Electrocardiographic imaging (ECGI) is a novel, noninvasive tool for imaging cardiac arrhythmia and defining electrophysiologic properties.1,2 ECGI combines multi-electrode body surface ECG recordings with three-dimensional anatomical heart-torso imaging to reconstruct an epicardial electroanatomical map (Figure 1). ECGI-reconstructed data can be presented as epicardial potential maps, electrograms, or isochrones during activation and repolarization. The ECGI procedure has been extensively tested and validated in experimental preparations with normal and abnormal canine hearts3–10. Recently, ECGI has been validated in humans1,2,11–13. To date, we have presented human ECGI studies of normal activation and repolarization 1,2, right and left bundle branch block1,11, various pacing protocols1,11,13, atrial flutter1, native sinus rhythm and bi-ventricular pacing for cardiac resynchronization (CRT) in heart failure patients11, and focal ventricular tachycardia12.
Figure 1.
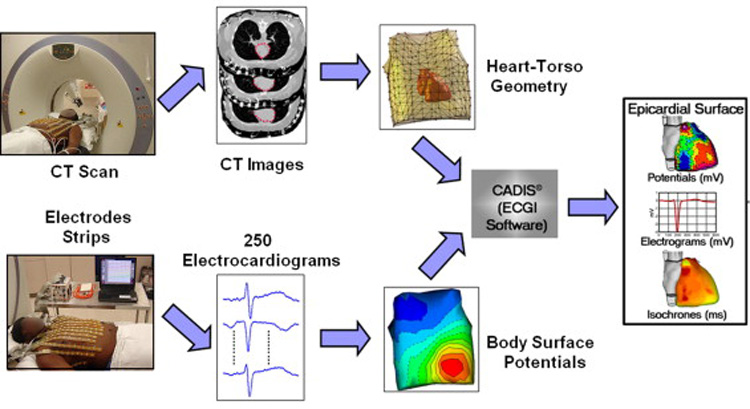
The ECGI procedure. Body surface potential maps (BSPM) are recorded using a multi-channel (256-electrode) mapping system. Non-contrast CT images with the body surface ECGI electrodes applied, simultaneously record the locations of the electrodes (shining dots in CT images) and the geometry of the heart surface. In this study, images from an ECG-gated CT scan were reconstructed at 20% and 70% of the RR interval. By combining the BSPM and heart-torso geometry information, ECGI reconstructs potential maps, electrograms, isochrones (activation sequences) and repolarization patterns on the epicardial surface of the heart. Modified from reference 1.
This report describes the first case in which ECGI was applied in a patient with a focal atrial tachycardia. In particular, ECGI accurately located the earliest site of activation in an atrium which had previously undergone two percutaneous pulmonary vein isolation procedures. The tachyarrhythmia was successfully terminated with radio frequency (RF) ablation in the ECGI-determined location.
Case Report
A fifty-five year old Caucasian female with a history of two prior pulmonary vein isolation procedures for long standing atrial fibrillation was evaluated for increasing palpitations and an episode of near syncope. She had a history of rheumatic mitral valve disease and had undergone a percutaneous valvuloplasty nine years prior. Since her valvuloplasty, she had an average of two admissions each year for symptomatic atrial fibrillation. She was cardioverted each time and, in addition to her oral anticoagulation, she was tried on a number of antiarrhythmic medications without sustained success. She underwent percutaneous pulmonary vein isolation procedures fourteen and six months prior to this admission. Low voltage electrograms were recorded throughout the left atrium during the first and second procedures. Transthoracic and transesophageal echocardiography demonstrated marked left atrial enlargement (~5.6 cm), mild mitral stenosis (mean gradient = 6–8 mmHg) and mild-to-moderate mitral regurgitation.
When she presented with recurring palpitations after the second pulmonary vein isolation procedure, an ECG revealed an ectopic atrial tachycardia with a rate of 136bpm. Figure 2 shows a 12 lead ECG recorded during the tachycardia that demonstrates the P-wave morphology. Neither sotalol nor dofetilide suppressed the arrhythmia. The decision was made to attempt an ablation procedure. Prior to the procedure, ECGI was performed to noninvasively localize the source of tachycardia.
Figure 2.
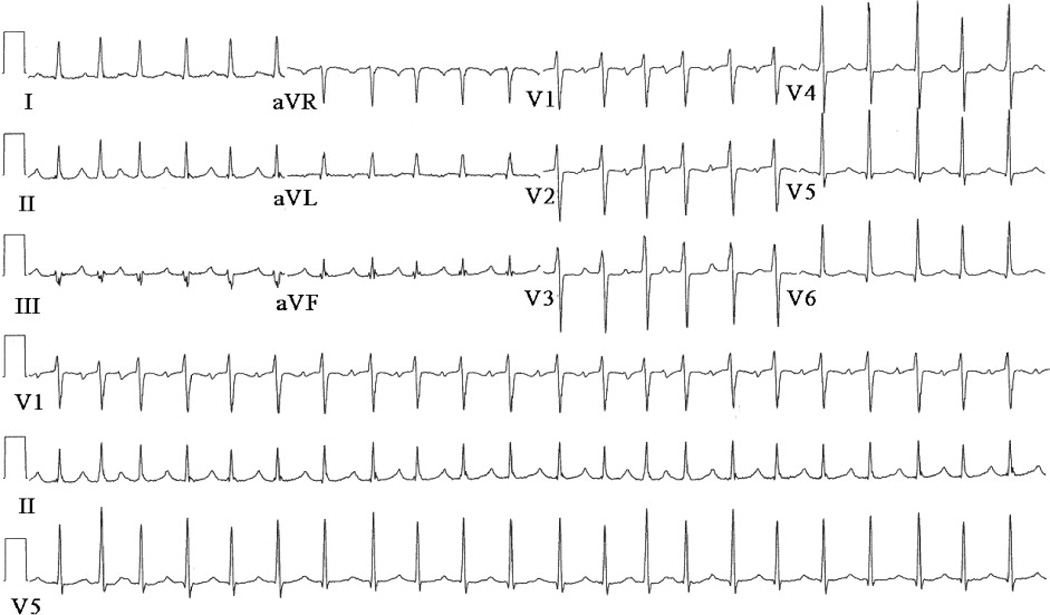
Patient’s 12-lead surface ECG
ECGI-reconstructed atrial epicardial surface voltage maps were produced for a single P-wave extracted during the tachycardia (Figure 3). At the onset of the surface P wave, an epicardial breakthrough (local potential minimum) was imaged on the left atrium, near the atrial septum (Figure 3A). During the end of the surface P wave, a repolarization pattern with reverse polarity (local potential maximum) appeared at the same site (Figure 3B). These observations strongly suggested the existence of an activation source at the breakthrough site. These findings were consistent for several ECGI imaged P waves during the tachycardia. Panel C superimposes the site of the breakthrough on a CT image. As shown, the earliest site of activation determined by ECGI was located on the roof of the left atrium, between the right superior pulmonary vein and the atrial septum (Figure 3C).
Figure 3.
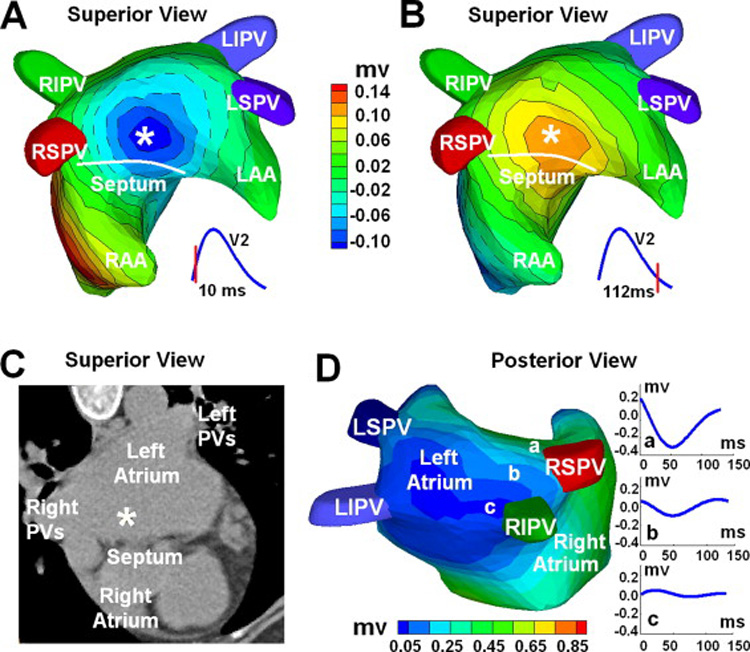
ECGI three-dimensional voltage and electrogram maps: Panel A and B show atrial epicardial potential maps at 10 ms and 112 ms after the onset of the surface P wave. Panel A captures the epicardial breakthrough pattern during activation, and Panel B shows the repolarization pattern with reverse polarity. The white asterisk indicates the site of earliest activation as predicted by ECGI. Panel C shows the ECGI-determined earliest activation site (white asterisk) on a CT image of the atria. Panel D is an electrogram magnitude map (peak-to-peak) reconstructed by ECGI (posterior view). The dark blue represents a region of low magnitude electrograms, indicating a scar region. Three electrograms selected from a non-scar region (a) and from the scar region (b, c) are shown. Location of low magnitude electrograms is consistent with prior pulmonary vein isolations and left atrial substrate modification. RIPV = right inferior pulmonary vein; LIPV = left inferior pulmonary vein; RSPV = right superior pulmonary vein; LSPV = left superior pulmonary vein; LAA = left atrial appendage; RAA = right atrial appendage;
Additionally, ECGI-reconstructed atrial epicardial potential maps were organized by location to provide temporal electrograms for any given site on the atrial epicardium. A three-dimensional map encoding electrogram magnitudes is shown in Figure 3D. Very low magnitude electrograms were reconstructed on the posterior left atrium, especially around the pulmonary veins, consistent with scar from the patient’s previous pulmonary vein isolation procedures. For reference, ECGI-imaged atrial electrogram magnitudes in normal subjects range from 0.6mV to 1mV. Diffuse low voltages were also observed on the posterior wall of the left atrium. This probably reflects the patient’s underlying disease and the effects of the two prior ablation procedures. Diffuse low voltage was observed during the initial study prior to the pulmonary vein isolation. The prior procedures induced wide circumferential ablation that contained much of the posterior wall, linear lesions connecting the superior veins and inferior veins, and ablation of residual potentials recorded on the posterior wall by a LASSO catheter (Biosense Webster, Diamond Bar, CA). During the ECGI study, potentials could not be identified within the pulmonary veins nor within the antra around the orifices, suggesting successful prior pulmonary vein isolation procedures. This was consistent with recordings made by a lasso catheter during the study.
At the onset of the EP study that followed the ECGI procedure, the patient was in an atrial tachycardia with a cycle length of 540ms. Using a lasso catheter, all four pulmonary veins were determined to be electrically isolated. Right and left atrial electroanatomic catheter mapping identified a focal tachycardia originating from the superior left atrium, between the right superior pulmonary vein and the atrial septum (Figure 4). Figure 4A is a catheter electroanatomic isochrone map (CARTO, Biosense Webster, Diamond Bar, CA) constructed in the electrophysiology (EP) laboratory during mapping and ablation of the tachycardia. The site of earliest activation is indicated by the white dot. RF energy was applied at this site and circumferentially around this site as indicated by the red markers. Diastolic potentials were recorded 58ms before the onset of the surface P-wave near the ECGI-predicted site (Figure 4B). The tachycardia was terminated with application of RF energy to this location, and the tachycardia did not recur with administration of isoproterenol. The patient concluded the study in sinus rhythm.
Figure 4.
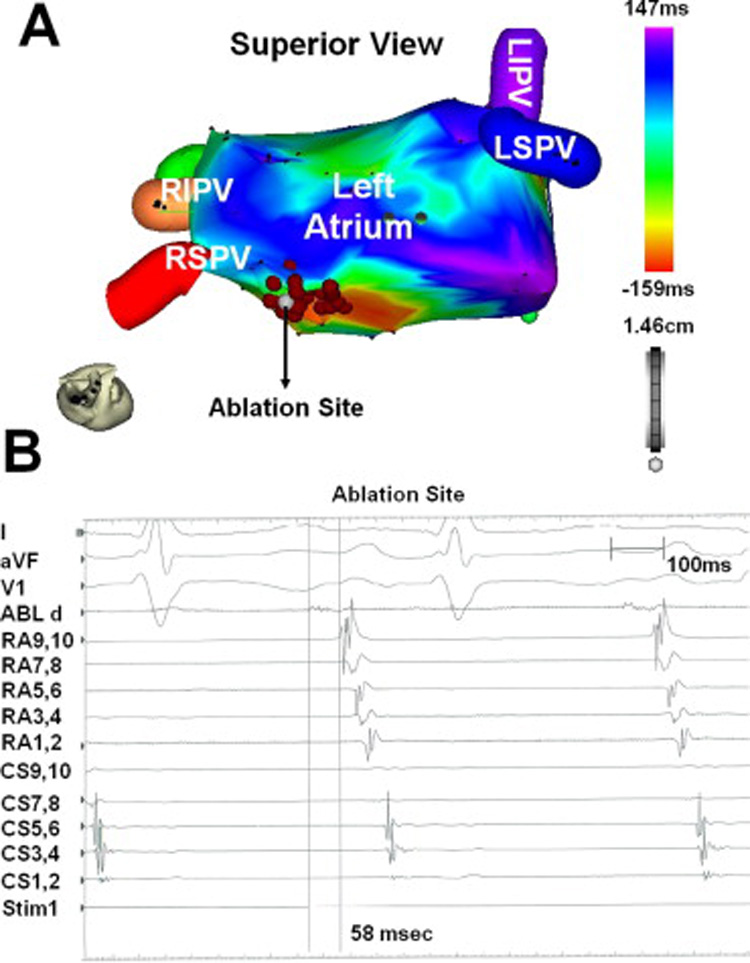
Electroanatomic isochrone map and multichannel intracardiac electrograms. Panel A is the left atrial electroanatomic isochrone map. The white marker (arrow) identifies the ablation site that successfully terminated the atrial tachycardia. The red markers are sites where RF energy was applied circumferentially around the site of earliest activation. Panel B is the multichannel endocardial electrogram recording prior to successful ablation, with the ablation catheter at the superior left atrium, near the activation site predicted by ECGI. Surface leads I, aVF, and V1 are followed by intracardiac recordings from the ablation catheter, a decapolar catheter located on the lateral wall of the right atrium, and a decapolar catheter positioned in the coronary sinus (proximal to distal). The ablation catheter measured spontaneous diastolic potentials 58 msec prior to the onset of the surface P wave.
Conclusion
This report describes a clinical application of the ECGI noninvasive electrical imaging system as an adjunctive technology to accurately identify the specific origin of a focal left atrial tachycardia and atrial electrophysiological substrate prior to catheter ablation. This case has several points of interest. Atrial tachycardias are relatively common in patients with atrial fibrillation who have undergone isolation of the pulmonary veins and often require additional mapping and ablation. Localization of an atrial tachycardia by analysis of the standard 12 lead ECG is difficult because the P-wave morphology can be affected by the T-wave. Moreover, the criteria for interpretation of P-wave morphology recorded on a standard lead ECG14 may not be valid in patients with diseased atria who have undergone circumferential pulmonary vein isolation combined with ablation of fragmented potentials. In the case under discussion, ECGI correctly located the arrhythmic focus to the roof of the left atrium, between the right superior pulmonary vein and atrial septum. Because the CT scan was not registered to the CARTO electroanatomic image, a precise quantitative measure of localization accuracy could not be obtained. ECGI also identified areas of low voltage on the posterior wall of the left atrium that were attributable to the patient’s prior ablation procedures and underlying atrial myopathy. The feasibility of assessing PV isolation noninvasively using ECGI will be further tested in a larger study. Despite an abnormal left atrial substrate with extensive scar tissue, ECGI was accurate in its localization of the focus of the tachycardia. Although further work remains to prove its clinical utility in large groups of patients, ECGI offers promise for improved noninvasive analysis of the origin and mechanisms of atrial arrhythmias.
ACKNOWLEDGMENTS
This study was supported by NIH-NHLBI Merit Award R37-HL-33343 and Grant R01-HL-49054 (to Yoram Rudy). Yoram Rudy is the Fred Saigh distinguished professor at Washington University in St Louis.
*Dr. Rudy received stock options in CardioInsight Inventors on patents related to ECGI that were submitted and owned by Case Western Reserve University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanathan C, Jia P, Ghanem RN, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc. Natl. Acad. Sci., U.S.A. (PNAS) 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oster HS, Taccardi B, Lux RL, Ershler PR, Rudy Y. Electrocardiographic imaging: noninvasive localization of pacing sites simulating ectopic foci. Circulation. 1994;90:I–437. [Google Scholar]
- 4.Oster HS, Taccardi B, Lux RL, Ershler PR, Rudy Y. Noninvasive electrocardiographic imaging: reconstruction of epicardial potentials, electrograms, and isochrones and localization of single and multiple electrocardiac events. Circulation. 1997;96:1012–1024. doi: 10.1161/01.cir.96.3.1012. [DOI] [PubMed] [Google Scholar]
- 5.Oster HS, Taccardi B, Lux RL, Ershler PR, Rudy Y. Electrocardiographic imaging: Noninvasive characterization of intramural myocardial activation from inverse-reconstructed epicardial potentials and electrograms. Circulation. 1998;97:1496–1507. doi: 10.1161/01.cir.97.15.1496. [DOI] [PubMed] [Google Scholar]
- 6.Burnes JE, Taccardi B, MacLeod RS, Rudy Y. Noninvasive ECG imaging of electrophysiologically abnormal substrates in infarcted hearts: A model study. Circulation. 2000;101:533–540. doi: 10.1161/01.cir.101.5.533. [DOI] [PubMed] [Google Scholar]
- 7.Burnes JE, Taccardi B, Rudy Y. A noninvasive imaging modality for cardiac arrhythmias. Circulation. 2000;102:2152–2158. doi: 10.1161/01.cir.102.17.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnes JE, Taccardi B, PR E, Rudy Y. Noninvasive electrocardiogram imaging of substrate and intramural ventricular tachycardia in infarcted hearts. Journal of the American College of Cardiology. 2001;38:2071–2078. doi: 10.1016/s0735-1097(01)01653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnes JE, Ghanem RN, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, I: comparison of body-surface and epicardial measures. Circulation. 2001;104:1299–1305. doi: 10.1161/hc3601.094276. [DOI] [PubMed] [Google Scholar]
- 10.Ghanem RN, Burnes JE, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, II: noninvasive reconstruction of epicardial measures. Circulation. 2001;104:1306–1312. doi: 10.1161/hc3601.094277. [DOI] [PubMed] [Google Scholar]
- 11.Jia P, Ramanathan C, Ghanem RN, Ryu K, Varma N, Rudy Y. Electrocardiographic imaging of cardiac resynchronization therapy in heart failure: Observation of variable electrophysiologic responses. Heart Rhythm. 2006;3:296–310. doi: 10.1016/j.hrthm.2005.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intini A, Goldstein RN, Jia P, Ramanathan C, Ryu K, Giannattasio B, Gilkeson R, Stambler BS, Brugada P, Stevenson WG, Rudy Y, Waldo AL. Electrocardiographic imaging (ECGI), a novel diagnostic modality used for mapping of focal left ventricular tachycardia in a young athlete. Heart Rhythm. 2005;2:1250–1252. doi: 10.1016/j.hrthm.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghanem RN, Jia P, Ramanathan C, Ryu K, Markowitz A, Rudy Y. Noninvasive electrocardiographic imaging (ECGI): comparison to intraoperative mapping in patients. Heart Rhythm. 2005;2:339–354. doi: 10.1016/j.hrthm.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kistler PM, Roberts-Thomson KC, Haqqani HM, Fynn SP, Singarayar S, Vohra JK, Morton JB, Sparks PB, Kalman JM. P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol. 2006;48:1010–1017. doi: 10.1016/j.jacc.2006.03.058. [DOI] [PubMed] [Google Scholar]


