Abstract
IL-10 is an anti-inflammatory cytokine that suppresses synthesis of proinflammatory cytokines and their receptors. Here we tested the possibility that TNFα-induced hormone resistance in myoblasts might be overcome by IL-10. We found that IL-10 restores myogenesis by suppressing the ability of exogenous TNFα to inhibit IGF-I-induced myogenin. This protection occurs without decreasing global activity of TNF receptors since IL-10 does not impair TNFα-induced IL-6 synthesis or ERK1/2 phosphorylation. Instead, IL-10 acts to prevent TNFα-induced phosphorylation of JNK. These findings demonstrate that IL-10 serves a previously unrecognized protective role in muscle progenitors by overcoming TNFα-induced resistance to IGF-I.
Keywords: inflammation, cytokines, hormone resistance, muscle, JNK, IGF-I
1. Introduction
It is now clear that cells of the immune system interact with muscle cells to regulate muscle development, including responses to injury and repair and a variety of dystrophies (Tidball, 2005). The potential role of inflammation in muscle development was most recently shown in young dystrophic mice by depletion of neutrophils or by injections of Etanercept, a soluble TNF receptor antagonist (Hodgetts et al., 2006): both treatments significantly reduced muscle necrosis. At the other end of the spectrum, prolonged elevations of proinflammatory cytokines are closely associated with muscle wasting that occurs during the sarcopenia of aging and in cachectic AIDS and cancer patients. These clinical disorders occur along with a decline in IGF-I anabolic activity, which is consistent with in vitro findings in muscle progenitor cells. Very low concentrations of TNFα (0.01-1 ng/ml) inhibit IGF-I-induced protein synthesis (Broussard et al., 2003; Strle et al., 2004) and expression of the critical muscle differentiation factors, MyoD (Strle et al., 2004) and myogenin (Broussard et al., 2003; Strle et al., 2004). This ability of TNFα to induce hormone resistance is mediated by c-Jun N-terminal kinase (JNK) (Strle et al., 2006). JNK is a stress-activated protein kinase (SAPK) that is implicated in the pathophysiology of major metabolic and inflammatory disorders mainly because it serves as a key signaling intermediate downstream of two major proinflammatory cytokine receptors, TNFα receptor (TNFR)1 and IL-1β receptor (Manning and Davis, 2003; Wajant and Scheurich, 2004). Potential treatments that might overcome TNFα-induced hormone resistance in myoblasts are unknown.
IL-10 is the protypical anti-inflammatory cytokine that suppresses the innate immune system and subsequently blunts Th1 activation (Moore et al., 2001; Strle et al., 2001). IL-10 acts in a multifaceted manner by inhibiting antigen presentation, decreasing cell-surface expression of cytokine receptors and inducing expression of endogenous cytokine antagonists (Moore et al., 2001). However, the hallmark of IL-10 anti-inflammatory activity is generally considered to reside in its ability to suppress the synthesis of chemokines and pro-inflammatory cytokines, particularly TNFα and IL-1β, because they are early-acting proteins that promote the synthesis of other cytokines (Fiorentino et al., 1991; Moore et al., 2001; Strle et al., 2001). Since IL-10 inhibits the synthesis of TNFα and IL-1β, it could play a protective role in muscle by abrogating the activation of key intermediary inhibitor proteins such as JNK, a possibility that has not yet been explored. This is surprising because IL-10 is expressed in vivo in skeletal muscle of young and elderly individuals (Nieman et al., 2003; Przybyla et al., 2006) and in vitro by myotubes derived from immortalized murine myoblasts (Alvarez et al., 2002). These results establish that muscle cells are capable of synthesizing IL-10 independently of myeloid cells such as macrophages that are known to reside in muscle. In addition, evidence is slowly accumulating that muscle cells not only produce IL-10 but also respond to it. For instance, IL-10 ameliorates insulin resistance in skeletal muscles of diabetic mice, as assessed by kinase activity of protein kinase C (PKC), phosphatidylinositol (PI) 3-kinase and AKT (Kim et al., 2004). Similarly, IL-10-encoding plasmids expressed in skeletal muscle notably improve whole-body insulin responses in diabetic animals (Zhang et al., 2003) and alleviate clinical severity of collagen-induced arthritis (Saidenberg-Kermanac’h et al., 2003). It has recently been shown that transfection of myoblasts with IL-10-encoding plasmids prevents weakness of the diaphragm and reduces mortality following infection with Pseudomonas aeruginosa (Divangahi et al., 2006). Collectively, these data establish that skeletal muscle is an IL-10 sensitive tissue, but the potential interaction of IL-10 with receptors for proinflammatory cytokines in development of muscle tissue has yet to be determined.
Here we show for the first time that IL-10 plays a protective role in skeletal muscle myoblasts by directly inhibiting TNFα-induced IGF-I resistance. This protective action of IL-10 is not caused by inhibition of pro-inflammatory cytokine synthesis or regulation of ERK 1/2 activation. Instead, IL-10 completely suppresses TNFα-induced phosphorylation of JNK. These data establish a novel protective activity for IL-10 in skeletal muscle progenitor cells which is to prevent TNFα-induced IGF-I resistance by a mechanism that is not caused by a reduction in cytokine or cytokine receptor expression.
2. Materials and Methods
2.1. Reagents
Recombinant murine TNFα and human IGF-I were purchased from Intergen (Purchase, NY) and recombinant murine IL-10 was from Pharmingen, (San Hose, CA). Enzyme-linked immunosorbent assay (ELISA) kits for measuring IL-6 and IL-1β concentrations were obtained from Pierce Biotechnology (Rockford, IL). Dulbecco’s Modified Eagle’s Medium (DMEM with 4.5 g/L glucose, 0.584 g/L glutamine), penicillin/streptomycin, sodium pyruvate and fetal bovine serum (FBS; < 0.25 EU/ml of endotoxin) were purchased from HyClone (Logan, UT). Mouse monoclonal antibodies were obtained from the following vendors: the IgG1 antibody to myogenin (F5D), the IgG2b antibody to TNF-receptor 1 (TNFR1; H-5) and the IgG2a antibody to phosphorylated ERK1/2 (E-4) were from Santa Cruz Biotechnology (Santa Cruz, CA); the IgG1 antibody to α-tubulin (B-5-1-2) was from Sigma Aldrich. Rabbit polyclonal antibodies to C-terminus (M-20, C-20) of the IL-10 receptor (IL-10R) and an antibody to ERK1 (K-23) were purchased from Santa Cruz Biotechnology. Horseradish peroxidase (HRP)-linked secondary antibodies were purchased from GE Healthcare (United Kingdom), Antibodies specific for JNK (9252) and phosphorylated JNK (P-JNK; 9251) were from Cell Signaling Biotechnology (Danvers, MA). All other reagents were obtained from Sigma Aldrich (St. Louis, MO).
2.2. Cell Culture
Skeletal murine C2C12 myoblasts were obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 4 mM L-glutamine, 4.5 g/L glucose and supplemented with 10% heat inactivated FBS, 1 mM sodium pyruvate, 100 U/ml of penicillin and 100 μg/ml of streptomycin at 37°C, 7% CO2 and 95% humidity. Myoblasts were grown to 70% confluence, washed three times in DMEM devoid of FBS to remove growth factors and deprived of serum for 4 h prior to initiation of experiments.
In all experiments using IL-10, myoblasts were pretreated with IL-10 (10, 25 or 50 ng/ml) for 1 h prior to addition of an optimal concentration of TNFα (1 ng/ml). For experiments measuring myogenin expression, myoblasts were treated with TNFα for 1 h before adding IGF-I (50 ng/ml) for an additional 24 h. In experiments measuring JNK and ERK 1/2 phosphorylation, myoblasts were pretreated with IL-10 (10 ng/ml) for 1 h prior to adding TNFα for another 10, 15 or 30 min. ELISA time-course experiments were conducted by pretreating myoblasts with IL-10 (10 ng/ml) for 1 h before adding TNFα for additional 24, 16, 8, 4 and 2 h. Myoblasts in control wells were left untreated for the duration of experiments. In ELISA experiments that utilized IL-10 dose-responses, myoblasts were pretreated with 0, 10, 25 and 50 ng/ml IL-10 for 1 h prior to treatment with TNFα for an additional 24 h. The highest concentration of IL-10 (50 ng/ml) served as a control in ELISA dose-response experiments. Whole cell lysates from untreated myoblasts were used to detect IL-10R1 and TNFα receptor 1 TNFR1 expression.
2.3. Western Blotting for Myogenin, JNK and ERK
At the termination of experiments, myoblasts were homogenized in 200 μl of homogenization buffer containing 50 mM HEPES, 150 mM NaCl2, 1 mM EGTA, 10 mM Na pyrophosphate, 1% glycerol, 1.5 mM MgCl2, 1% Na deoxycholate, 100 mM NaF, 0.1% sodium dodecylsulfate (SDS), 1% Triton X-100, 0.1 mM sodium orthovanadate, 2 μg/mL aprotinin, 40 nM leupeptin, 2 μg/mL pepstatin and 1 mM phenylmethylsulfonyl fluoride, pH 7.4. To confirm that equal amounts of protein were loaded onto the polyacrylamide gels, protein concentrations of the samples were determined using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Protein concentrations did not differ in lysates from myoblasts treated with IL-10 or TNFα compared to untreated control samples (data not shown). Proteins from whole cell lysates were separated using SDS-containing polyacrylamide gel electrophoresis (SDS-PAGE). For western blotting experiments measuring myogenin (MW = 35 kDa) proteins were separated on 12.5% polyacrylamide gels. To measure IL-10R (MW = 120 kDa), TNFR1 (MW = 55 kDa), ERK1/2 (MW = 44 and 42 kDa respectively) and JNK (MW = 46 and 54 kDa), proteins were separated on 10% polyacrylamide gels. Following SDS-PAGE, proteins were transferred to Trans-Blot polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories) using a Mini Protean 3 Cell transfer system from Bio-Rad Laboratories. Membranes were then rinsed in TBS-T (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20) and incubated for 1 h in TBS-T containing 2% bovine serum albumin (BSA) to block non-specific antibody-binding interactions. After blocking, membranes were incubated with antibodies specific to myogenin, phosphorylated JNK, JNK, phosphorylated ERK1/2, ERK1/2, IL-10R, TNFR1, or α-tubulin at 4°C overnight. These primary antibodies were diluted 1/1000 or 1/5000 (for α-tubulin) in TBS-T containing 2% BSA. Membranes were washed in TBS-T 4 times for 5 min by shaking on an orbital rotator at 25°C and then incubated with the secondary anti-mouse or anti-rabbit HRP-labeled IgG antibodies diluted 1/2000 in TBS-T containing 2% BSA for another 2 h at 25°C. Blots were once again washed 4 times 5 min in TBS-T, and incubated for 1 min with chemiluminescence western Blotting Detection Reagents (RPN2106) from GE Healthcare. Western blots were exposed for up to 15 min on autoradiographic B-Plus X-ray film obtained from Central Illinois X-Ray (Bloomington, IL) and the films were developed using a Fischer model K-Plus, automatic X-Ray film processor from Fischer Industries Inc. (Geneva, IL). Band intensity of specific proteins was quantified by densitometric analysis of scanned autoradiograms with IMAGEJ software from National Institute of Health (Bethesda, MD). Densitometric summaries were presented as ratios of the densitometric values of myogenin to α-tubulin, Thr183/Tyr185 phosphorylated JNK to total JNK and Tyr204 phosphorylated ERK1/2 to total ERK1/2. Data from experiments over time were standardized by dividing the individual sample ratios by the mean of that entire experiment.
2.4. ELISA
After treatment, cell culture supernatants were collected from 6-well culture plates and 50 μl of each sample was added to ELISA plates in duplicates. ELISA was performed as recommended by the manufacturer (Pierce Biotechnology). The lower limit of sensitivity for IL-6 was 7 pg/ml and for IL-1β the lower limit of sensitivity was 3 pg/ml. Absorbance at 450 nm minus 550 nm was measured on a Molecular Devices OPTImax ELISA plate reader (Sunnyvale, CA). The sample values were expressed pg/ml.
2.5. Statistical analysis
All data were analyzed by ANOVA using the Statistical Analysis System (SAS Version 8) (SAS, 2003) with a general linear model. F-protected Duncan’s multiple range test was utilized to detect differences among treatments. Results are expressed as the mean ± the standard error of the mean.
3. Results
3.1. C2C12 myoblasts express receptors for both IL-10 and TNFα
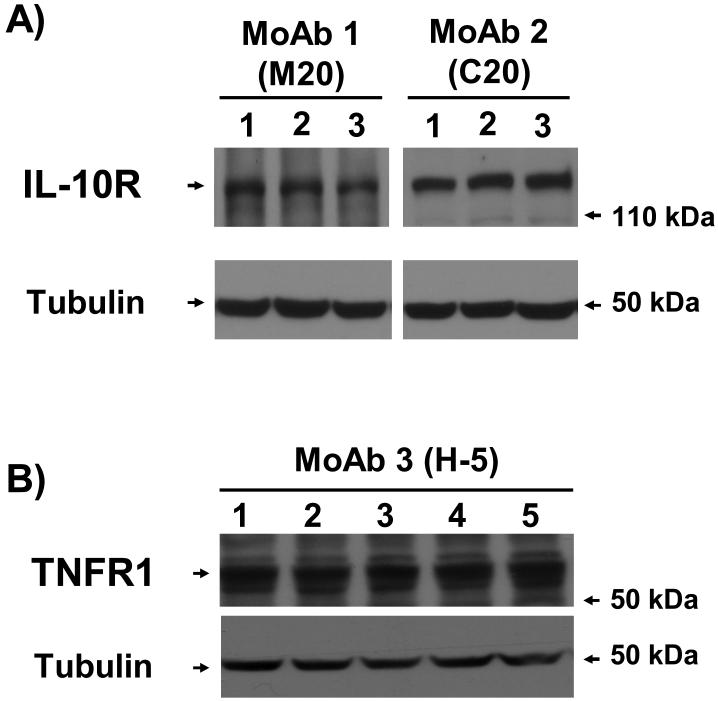
To determine if C2C12 myoblasts have the machinery to respond to IL-10 by expressing IL-10 receptors, whole cell lysates from untreated C2C12 myoblasts were separated on SDS-PAGE, transferred to PVDF membranes and then blotted with two different antibodies specific for IL-10R (M-20 and C-20). As a positive control, lysates were also blotted with an antibody specific for TNFR1 (H5), as described in methods. Representative Western blots show that both IL-10Rs (Fig. 1A) and TNFR1s (Fig. 1B) are expressed by these cells. Tubulin served as a control to ensure that all lanes contain equal amounts of total protein (lower bands in Fig. 1A and Fig. 1B). These results clearly establish that C2C12 myoblasts express the necessary receptors to respond to IL-10 and TNFα.
Figure 1. C2C12 myoblasts express IL-10R and TNFR1.
Proteins from whole cell lysates of untreated C2C12 myoblasts were separated on 10% SDS-PAGE gels and transferred to PVDF membranes. Membranes were then probed with antibodies specific for the IL-10R (A; MoAb 1 (M20) lanes 1-3, MoAb 2 (C20) lanes 4-6) or for the TNFR1 (B; MoAb 3 (H-5) lanes 1-5,), stripped and re-probed with an antibody to α-tubulin (B-5-1-2). Representative western blots show that C2C12 myoblasts express both IL-10Rs (A) and TNFR1s (B). Proteins detected with α-tubulin-specific antibody show that equal amounts of total protein were added to each well.
3.2. IL-10 plays a protective role during myogenesis by suppressing TNFα inhibition of IGF-I-induced myogenin
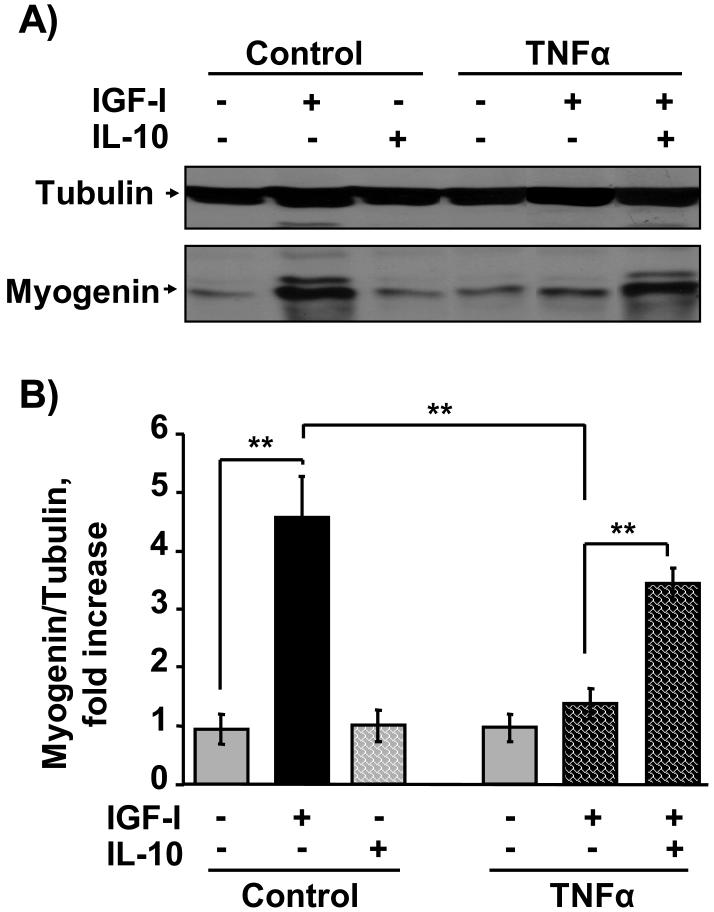
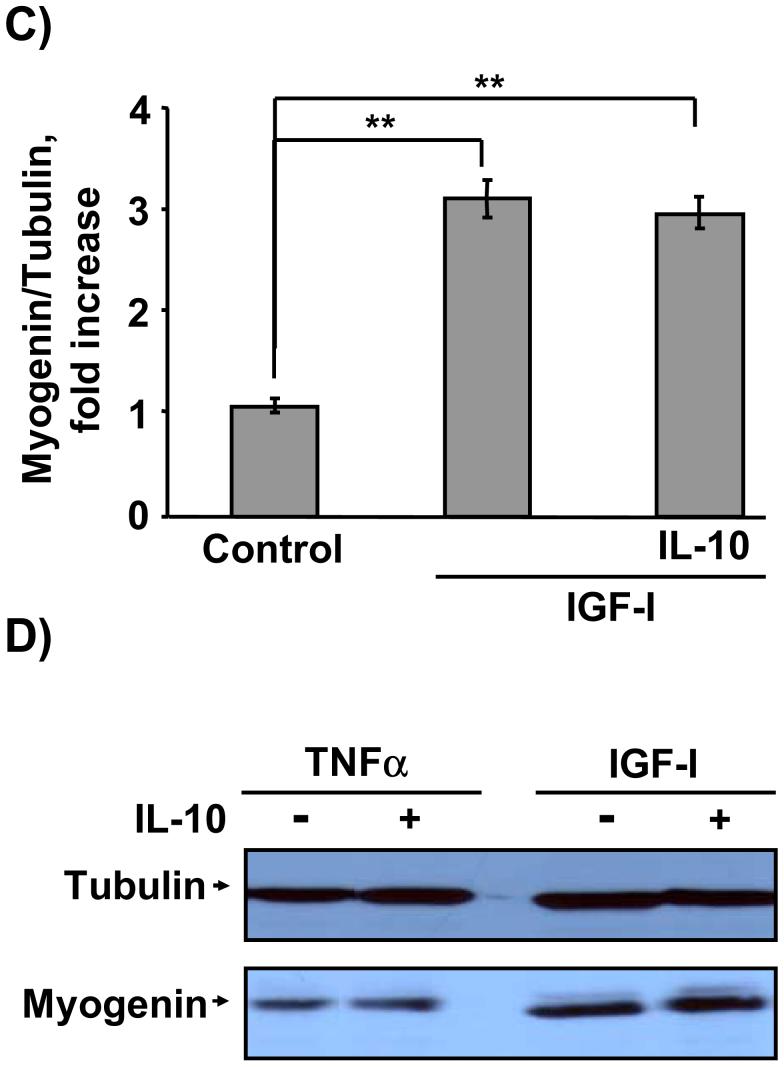
Myogenin is a critical muscle-specific transcription factor that is required for differentiation of progenitor muscle cells into myotubes. TNFα is closely implicated in the pathophysiology of muscle wasting disorders and is thought to act in part by blocking the actions of growth factors such as IGF-I, thereby inducing hormone resistance (Broussard et al., 2003; Strle et al., 2004). Here we tested the possibility that IL-10 might overcome this type of hormone resistance. As expected, TNFα (1 ng/ml) significantly inhibited the ability of IGF-I to promote expression of myogenin (Fig. 2). We then found that pretreatment with the anti-inflammatory cytokine, IL-10, revealed a novel protective role for IL-10 in C2C12 myoblasts by directly suppressing activity of a proinflammatory cytokine, TNFα (Fig. 2). Preliminary dose-response experiments established that the effective range of IL-10 concentrations that suppress TNFα activity ranged from 5 to 20 ng/ml (data not shown). A representative western blot using IL-10 at 10 ng/ml is shown in Fig. 2A. A densitometric summary of five similar but independent experiments expressed as a ratio of myogenin to tubulin is presented in Fig. 2B. Consistent with previously published results (Strle et al., 2006), IGF-I (50 ng/ml) induced a 4-fold increase in myogenin expression after 24 h of treatment (P < 0.01), and pretreatment with TNFα (1 ng/ml) abolished this increase (P < 0.01). As a direct test of our hypothesis, these experiments established that IL-10 (10 ng/ml) completely blocked (P < 0.01) the ability of TNFα to inhibit IGF-I-induced myogenin expression since this treatment was not statistically different from the response of cells treated with IGF-I alone. In the absence of IGF-I, neither TNFα nor IL-10 altered myogenin expression compared to untreated control cells. Furthermore, co-treatment with both IL-10 and IGF-I (Fig. 2C) or IL-10 and TNFα (Fig. 2D) also did not change myogenin expression. This is the first evidence that IL-10 plays a protective role in skeletal myoblasts by inhibiting the activity of an exogenously added proinflammatory cytokine, TNFα.
Figure 2. IL-10 suppresses TNFα inhibition of IGF-I-induced myogenin expression.
C2C12 myoblasts were pretreated with IL-10 (10 ng/ml) for 1 h before adding TNFα (1 ng/ml) for another 1 h. C2C12 myoblasts were then incubated with or without IGF-I (50 ng/ml) for an additional 24 h. Myogenin expression was determined by incubating blots with a myogenin-specific antibody. Blots were then stripped and re-probed with an anti-α-tubulin antibody. A, Representative western blot and B, densitometric summary of five independent experiments demonstrates that IL-10 significantly ameliorated TNFα inhibition of IGF-I-induced myogenin expression. IGF-I induced a 4-fold increase in myogenin expression and this increase was blocked by TNFα. More importantly, IL-10 suppressed the ability of TNFα to inhibit myogenin expression. C, IL-10 did not alter myogenin expression in the presence of IGF-I but in the absence of TNFα, as shown by a densitometric summary of three independent experiments. and D, Representative western blot of two independent experiments demonstrates that co-treatment with IL-10 and TNFα alone or IL-10 and IGF-I alone did not alter myogenin expression. Tubulin served as a control to ensure that equal amounts of proteins were added to each well. ** P < 0.01
3.3. TNFα-induced expression of IL-6 in C2C12 myoblasts is not inhibited by the low concentrations of IL-10 that block JNK activation
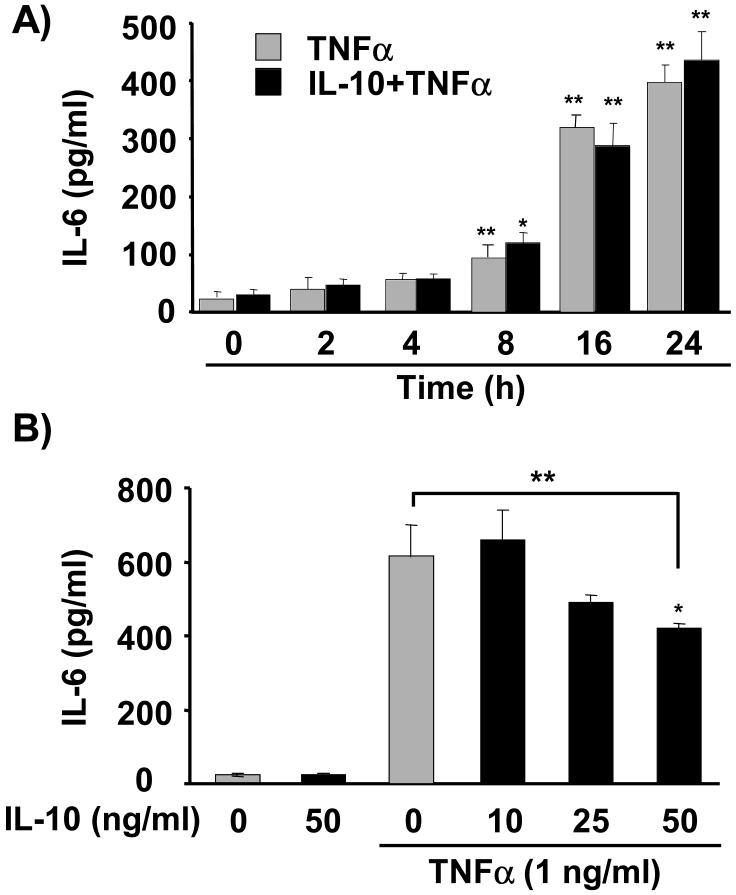
A number of recent studies have established that a variety of tissues, including skeletal muscle cells, are capable of producing and responding to proinflammatory cytokines (Alvarez et al., 2002; Broussard et al., 2003; Frost and Lang, 2005; Kim et al., 2004; Moore et al., 2001; Rasley et al., 2006; Strle et al., 2004; Strle et al., 2002; Strle et al., 2001). Consequently, we first tested the possibility that TNFα induces expression of IL-6 and IL-1β at the protein level. C2C12 myoblasts were treated with TNFα (1 ng/ml) for 0, 2, 4, 8, 16 and 24 h (Fig. 3A). TNFα caused a time-dependent increase in expression of IL-6 that reached 450 ± 35 pg/ml after 24 h (Fig. 3A; gray bars). In contrast, TNFα did not induce expression of IL-1β at any time-points tested (n = 3; data not shown). These results are consistent with previous findings showing that IL-6 is the primary cytokine expressed in muscle (Alvarez et al., 2002; Frost et al., 2003).
Figure 3. TNFα-induced IL-6 expression is not inhibited by low concentrations of IL-10.
A, C2C12 myoblasts were treated with TNFα for 0, 2, 4, 8, 16 and 24 h (gray bars) or pre-treated with IL-10 (10 ng/ml) for 1 h prior to treatment with TNFα (black bars). Expression of IL-6 protein in culture supernatants was determined by an ELISA. TNFα induced a time-dependent increase in IL-6 expression that peaks at 24 h (N = 4; gray bars). IL-10 (10 ng/ml) did not inhibit the expression of IL-6 (N = 3; black bars). B, To test the ability of IL-10 at higher concentrations to inhibit IL-6 expression, C2C12 myoblasts were pre-treated with three doses of IL-10 (10, 25 and 50 ng/ml) for 1 h prior to treatment with TNFα (1 ng/ml) for an additional 24 h (N = 4). Consistent with results in A, IL-10 at the lower concentration (10 ng/ml) did not inhibit the ability of TNFα to induce IL-6 expression. However, a higher concentration of IL-10 (50 ng/ml) inhibited TNFα-induced expression of IL-6 by 33% (50 ng/ml). IL-10 at 25 ng/ml reduced IL-6 expression by 22%, but this decrease was not significant (P < 0.06). In the absence of TNFα, IL-10 (50 ng/ml) did not alter IL-6 expression. * P < 0.05; ** P < 0.01
Although the role of IL-6 in skeletal muscle development is only now being determined, several reports indicate that IL-6 is associated with muscle wasting and might mediate the catabolic actions of other proinflammatory cytokines. Because our results show that IL-10 directly blocks the activity of exogenous TNFα (Fig. 2), we tested the possibility that the protective activity of IL-10 is simply mediated by IL-10 acting in a classical manner to inhibit the synthesis of catabolic factors such as IL-6. We therefore pretreated C2C12 myoblasts with IL-10 (10 ng/ml) for 1 h prior to treatment with TNFα for additional 0, 2, 4, 8, 16, and 24 h (Fig. 3A, black bars). This lower, more physiological concentration of IL-10 (10 ng/ml) did not suppress the ability of TNFα to induce IL-6 expression at any of the time-points tested (Fig. 3A). Since a major mode of IL-10 action is to suppress synthesis of proinflammatory cytokines, we also tested higher concentrations of IL-10 (25 and 50 ng/ml) (Fig. 3B; black bars). For this experiment, C2C12 myoblasts were pretreated with IL-10 at 10, 25 and 50 ng/ml or control medium for 1 h prior to treatment with TNFα for an additional 24 h. Only the highest concentration of IL-10 (50 ng/ml) significantly reduced TNFα-induced expression of IL-6 (P < 0.05; Fig. 3B). In the absence of TNFα, IL-10 (10 ng/ml, Fig. 3A; and 50 ng/ml, Fig. 3B) did not alter IL-6 expression. These data establish that IL-10 acts via two separate mechanisms in skeletal myoblasts: at the lower concentration of 10 ng/ml, IL-10 plays a protective role in myogenesis by directly suppressing activity of exogenous TNFα, while the higher IL-10 concentrations of 25-50 ng/ml can also act in a more classical manner by inhibiting cytokine expression.
3.4. IL-10 does not impair global activity of TNF receptor signaling, suppressing TNFα-induced JNK but not ERK1/2 phosphorylation
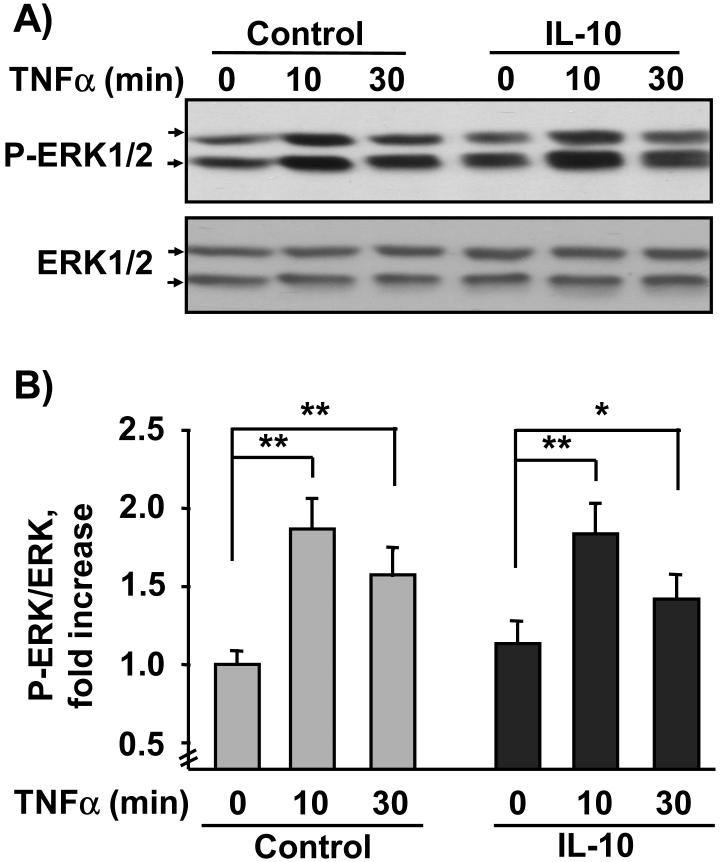
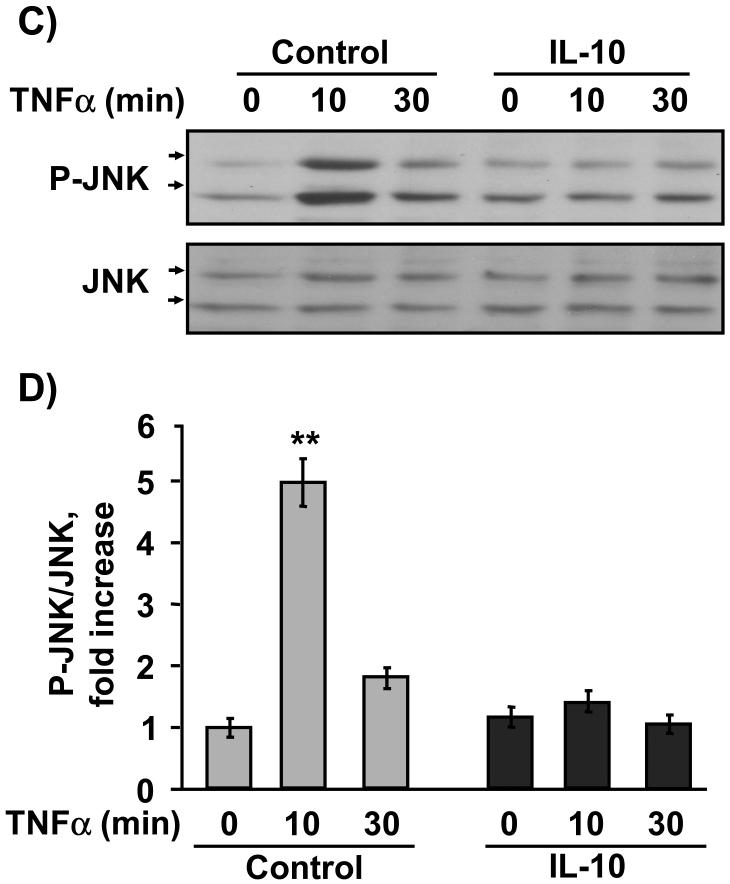
We next determined if IL-10 equally impairs two primary intracellular events downstream of activation of TNFR1: TNFα-induced phosphorylation of ERK1/2 (Kyriakis and Avruch, 2001) and JNK (Strle et al., 2006). These two kinases are associated with inhibition of muscle development (Strle et al., 2006; Tortorella et al., 2001), whereas p38 promotes differentiation (Cuenda and Cohen, 1999). For these experiments, C2C12 myoblasts were pretreated with IL-10 for 1 h prior to treatment with TNFα for another 10 and 30 min. The sample lysates were evaluated for both ERK1/2 (Fig. 4A, B) and JNK (Fig. 4C, D) by western blotting. Densitometric summaries of four independent experiments were expressed as ratios of the slower migrating (44 kDa) P-ERK1/2 to ERK1/2 (Fig. 4B) or as ratios of the slower migrating (54 kDa) P-JNK to JNK (Fig. 4D). Similar results were obtained from densitometric summaries of faster migrating ERK1/2 (42 kDa) and JNK (46 kDa). TNFα induced a significant increase in ERK1/2 Tyr204 phosphorylation at 10 min (P < 0.01; Fig. 4A, B) which began to decay by 30 min (P < 0.01; Fig. 4A, B). Consistent with previously published results (Strle et al., 2006), TNFα (1 ng/ml) induced a 5-fold increase in Thr183/Tyr185 phosphorylation JNK at 10 min, but not after 30 min of treatment (P < 0.01; Fig. 4C, D).
Figure 4. IL-10 selectively suppresses JNK activity.
C2C12 myoblasts were pre-treated with IL-10 (10 ng/ml) for 1 h prior to addition of TNFα (1 ng/ml) for another 10 or 30 min. Phosphorylation of ERK1/2 and JNK was detected by probing membranes with P-ERK1/2 or P-JNK specific antibodies. Densitometric summaries were expressed as ratios of P-ERK to ERK (A, B) or as ratios of P-JNK to JNK (C, D). A, Representative western blot and B, densitometric summary of four independent experiments showed that TNFα induced a significant increase in ERK1/2 phosphorylation at 10 min and to lesser extent following 30 min of treatment. This increase in ERK1/2 phosphorylation was not inhibited by IL-10 at any time. C, Representative western blot and D, densitometric summary of four independent experiments demonstrate that TNFα induced a 5-fold increase in JNK phosphorylation at 10 min which returned to basal levels at 30 min. Although IL-10 did not inhibit the ability of TNFα to induce ERK1/2 phosphorylation, IL-10 completely blocked TNFα-induced JNK phosphorylation. * P < 0.05; **P < 0.01
IL-10 did not suppress ERK1/2 phosphorylation (Fig. 4A, B) in either the presence or absence of TNFα. However, in these same sample lysates, IL-10 potently suppressed the ability of TNFα to induce JNK phosphorylation (P < 0.01; Fig. 4C, D). These findings are consistent with our unpublished observations which demonstrate that TNFα-induced IL-6 expression is suppressed by an inhibitor of ERK1/2 PD98059 (10 μM; data not shown). Collectively, these data indicate that IL-10 (10 ng/ml) does not suppress TNFR1 expression or sensitivity since the ability of TNFR1 to induce ERK1/2 was not impaired by IL-10. Instead, the results demonstrate that IL-10 selectively suppresses the ability of TNFα to induce phosphorylation of JNK.
4. Discussion
When combined with growth hormone, IGF-I is responsible for nearly 80% of postnatal growth (Lupu et al., 2001). Myoblasts are well-known to express functional IGF receptors, and data in this report establish that myoblasts also express IL-10Rs and TNFR1s. However, the potential biological role of the anti-inflammatory cytokines such as IL-10 in myoblasts is only now being unraveled. Here we address this void of knowledge by showing for the first time that IL-10 can protect skeletal muscle progenitor cells, thereby promoting their differentiation, but that this occurs only in the presence of IGF-I and TNFα. Incubation of these myoblasts with IGF-I promotes their differentiation by increasing expression of myogenin. TNFα blocks this event and causes IGF-I resistance. The previously unknown protective activity of IL-10 occurs because it acts on myoblasts to prevent TNFα from causing IGF-I resistance. The second major finding in these experiments is that IL-10 is shown to act by a previously undefined mechanism: direct inhibition of cytokine receptor signaling at concentrations that do not inhibit cytokine synthesis.
Skeletal muscle development is mediated by a complex network of signaling interactions downstream of immune and endocrine factor receptors. Skeletal muscle is the largest tissue in the body, and it impacts whole-body physiology by synthesizing and responding to inflammatory cytokines (Frost and Lang, 2005). However, the important possibility that IL-10 affects development of skeletal muscle has not yet been demonstrated. Muscle development and hypertrophy are dependent on sequential expression of myogenic regulatory factors, such as MyoD and myogenin, which are required for differentiation of myoblasts into myotubes (Buckingham, 2001; Sabourin and Rudnicki, 2000). This process is tightly regulated by the anabolic actions of growth hormone and IGF-I and tempered by the anti-anabolic activity of proinflammatory cytokines (Broussard et al., 2004; Broussard et al., 2003; Frost and Lang, 2005; Strle et al., 2004; Strle et al., 2006). Actions of proinflammatory cytokines in myoblasts are in part mediated by JNK, since a cell-permeable peptide inhibitor of JNK ameliorates TNFα inhibition of IGF-I-induced myogenin expression and suppression of muscle differentiation (Strle et al., 2006). Here we significantly extend these results by showing that, in addition to expressing the IGF-I receptor (Broussard et al., 2003), murine myoblasts express receptors for both IL-10 (IL-10R1, Fig. 1A) and TNFα (TNFR1, Fig. 1B). These results indicate that skeletal muscle progenitors are capable of directly responding to both endocrine and immune stimuli, including IGF-I, TNFα and IL-10. Unfortunately, the mechanisms by which both hormone and cytokine regulatory signals are integrated in a single cell are only just now beginning to be understood (Janes et al., 2005). We demonstrate that a physiological concentration of IL-10 (10 ng/ml) restores the ability of IGF-I to induce myogenin expression (Fig. 2). IL-10 inhibits TNFα-induced JNK, but not ERK1/2, phosphorylation (Fig. 4) and suppresses the inhibitory actions of TNFα (Fig. 2). Importantly, IL-10 does not change IGF-I-induced myogenin expression in absence of TNFα. Similarly, neither TNFα nor IL-10 directly alter expression of myogenin in the absence of IGF-I (Fig. 2). These new findings demonstrate that IL-10 plays a protective role in skeletal muscle progenitors and that it does so by directly inhibiting a specific component of cytokine receptor signaling. These results are consistent with the emerging concept that a single cell can integrate multiple diverse signals from both the immune and the endocrine system.
The first direct relationship between the mitogen activated protein kinase (MAPK) family of proteins and IL-10 was reported by Geng et.al. in 1994 (Geng et al., 1994). These scientists determined that in monocytes, IL-10 directly inhibits LPS-induced tyrosine kinase p56lyn and other subsequent steps in this MAPK signaling pathway, including the activation of GTPase, Ras and MAPK. Because IL-10 also suppresses LPS-induced IL-1β mRNA expression, these findings indicated that the Ras-MAPK pathway may be involved in cytokine synthesis. Indeed, a few later reports confirmed that MAPK pathways play a role in the classical anti-inflammatory property of IL-10. For example, IL-10 inhibits cytokine synthesis, expression of cell surface markers and co-stimulatory molecules in hematopoietic cells (Geng et al., 1994; Haddad et al., 2003; Sato et al., 1999). Our findings in myoblasts significantly extend these results by showing that IL-10 plays a protective role in non-hematopoietic cells by directly suppressing specific downstream signaling events following activation of the TNFR1. We demonstrate that IL-10 specifically inhibits TNFα-induced phosphorylation of JNK, but not phosphorylation of ERK1/2 (Fig. 4), without reducing global TNFR signaling. We believe this is an important finding because IL-10 inhibits TNFα-induced JNK activity at a five-fold lower concentration than is required for IL-10 to inhibit cytokine synthesis (Fig. 3B), which is the classical activity attributed to IL-10. The selective role of low concentrations of IL-10 in inhibiting phosphorylation of JNK, but not ERK1/2, raises an interesting conceptual possibility. For example, suppression of proinflammatory cytokine synthesis by high concentrations of IL-10 would inhibit all the biological activities of the proinflammatory cytokine. In contrast, our results indicate that the effects of IL-10 depend upon its concentration, whereby lower doses of IL-10 inhibit only specific aspects of cytokine receptor signaling pathways without obliterating all cytokine activity. These findings are supported by results demonstrating that TNFα-dependent IL-6 expression is suppressed in presence of ERK1/2 inhibitor, but not by IL-10 or the peptide inhibitor of JNK (data not shown). Consequently, these experiments reveal that activation of ERK1/2 and JNK have different functional outcomes in progenitor muscle cells.
JNK is a SAPK member of the MAPK family that is activated in response to a plethora of stress stimuli, including proinflammatory cytokines (Huwiler et al., 2004; Kyriakis and Avruch, 2001; Roux and Blenis, 2004). The therapeutic potential of targeting JNK has recently gained significant clinical attention because of the observation that chemical and peptide inhibitors of JNK ameliorate clinical symptoms of a broad spectrum of inflammatory, neurodegenerative and metabolic disorders including Alzheimer’s disease, diabetes and stroke (Manning and Davis, 2003; Wajant and Scheurich, 2004). These multifaceted actions of JNK strongly suggest that JNK is a convergence point for a variety of stress and inflammatory stimuli, which might be due to the ability of JNK to mediate cytokine-induced resistance to several hormones.
Most inflammatory responses consist of a common repertoire of mediators, including growth factors and pro- and anti-inflammatory cytokines (Kaminska, 2005). Both IL-10 and TNFα play a role in Alzheimer’s disease, stroke, diabetes and microbial infections. Activation of JNK primarily worsens clinical symptoms in these conditions, whereas IL-10 ameliorates them. It is generally considered that IL-10 acts exclusively by inhibiting the synthesis of proinflammatory cytokines. The important possibility that IL-10 directly and specifically inhibits the ability of exogenous TNFα to induce IGF-I resistance has not been reported. We address this paucity of information by demonstrating three new findings: (a) IL-10 restores the ability of IGF-I to induce myogenesis by suppressing TNFα inhibition of a critical muscle-specific transcription factor, myogenin. In the absence of IGF-I and TNFα, IL-10 has no effect. (b) IL-10 plays a protective role in muscle progenitors by inhibiting proinflammatory cytokine receptor signaling at low concentrations that do not inhibit cytokine synthesis. (c) IL-10 does not globally impair expression or sensitivity of TNFR1 because it blocks the ability of TNFα to phosphorylate only JNK, but not ERK1/2. Collectively, these findings demonstrate a novel activity for a classic anti-inflammatory cytokine by establishing that IL-10 directly suppresses the ability of TNFα to cause resistance of muscle cell progenitors to respond to IGF-I.
The abbreviations used in this article are
- SAPK
stress-activated protein kinase
- JNK
c-jun N-terminal kinase
- IL-10
interleukin 10
- IGF-I
insulin like growth factor I
- TNFα
tumor necrosis factor α
- IL-1β
interleukin-1 β
Footnotes
This research was supported by grants from National Institutes of Health to 1K.W.K. (AI50442 and MH51569), 2R.W.J. (AG16710 and AG023580), 3G.G.F. (DK064862), 1R.D. (MH071349) and the USDA to 1R.H.M. (2004-35206-14144)
Disclosure Statement: The authors have read the Disclosure of Potential Conflicts of Interest and have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez B, Quinn LS, Busquets S, Lopez-Soriano FJ, Argiles JM. TNF-alpha modulates cytokine and cytokine receptors in C2C12 myotubes. Cancer Lett. 2002;175:181–185. doi: 10.1016/s0304-3835(01)00717-0. [DOI] [PubMed] [Google Scholar]
- Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Dantzer R, Kelley KW. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. J Immunol. 2004;172:7713–7720. doi: 10.4049/jimmunol.172.12.7713. [DOI] [PubMed] [Google Scholar]
- Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Cytokine-hormone interactions: tumor necrosis factor alpha impairs biologic activity and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. Endocrinology. 2003;144:2988–2996. doi: 10.1210/en.2003-0087. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Skeletal muscle formation in vertebrates. Curr Opin Genet Dev. 2001;11:440–448. doi: 10.1016/s0959-437x(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- Divangahi M, Demoule A, Danialou G, Yahiaoui L, Bao W, Xing Z, Petrof BJ. Impact of IL-10 on Diaphragmatic Cytokine Expression and Contractility During Pseudomonas Infection. Am J Respir Cell Mol Biol. 2006 doi: 10.1165/rcmb.2006-0038OC. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8:255–263. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1153–1164. doi: 10.1152/ajpregu.00164.2003. [DOI] [PubMed] [Google Scholar]
- Geng Y, Gulbins E, Altman A, Lotz M. Monocyte deactivation by interleukin 10 via inhibition of tyrosine kinase activity and the Ras signaling pathway. Proc Natl Acad Sci U S A. 1994;91:8602–8606. doi: 10.1073/pnas.91.18.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Saade NE, Safieh-Garabedian B. Interleukin-10 and the regulation of mitogen-activated protein kinases: are these signalling modules targets for the anti-inflammatory action of this cytokine? Cell Signal. 2003;15:255–267. doi: 10.1016/s0898-6568(02)00075-x. [DOI] [PubMed] [Google Scholar]
- Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord. 2006;16:591–602. doi: 10.1016/j.nmd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Xin C, Brust AK, Briner VA, Pfeilschifter J. Differential binding of ceramide to MEKK1 in glomerular endothelial and mesangial cells. Biochim Biophys Acta. 2004;1636:159–168. doi: 10.1016/j.bbalip.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Janes KA, Albeck JG, Gaudet S, Sorger PK, Lauffenburger DA, Yaffe MB. A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science. 2005;310:1646–1653. doi: 10.1126/science.1116598. [DOI] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, Sullivan DH, Peterson CA, Dennis RA. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol. 2006;41:320–327. doi: 10.1016/j.exger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Rasley A, Tranguch SL, Rati DM, Marriott I. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia. 2006;53:583–592. doi: 10.1002/glia.20314. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- Saidenberg-Kermanac’h N, Bessis N, Deleuze V, Bloquel C, Bureau M, Scherman D, Boissier MC. Efficacy of interleukin-10 gene electrotransfer into skeletal muscle in mice with collagen-induced arthritis. J Gene Med. 2003;5:164–171. doi: 10.1002/jgm.321. [DOI] [PubMed] [Google Scholar]
- SAS. User’s guide. SAS Institute; Cary, NC: 2003. 8.2, V. [Google Scholar]
- Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–3872. [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602. doi: 10.1210/en.2003-1749. [DOI] [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW. C-jun N-terminal kinase mediates tumor necrosis factor-alpha suppression of differentiation in myoblasts. Endocrinology. 2006;147:4363–4373. doi: 10.1210/en.2005-1541. [DOI] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Broussard SR, Venters HD, Johnson RW, Freund GG, Dantzer R, Kelley KW. IL-10 promotes survival of microglia without activating Akt. J Neuroimmunol. 2002;122:9–19. doi: 10.1016/s0165-5728(01)00444-1. [DOI] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem. 2001;276:13709–13717. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- Wajant H, Scheurich P. Analogies between Drosophila and mammalian TRAF pathways. Prog Mol Subcell Biol. 2004;34:47–72. doi: 10.1007/978-3-642-18670-7_3. [DOI] [PubMed] [Google Scholar]
- Zhang ZL, Shen SX, Lin B, Yu LY, Zhu LH, Wang WP, Luo FH, Guo LH. Intramuscular injection of interleukin-10 plasmid DNA prevented autoimmune diabetes in mice. Acta Pharmacol Sin. 2003;24:751–756. [PubMed] [Google Scholar]