ground‐level concentration. Using this assumption, it would be possible to identify sites of probable local impact, even if emissions from each source had to be assumed to be similar. Here, the high effective stack height of the coal‐fired power station (tall stack, high temperature of release and high vertical velocity) would be expected to result in much lower ground‐level concentrations than around the chlor alkali plant (short stack and low temperature of release).
The predominant wind direction could also have been used to provide a more realistic indication of dispersion from the point sources. In this instance, the predominant wind directions were from the north‐west and from the south‐east; elongating the circle in these predominant wind directions would be a simple way to improve the proxy approach. Alternatively, a wind rose (a radial graph showing the number of hours the wind blows from each sector) could be transformed to show where the wind blows to, giving a much better idea of the expected pattern of dispersion.
Conclusions
It is clear that a proxy measure of exposure can provide a relatively quick and cheap way of predicting zones of potential impact around a point source; however, this modelling exercise has shown that a more informative indication of exposure will be derived if point source characteristics (especially emissions and stack height) can be incorporated into the exposure measure, and if consideration can be given to local meteorology and topography. Where modelling is not possible, modification to the distance as a proxy measure based on even limited information on the factors known to influence dispersion can also greatly improve the crude measure and reduce exposure misclassification, and increase the interpretability of the resulting epidemiological findings.
Acknowledgements
SAHSU is funded by a grant from the Department of Health; the Department for Environment, Food and Rural Affairs; the Environment Agency; the Scottish Executive; the Welsh Assembly Government and Northern Ireland Department of Health, Social Services and Public Safety. SH was supported by an MRC PhD studentship. We thank Casella Stanger for undertaking the air monitoring campaign; the Met Office/BADC for providing access to meteorological data, Ian Taylor and colleagues (Ineos chlor) for their willing help, and John Gulliver/Kees De Hoogh for advice on ADMS/GIS.
Abbreviations
ADMS - Atmospheric Dispersion Modelling System
DETR - Department of the Environment, Transport and the Regions
EA - Environment Agency
TCA - total cloud amount
Footnotes
Competing interests: None.
References
- 1.Anonymous The sanitary state of Runcorn. Lancet 18802442–143. [Google Scholar]
- 2.Harland B J, Taylor D, Wither A. The distribution of mercury and other trace metals in the sediments of the Mersey Estuary over 25 years 1974–1998. Sci Total Environ 200025345–62. [DOI] [PubMed] [Google Scholar]
- 3.Environment Agency Pollution Inventory http://www.environment‐agency.gov.uk/business/444255/446867/255244/ (accessed 27 March 2007)
- 4.Williams F L, Ogston S A. Identifying populations at risk from environmental contamination from point sources. Occup Environ Med 2002592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aylin P, Maheswaran R, Wakefield J.et al A national facility for small area disease mapping and rapid initial assessment of apparent disease clusters around a point source: the UK Small Area Health Statistics Unit. J Public Health Med 199921289–298. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson S, Nieuwenhuijsen M J, Hansell A.et al Excess risk of kidney disease in a population living near industrial plants. Occup Environ Med 200461717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams M L. Atmospheric dispersal of pollutants and the modelling of air pollution. In: Harrison RM, ed. Pollution, causes, effects and control. Cambridge, UK: Royal Society of Chemistry, 1990201–219.
- 8.Cora M G, Hung Y T. Air dispersion modeling: a tool for environmental evaluation and improvement. Environment Quality Management 20031275–86. [Google Scholar]
- 9.Bellander T, Berglind N, Gustavsson P.et al Using geographic information systems to assess individual historical exposure to air pollution from traffic and house heating in Stockholm. Environ Health Perspect 2001109633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colvile R N, Stevens E C, Nieuwenhuijsen M J.et al Atmospheric dispersion modeling for assessment of exposure to arsenic for epidemiological studies in the Nitra Valley, Slovakia. J Geophys Res 200110617421–17431. [Google Scholar]
- 11.Ihrig M M, Shalat S L, Baynes C. A hospital‐based case‐control study of stillbirths and environmental exposure to arsenic using an atmospheric dispersion model linked to a geographical information system. Epidemiology 19989290–294. [PubMed] [Google Scholar]
- 12.Reynolds P, Von Behren J, Gunier R B.et al Childhood cancer incidence rates and hazardous air pollutants in California: an exploratory analysis. Environ Health Perspect 2003111663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassilev Z P, Robson M G, Klotz J B. Outdoor exposure to airborne polycyclic organic matter and adverse reproductive outcomes: a pilot study. Am J Ind Med 200140255–262. [DOI] [PubMed] [Google Scholar]
- 14.European mercury emissions from chlor alkali plants (EMECAP) [EMECAP website] http://www.emecap.com (accessed 23 Feb 2007)
- 15.Richardson G M.Assessment of mercury exposure and risks from dental amalgam. Ottawa: Medical Devices Bureau, Environmental Health Directorate, Health Canada, 1995
- 16.Skare I, Engqvist A. Human exposure to mercury and silver released from dental amalgam restorations. Arch Environ Health 199449384–394. [DOI] [PubMed] [Google Scholar]
- 17. WHO Environmental Health Criteria 118—Inorganic mercury. United Nations Environment Programme, the International Labour Organisation, and the World Health Organization 1991
- 18.Carpi A, Chen Y F. Gaseous elemental mercury as an indoor air pollutant. Environ Sci Technol 2001354170–4173. [DOI] [PubMed] [Google Scholar]
- 19.Nusslein F, Feicht E A, Schulte‐Hostede S.et al Exposure analysis of the inhabitants living in the neighbourhood of a mercury‐contaminated industrial site. Chemosphere 1995302241–2248. [DOI] [PubMed] [Google Scholar]
- 20.ADMS Urban [computer program] Version 2. Cambridge: Cambridge Environmental Research Consultants, 2003
- 21.Environment Protection Agency Mercury study report to congress. Vol III: fate and transport of mercury in the environment. UK: Environment Protection Agency, 1997, (EPA‐452/R‐97‐005)
- 22.Ebinghaus R, Kock H H, Schmolke S R. Measurements of atmospheric mercury with high time resolution: recent applications in environmental research and monitoring. Fresenius J Anal Chem 2001371806–815. [DOI] [PubMed] [Google Scholar]
- 23.Lee D S, Dollard G J, Pepler S. Gas‐phase mercury in the atmosphere of the United Kingdom. Atmos Environ 199832855–864. [Google Scholar]
- 24.Schroeder W H, Munthe J. Atmospheric mercury—an overview. Atmos Environ 199832809–822. [Google Scholar]
- 25.Bullock O. Modeling assessment of transport and deposition patterns of anthropogenic mercury air emissions in the United States and Canada. Sci Total Environ 20002599145–157. [DOI] [PubMed] [Google Scholar]
- 26.Pai P, Karamchandani P, Seigneur C. Simulation of the regional atmospheric transport and fate of mercury using a comprehensive Eulerian model. Atmos Environ 1997312717–2732. [Google Scholar]
- 27.British Atmospheric Data Centre Land surface meteorological observations data. http://badc.nerc.ac.uk/data/surface_data.html (accessed 27 March 2007)
- 28.ApSimon H M, Goddard A J H, Holmes R E.Meteorological data correlation and computed concentrations at sites for Oct and Nov 1975 for ICI ltd. Crown house, Copthorne Bank, Crawley, Sussex, England: PPC Computing, 1975
- 29.Maggs R, Mann C, Booker J.et al Monitoring of lead, arsenic, cadmium, nickel and mercury around industrial sites [Stanger Science and Environment website]. 4 Sep 2001. http://www.stanger.co.uk/airqual/metals/ (accessed 23 Feb 2007)
- 30.Office for National Statistics Census 2001 results show decline in population of the North West. 2004. http://www.statistics.gov.uk/census2001/press_release_nw.asp (accessed 23 Feb 2007)
- 31.Barregard L, Sallsten G, Mazzolai B.et al Report on the comparison between estimates of average air mercury levels and the internal dose in populations living close to MCCA plants. Work Package number 3, deliverable D3.2. 2004
- 32.Office for National Statistics 2001 census [ONS Census website]. http://www.statistics.gov.uk/census/ (accessed 23 Feb 2007)
- 33.Kruger O, Ebinghaus R, Kock H H.et al Estimation of gaseous mercury emissions in Germany: inverse modelling of source strengths at the contaminated Industrial Site BSL Werk Schkopau. In: Ebinghaus R, Turner R, de Lacerda LD, et al eds. Mercury contaminated sites; characterisation, risk assessment and remediation. Heidelberg: Springer 1999377–392.
- 34.Wangberg I, Edner H, Ferrara R.et al Atmospheric mercury near a chlor‐alkali plant in Sweden. Sci Total Environ 200330429–41. [DOI] [PubMed] [Google Scholar]
- 35.Lines I G, Deaves D M, Atkins W S. Practical modelling of gas dispersion in low wind speed conditions, for application in risk assessment. J Hazard Mater 199754201–226. [Google Scholar]
- 36.Lines I G, Daycock J H, Deaves D M. Guidelines for the inclusion of low wind speed conditions into risk assessments. J Hazard Mater 200183153–179. [DOI] [PubMed] [Google Scholar]
- 37.Atmospheric Dispersion Modelling Liaison Committee Atmospheric dispersion at low wind speed. Annex A, Annual Report 1996/97. NRPB R‐302. UK: National Radiological Protection Board