Abstract
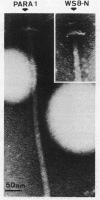
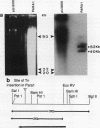
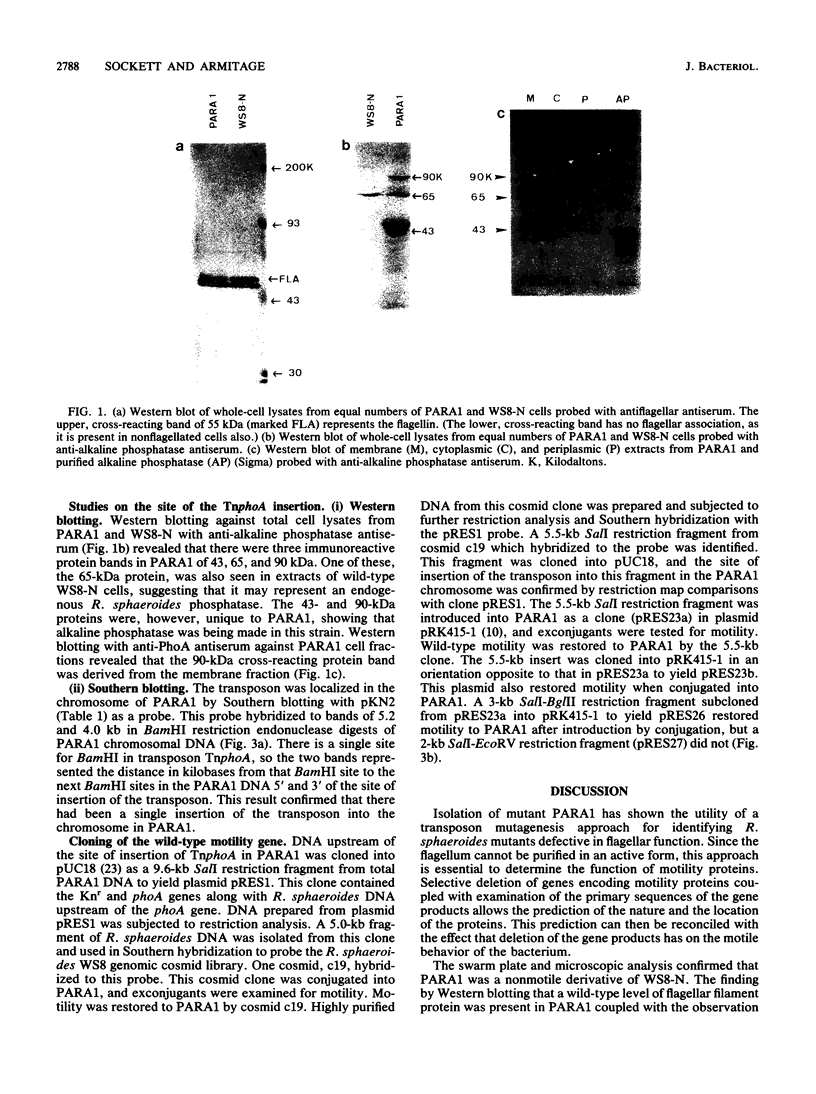
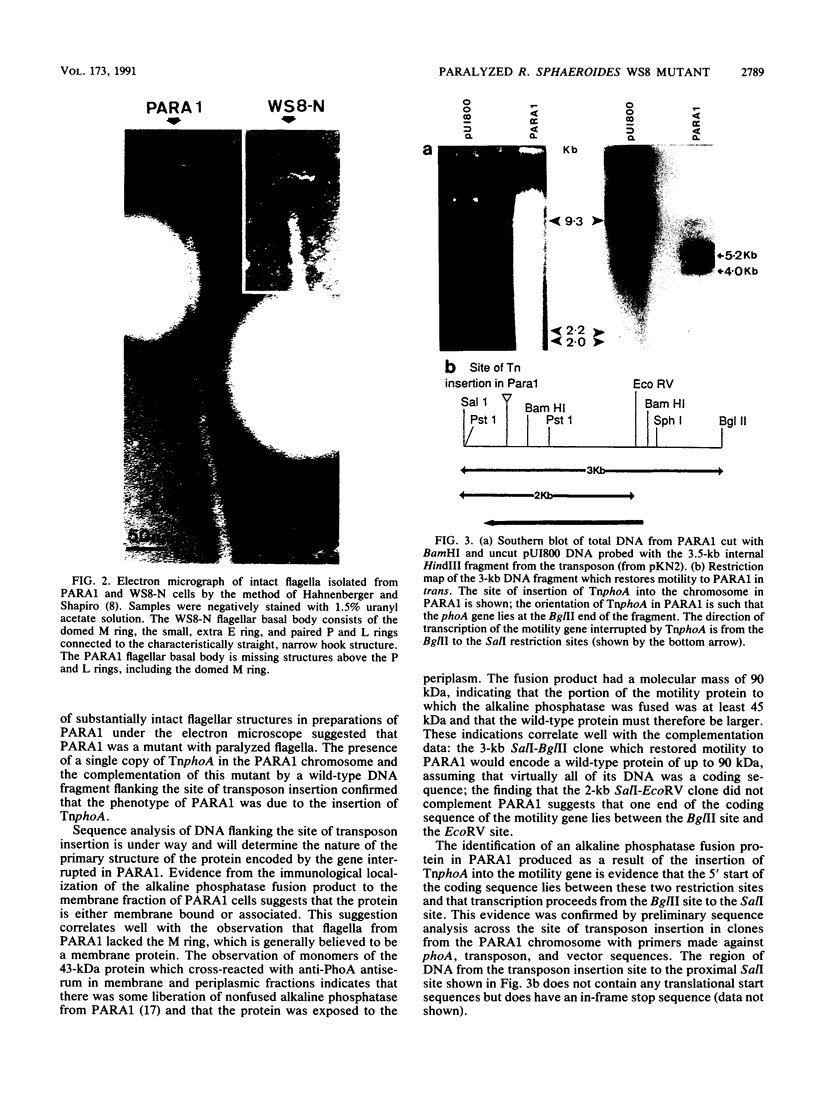
A paralyzed Rhodobacter sphaeroides mutant strain (PARA1) was isolated by a motility screening procedure following mutagenesis of wild-type R. sphaeroides WS8-N with the transposable element TnphoA (Tn5 IS50L::phoA). PARA1 synthesized a wild-type level of flagellin, as detected by Western immunoblotting with antiflagellar antiserum. Flagellar staining showed that flagellin was assembled into apparently normal external flagellar filaments. Electron micrographs of basal body structures from PARA1 showed that some ring structures that were present were similar to those in wild-type R. sphaeroides WS8-N. PARA1 cells were nonmotile under all growth conditions. No pseudorevertants to motility were seen when PARA1 was grown in the presence of kanamycin to select for the presence of the transposon. The presence of the single copy of TnphoA in the PARA1 chromosome was demonstrated by Southern blotting. Western blotting of cytoplasmic, periplasmic, and membrane fractions of PARA1 with anti-alkaline phosphatase antiserum showed that the transposon had been inserted in-frame into a gene encoding a membrane protein. A SalI restriction endonuclease fragment was cloned from the chromosome of PARA1; this fragment contained a portion of the transposon and R. sphaeroides DNA sequence 5' of the site of insertion. This flanking R. sphaeroides DNA sequence was used to probe an R. sphaeroides WS8 cosmid library. A cosmid designated c19 hybridized to the probe, and a SalI restriction endonuclease fragment derived from this cosmid restored wild-type motility to PARA1 when introduced into this mutant strain by conjugation. The significance of this finding in a bacterium with unidirectionally rotating flagella is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J. P., Macnab R. M. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):514–518. doi: 10.1128/jb.169.2.514-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. M., Segall J. E., Berg H. C. Impulse responses in bacterial chemotaxis. Cell. 1982 Nov;31(1):215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- Davis J., Donohue T. J., Kaplan S. Construction, characterization, and complementation of a Puf- mutant of Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., McEwan A. G., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986 Nov;168(2):962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnenberger K. M., Shapiro L. Identification of a gene cluster involved in flagellar basal body biogenesis in Caulobacter crescentus. J Mol Biol. 1987 Mar 5;194(1):91–103. doi: 10.1016/0022-2836(87)90718-2. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L. The bacterial chemosensory system. Can J Microbiol. 1988 Apr;34(4):466–474. doi: 10.1139/m88-080. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W., Vogler A. P. Molecular mechanisms of bacterial chemotaxis towards PTS-carbohydrates. FEMS Microbiol Rev. 1989 Jun;5(1-2):81–92. doi: 10.1016/0168-6445(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Moore M. D., Kaplan S. Construction of TnphoA gene fusions in Rhodobacter sphaeroides: isolation and characterization of a respiratory mutant unable to utilize dimethyl sulfoxide as a terminal electron acceptor during anaerobic growth in the dark on glucose. J Bacteriol. 1989 Aug;171(8):4385–4394. doi: 10.1128/jb.171.8.4385-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole P. S., Armitage J. P. Role of metabolism in the chemotactic response of Rhodobacter sphaeroides to ammonia. J Bacteriol. 1989 May;171(5):2900–2902. doi: 10.1128/jb.171.5.2900-2902.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E., Armitage J. P., Evans M. C. Methylation-independent and methylation-dependent chemotaxis in Rhodobacter sphaeroides and Rhodospirillum rubrum. J Bacteriol. 1987 Dec;169(12):5808–5814. doi: 10.1128/jb.169.12.5808-5814.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E., Donohue T. J., Varga A. R., Kaplan S. Control of photosynthetic membrane assembly in Rhodobacter sphaeroides mediated by puhA and flanking sequences. J Bacteriol. 1989 Jan;171(1):436–446. doi: 10.1128/jb.171.1.436-446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S. P., Kaplan S. Intracellular localization of phospholipid transfer activity in Rhodopseudomonas sphaeroides and a possible role in membrane biogenesis. J Bacteriol. 1985 Oct;164(1):181–186. doi: 10.1128/jb.164.1.181-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]