Abstract
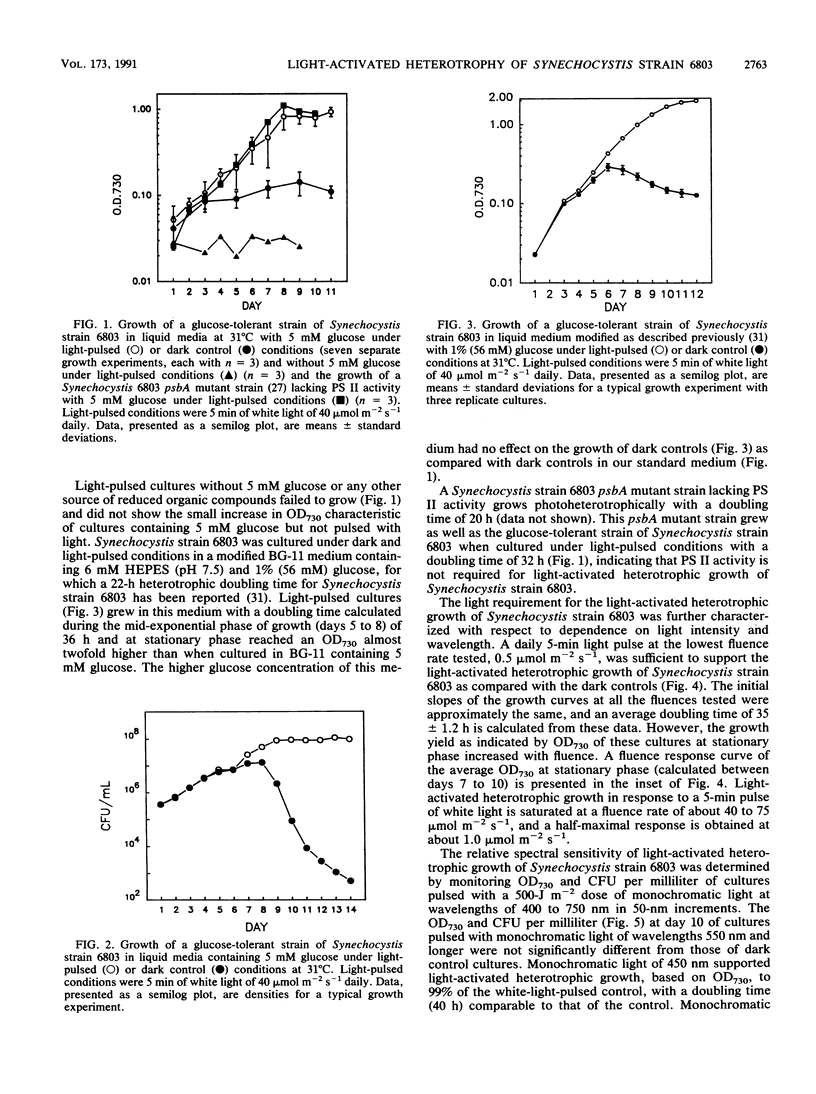
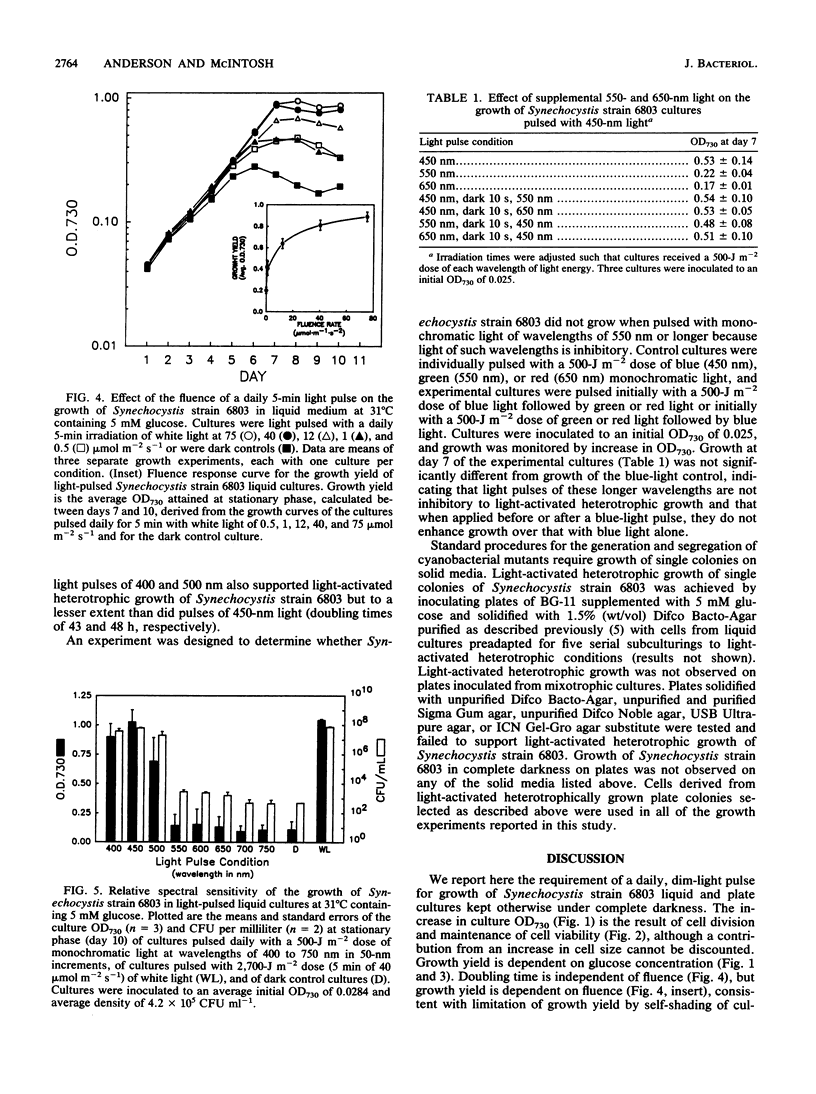
A glucose-tolerant strain of Synechocystis sp. strain 6803 will not grow on glucose under complete darkness unless given a daily pulse of white light, typically 5 min of 40 mumol m-2 s-1 (light-pulsed conditions). The light pulse is insufficient for photoautotrophy, as glucose is required and growth yield is dependent on glucose concentration. Growth rate is independent of fluence, but growth yield is dependent on fluence, saturating at 40 to 75 mumol m-2 s-1. A Synechocystis strain 6803 psbA mutant strain grows under light-pulsed conditions at rates similar to those for the glucose-tolerant strain, indicating that photosystem II is not required for growth. The relative spectral sensitivity of the growth of light-pulsed cultures (growth only in blue light, 400 to 500 nm, maximum at 450 nm) precludes energetic contribution from cyclic electron transport around photosystem I. Pulses of long-wavelength light (i.e., 550 and 650 nm) did not support the growth of Synechocystis strain 6803 and, when supplied before or after a blue-light pulse, did not inhibit blue-light-stimulated growth of Synechocystis strain 6803. We conclude that the required blue-light pulse does not support growth via photosynthetic electron transport but appears instead to function as an environmental signal regulating heterotrophic metabolism, cell division, or other photomorphogenic processes. We have termed the growth of Synechocystis strain 6803 pulsed with light and kept otherwise in complete darkness light-activated heterotrophic growth. This observation of a blue-light requirement for the growth of Synechocystis strain 6803 represents a novel blue light effect on the growth of a cyanobacterium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asato Y. Macromolecular synthesis in synchronized cultures of Anacystis nidulans. J Bacteriol. 1979 Oct;140(1):65–72. doi: 10.1128/jb.140.1.65-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier C., Elmorjani K., Meyer I., Joset F., Herdman M. Photosynthetic mutants of the cyanobacteria Synechocystis sp. strains PCC 6714 and PCC 6803: sodium p-hydroxymercuribenzoate as a selective agent. J Bacteriol. 1984 May;158(2):659–664. doi: 10.1128/jb.158.2.659-664.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN A. C., WOOD H. N. On the activation of certain essential biosynthetic systems in cells of Vinca rosea L. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1776–1782. doi: 10.1073/pnas.48.10.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A. The photoregulated expression of multiple phycocyanin species. A general mechanism for the control of phycocyanin synthesis in chromatically adapting cyanobacteria. Eur J Biochem. 1981 Oct;119(2):425–429. doi: 10.1111/j.1432-1033.1981.tb05625.x. [DOI] [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Miedel M. C., Nelson N. Structure and targeted mutagenesis of the gene encoding 8-kDa subunit of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18374–18380. [PubMed] [Google Scholar]
- Chitnis P. R., Reilly P. A., Nelson N. Insertional inactivation of the gene encoding subunit II of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1989 Nov 5;264(31):18381–18385. [PubMed] [Google Scholar]
- Cosgrove D. J. Kinetic separation of phototropism from blue-light inhibition of stem elongation. Photochem Photobiol. 1985;42(6):745–751. doi: 10.1111/j.1751-1097.1985.tb01642.x. [DOI] [PubMed] [Google Scholar]
- Crawford N. A., Sutton C. W., Yee B. C., Johnson T. C., Carlson D. C., Buchanan B. B. Contrasting modes of photosynthetic enzyme regulation in oxygenic and anoxygenic prokaryotes. Arch Microbiol. 1984 Oct;139(2-3):124–129. doi: 10.1007/BF00401986. [DOI] [PubMed] [Google Scholar]
- Csatorday K., Horváth G. Synchronization of Anacystis nidulans. Oxygen evolution during the cell cycle. Arch Microbiol. 1977 Jan 11;111(3):245–246. doi: 10.1007/BF00549361. [DOI] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Babcock G. T., McIntosh L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1988 Jan;85(2):427–430. doi: 10.1073/pnas.85.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Sithole I., Babcock G. T., McIntosh L. Directed mutagenesis indicates that the donor to P+680 in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry. 1988 Dec 27;27(26):9071–9074. doi: 10.1021/bi00426a001. [DOI] [PubMed] [Google Scholar]
- Federspiel N. A., Grossman A. R. Characterization of the light-regulated operon encoding the phycoerythrin-associated linker proteins from the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1990 Jul;172(7):4072–4081. doi: 10.1128/jb.172.7.4072-4081.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr Robert, Moses Phyllis, Morelli Giorgio, Coruzzi Gloria, Chua Nam-Hai. Expression dynamics of the pea rbcS multigene family and organ distribution of the transcripts. EMBO J. 1986 Sep;5(9):2063–2071. doi: 10.1002/j.1460-2075.1986.tb04467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Mullet J. E. Blue light regulates the accumulation of two psbD-psbC transcripts in barley chloroplasts. EMBO J. 1989 Oct;8(10):2785–2794. doi: 10.1002/j.1460-2075.1989.tb08424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson C., Debus R. J., Osiewacz H. D., Gurevitz M., McIntosh L. Construction of an Obligate Photoheterotrophic Mutant of the Cyanobacterium Synechocystis 6803 : Inactivation of the psbA Gene Family. Plant Physiol. 1987 Dec;85(4):1021–1025. doi: 10.1104/pp.85.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROFF N., VISHNIAC W. The effect of light on this developmental cycle of Nostoc muscorum, a filamentous blue-green alga. J Gen Microbiol. 1961 Jul;25:365–374. doi: 10.1099/00221287-25-3-365. [DOI] [PubMed] [Google Scholar]
- Labarre J., Thuriaux P., Chauvat F. Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis sp. strain 6803. J Bacteriol. 1987 Oct;169(10):4668–4673. doi: 10.1128/jb.169.10.4668-4673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaroff N., Schiff J. Action Spectrum for Developmental Photo-Induction of the Blue-Green Alga Nostoc muscorum. Science. 1962 Aug 24;137(3530):603–604. doi: 10.1126/science.137.3530.603. [DOI] [PubMed] [Google Scholar]
- Pelroy R. A., Bassham J. A. Photosynthetic and dark carbon metabolism in unicellular blue-green algae. Arch Mikrobiol. 1972;86(1):25–38. doi: 10.1007/BF00412397. [DOI] [PubMed] [Google Scholar]
- Russo V. E. Blue light induces circadian rhythms in the bd mutant of Neurospora: double mutants bd,wc-1 and bd,wc-2 are blind. J Photochem Photobiol B. 1988 Jul;2(1):59–65. doi: 10.1016/1011-1344(88)85037-1. [DOI] [PubMed] [Google Scholar]
- Schaeffer F., Stanier R. Y. Glucose-6-phosphate dehydrogenase of Anabaena sp. Kinetic and molecular properties. Arch Microbiol. 1978 Jan 23;116(1):9–19. doi: 10.1007/BF00408728. [DOI] [PubMed] [Google Scholar]
- Van Baalen C., Hoare D. S., Brandt E. Heterotrophic growth of blue-gren algae in dim light. J Bacteriol. 1971 Mar;105(3):685–689. doi: 10.1128/jb.105.3.685-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaas W. F., Williams J. G., Rutherford A. W., Mathis P., Arntzen C. J. Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9474–9477. doi: 10.1073/pnas.83.24.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermass W. F., Rutherford A. W., Hansson O. Site-directed mutagenesis in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: Donor D is a tyrosine residue in the D2 protein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8477–8481. doi: 10.1073/pnas.85.22.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]


