Abstract
This study is a cost-benefits analysis of the recommendations of the Centers for Disease Control and Prevention for presumptive anti-malarial treatment among departing West African refugees. We conducted a retrospective chart review of symptomatic, blood smear-positive cases of malaria seen in Minneapolis, Minnesota, from 1996 through 2005. Billing charges of U.S. care were compared with estimates of implementation costs for overseas treatment. Fifty-eight symptomatic malaria infections occurred among West African refugees. After overseas pre-departure presumptive treatment, symptomatic malaria in arriving refugees decreased from 8.2% to 0%. The pre-departure number needed to treat to prevent one case of symptomatic malaria is 13.9 (95% confidence interval = 9.8–24). The average U.S. billing charge for each malaria case is $1,730. Overseas implementation costs for presumptive treatment are estimated to be between $141 and $346 to prevent one U.S. malaria case. Overseas presumptive pre-departure anti-malarial therapy prevents clinical malaria in refugees and results in cost-benefits when the malaria prevalence is > 1%. Overseas presumptive therapy has greater cost-benefits than U.S. based screening and treatment strategies.
INTRODUCTION
There are approximately 500 million cases of malaria worldwide resulting in an estimated 1.1 million deaths annually.1 Military conflicts and adverse economic conditions in west Africa since the 1990s resulted in large numbers of refugees seeking a better life in the United States. Malaria is no longer endemic in the United States, but increasing international travel, military operations, and immigration are responsible for imported cases each year. Between 1996 and 2004, an average of 1,381 malaria cases was reported annually to the Centers for Disease Control and Prevention (CDC).2,3
In response to concern over malaria importation, the CDC issued recommendations in 1999 that all non-pregnant sub-Saharan African refugees more than two years of age receive pre-departure presumptive anti-malarial therapy prior to departure to the United States. The International Organization of Migration (IOM) began implementing these recommendations in May 1999. No published data have evaluated the clinical and economic impact of these CDC recommendations.
This paper analyzes the cost-benefits of this program in West African refugees by evaluating changes in malaria epidemiology at Hennepin County Medical Center (HCMC) and affiliated clinics between 1996 and 2005. Addressing refugee malaria is important not only because of financial implications, but also because of the indirect costs of productive time lost in a population already faced with multiple obstacles to integration into our society. At least 60% of Liberian refugee children had smear positive malaria one month after arrival in the late 1990s.4 The Anopheles quadrimaculatus mosquito, which is endemic to large areas of the United States, including Minnesota, is a competent vector for malaria transmission.5 Although autochthonous transmission has not been reported in Minnesota since the 1930s, local U.S. transmission has occurred in 63 U.S. outbreaks responsible for 156 known malaria cases in the past 50 years.6,7 Reducing potential malaria reservoirs is important as average temperatures warm and increase the potential for malarial transmission.
METHODS
Population studied
Hennepin County is the major resettlement destination in Minnesota for newly arriving sub-Saharan African refugees and receives more refugees than many states. HCMC is an urban teaching hospital that serves most new refugees in Hennepin County (Minneapolis, MN). This study is a retrospective chart review of symptomatic cases of smear-positive malaria in West African refugees seen at HCMC between January 1, 1996 and December 31, 2005. Malaria cases were detected using hospital laboratory records.
According to CDC definitions, each symptomatic or asymptomatic patient with smear-positive malaria is reported as a malaria case only once, even if treated multiple times. However, because the focus was on the economic cost of malaria, each full treatment course was counted individually in this study. Asymptomatic malaria parasitemias detected by refugee screening for other scientific studies were excluded because these patients would not have been treated for malaria in the absence of these studies.
Measurements
Data from patient charts with documented malaria were collected in a standard format that included age, sex, origin, travel itinerary, Plasmodium species, infection severity, and personal history of malaria. Treatment type and location (e.g., outpatient clinic, inpatient hospital) were recorded. Refugee status was cross-checked with Minnesota Department of Health data. The incidence of malaria diagnosed among West African refugees before and after implementation of presumptive anti-malarial treatment was studied.
The billing department of HCMC provided health care charges and reimbursements for inpatients and outpatients. Complete billing information was available for 51 patients from January 1, 1998 onward. U.S.-based therapy charges were derived from actual HCMC treatment charges.
The cost of pre-departure presumptive treatment was estimated using wholesale drug prices and overhead costs based on the published costs for delivering malaria care in Africa multiplied two-fold in an effort to account for unforeseen costs. Sulfadoxine-pyrimethamine (SP) was used alone as pre-departure anti-malaria treatment from May 1999 until October 2003, when some refugees may have begun to receive SP and artesunate. Given the increase in SP resistance in parts of Africa and the recent World Health Organization (WHO) recommendation of artemisinin-based combination therapy (ACT) for treatment of symptomatic malaria in sub-Saharan Africa, hypothetical treatment costs using artemether-lumefantrine (AL) (Coartem®; Novartis, Basel, Switzerland) were estimated. These costs were adjusted to account for potential treatment failures. All charges and costs in this study were adjusted to constant 2005 U.S. dollars.
Statistical analysis
We calculated case prevalence rates for a given time period using malaria cases treated at HCMC as the numerator and the number of refugees with Hennepin County, MN as their primary port of entry (primary refugees) as the denominator. Confidence intervals (CIs) were calculated using the Newcombe-Wilson score method8 without continuity correction because normal approximation methods perform poorly with proportions that are close to zero. P values were calculated by using a Monte Carlo approximation to the chi-square statistic sampling distribution because of the small number of malaria cases in each year. The malaria prevalence breakpoint for cost-benefits is calculated from the formula cost-benefit breakpoint = presumptive malaria therapy cost/U.S. malaria treatment cost.
RESULTS
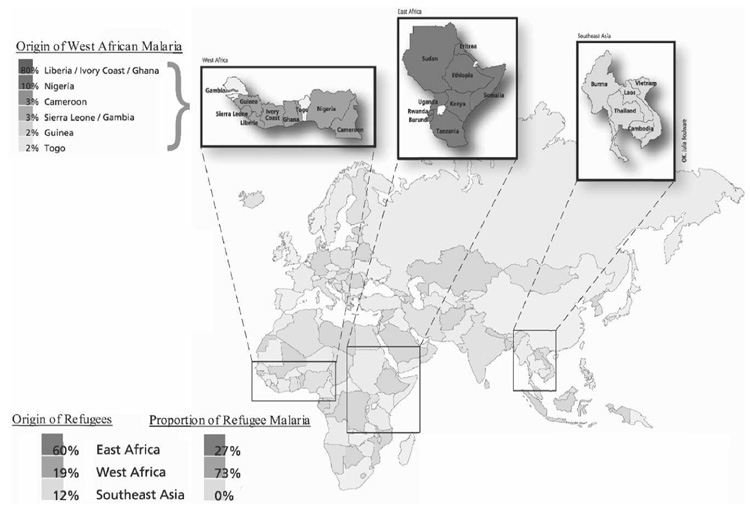
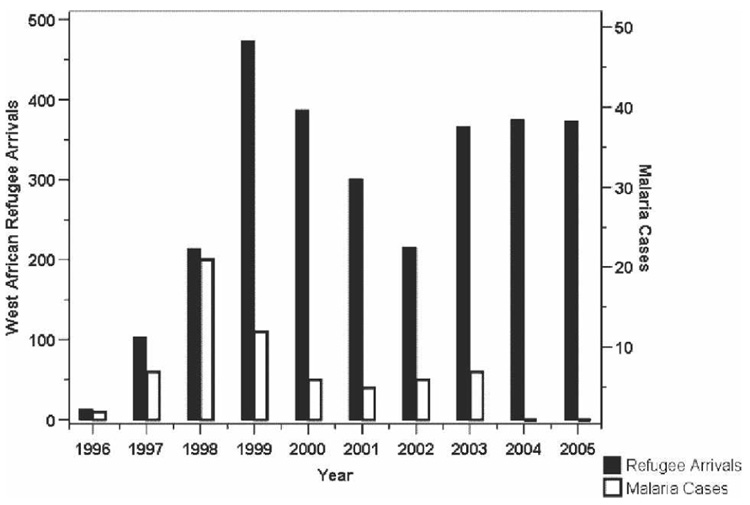
Between 1996 and 2005, 2,821 West African primary refugees resettled in Hennepin County.9 During the study period, 58 cases of symptomatic smear-positive malaria occurred in West African refugees, accounting for 73% of malaria cases seen among refugees and 46% of all malaria cases seen at HCMC (Figure 1). Peak years for West African refugee malaria cases seen at HCMC were 1997–1999 (Figure 2). West African refugee arrivals decreased significantly during 2001–2002 because of concomitant federal policy shifts. Although West African refugee arrivals increased and exceeded pre 1999 levels in 2004–2005, no refugee cases of malaria were detected from January 1, 2004 through December 31, 2005.9
FIGURE 1.
Origin of refugees and proportion of refugee malaria in Hennepin County, Minnesota, by regions, 1996–2005.
FIGURE 2.
Incidence of refugee arrivals in Hennepin County, Minnesota, and malaria cases at the Hennepin County Medical Center and affiliated clinics, 1996–2005.
All refugee screening in Hennepin County occurs in HCMC-affiliated clinics, accounting for 78% of West African refugee medical screening in the state in 1997–1998 and 76% between 1999 and 2005. In 1997–1998, 79% of West African refugees in Hennepin County were screened versus 83% between 1999 and 2005. No screening occurred for this population in Hennepin County in 1996. However, because of the complexity and cost of screening asymptomatic individuals, < 5% of arriving refugees are tested for malaria at the medical screening examination. Between 1988 and 1998, 113 (88%) of 129 malaria cases in Hennepin County were diagnosed at HCMC.10 During incremental implementation of overseas presumptive treatment between 1999 and 2004, HCMC diagnosed 66 (69%) of the 95 malaria cases in the county. Non-refugee malaria remained stable at HCMC from 1988 to 2005, averaging 4.8 cases annually (median = 5.5, range = 1–12), 4.9 cases annually until 1998 (median = 5.5, range = 3–12), and 4.7 thereafter (median = 4.5, range = 1–11) (P = 0.8).
Patient characteristics
Of the 58 refugee malaria cases, 55% were male, 59% were ≤ 15 years of age, 26% were treated as inpatients, and 74% were treated as outpatients. Thirty-five (60%) were infected with Plasmodium falciparum, 9 (16%) with P. ovale, 4 (7%) with P. malariae, and 2 (3%) were co-infected with P. falciparum and P. ovale. In eight cases (14%), the species was not determined. Refugees who originated and/or transited through Liberia, Côte d’Ivoire, and Ghana made up 79% (46 of 58) of malarial cases from west Africa (Figure 1). During the study period, 63% of malaria cases occurred in primary refugees resettling in Minnesota and 37% entered the U.S. via ports outside Minnesota (secondary refugees).
Pre-departure treatment
Presumptive anti-malarial treatment of West African refugees with SP before departure began in 1999. Between October 1, 2001 and June 30, 2002, the IOM treated 225 (69%) of 324 West African refugees immigrating to the United States. In October 2003, the CDC recommended the use of ACT. Between October 1, 2003 and September 30, 2004, the IOM reported treating 8,396 (99%) of 8,479 West African refugees with either SP or ACT. Children less than 15 years of age accounted for 23–38% of those treated.11 Incremental implementation of pre-departure treatment was associated with incremental reduction in refugee malaria cases in our study (Figure 3).
FIGURE 3.
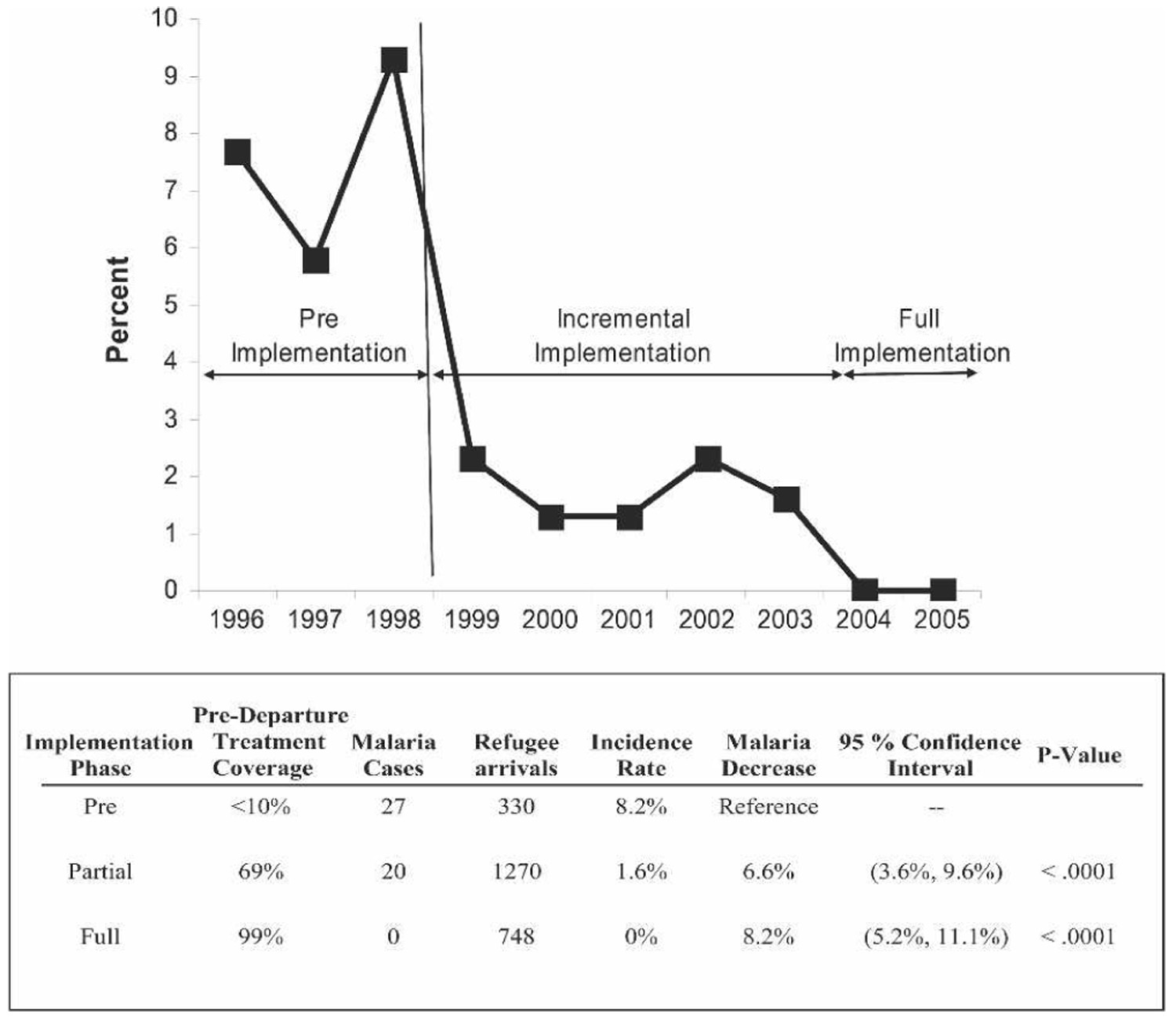
Association between malaria rates and incremental implementation of mass pre-departure presumptive treatment among West African refugees in Hennepin County (Minneapolis), Minnesota.
U.S. medical billing data
The average billing charge of treating refugee malaria was $1,730. Treatment charges for the 11 inpatients averaged $6,290/case (range = $1,345–$18,175). Reimbursement occurred for 70% of total charges with 20% provided as charity care, 6% denied by insurers, and 4% written off. The average outpatient charge was $475 per patient. Average charges for smear sets were $48 (range = $19.80–$80). The total charges for symptomatic malaria treatment in West African refugees during 1998–2005 were $88,205. The HCMC inpatient billing charges were < 60% of the average 2005 U.S. Medicare-covered charges by diagnosis-related group coding. Length of hospitalization was also shorter in this semi-immune population, averaging 2.3 days versus 3.2 days nationally.12 Actual billing charges were used in our model rather than the generalized covered charges based on Medicare Provider Analysis and Review (MedPar) to remain consistently conservative.
Plasmodium ovale infection accounted for 23% of cases (7 of 31) between 1999 and 2005 during incremental implementation of presumptive treatment. This high proportion of P. ovale is most likely artifactual. The background rate in travelers to West Africa during 1988–2005 and in West African refugees during 1988–1998 was 12% (10 of 82). Plasmodium ovale has a prolonged hepatic stage (hypnozoite) and later relapse would not necessarily have been avoided with AL, which has only blood-phase anti-malarial activity. However, P. ovale relapse after blood-phase anti-malarial treatment is 5–10%.13
Cost estimates of presumptive anti-malarial treatment and statistical analysis
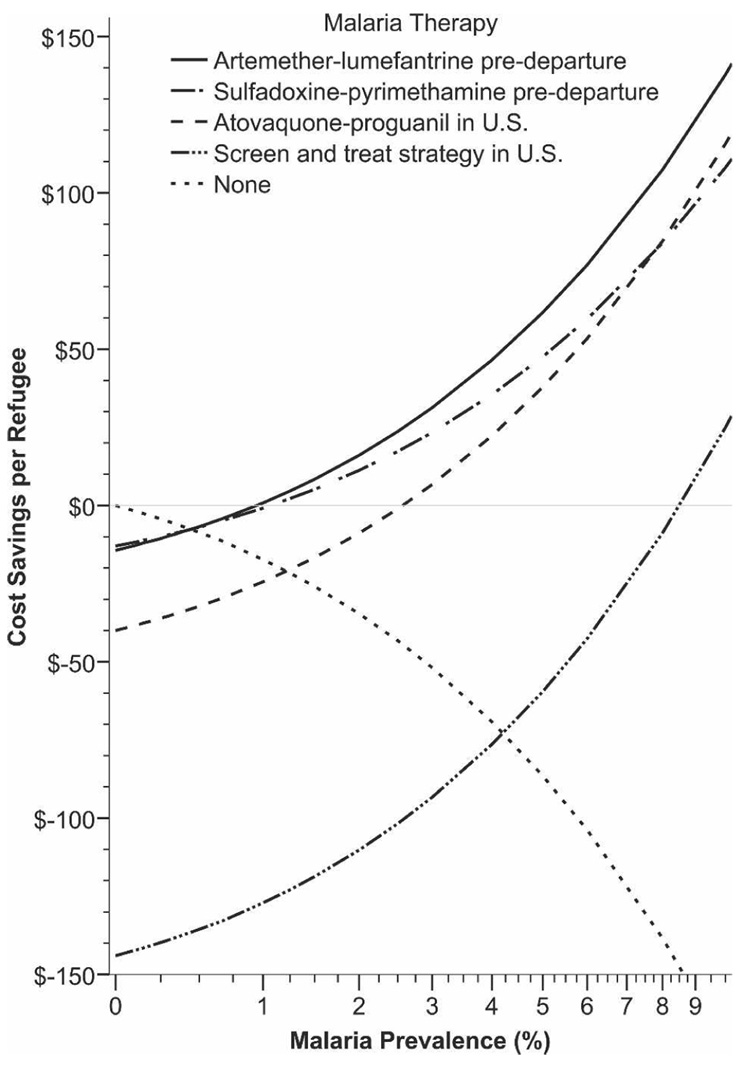
The drug cost per adult refugee for a six-dose regimen of AL is $2.40.14 Prices will likely decrease with increased production and patent expirations.15–17 Published costs for the administration of AL in Africa cite overhead costs of approximately $5–$6 per person treated (Table 1).18,19 Our estimation uses a two-fold higher overhead cost ($12) and the international AL cost ($2.40). Using WHO criteria, we calculated that AL has a 28-day efficacy between 97.2% and 100% for P. falciparum.16,19–25 Comparative costs and charges for overseas and domestic treatment are shown in Table 2 and Figure 4.
TABLE 1.
Itemized budget for overseas provision of ACT per person*
| Category | Wiseman and others18 estimate in Tanzania | Candra and others19 estimate in Zambia | Collinet-Adler presumptive therapy estimate |
|---|---|---|---|
| Drug (artemether-lumefantrine)† | $0.91 | $1.33 | $2.40 |
| Staff salary | $3.98 | $0.84 | $7.96 |
| Building rental | $0.17 | $0.38 | $0.76 |
| Overhead (utilities, consumables) | $0.57 | $0.54 | $1.14 |
| Microscope, diagnostics | $0.33 | $4.25 | – |
| Transport, miscellaneous costs | – | – | $2.14 |
| Total adult cost | $5.96 | $7.34 | $14.40 |
ACT = artemisinin combination therapy.
Subsidized cost of artemether-lumefantrine. The non-subsidized cost is $2.40 per treatment course. Our estimates are double prior published estimates for ACT in Africa.
TABLE 2.
Comparative costs for malaria treatment of West African refugees by strategy, 1998–2004*
| Strategy | Artemether-lumefantrine presumptive treatment | Observed U.S. malaria cases | Expected U.S. malaria cases |
|---|---|---|---|
| Presumptive overseas Pre-departure treatment | All† | Actual | None† |
| Refugees treated | 2,332 | 51 | 191‡ |
| Cost per treatment course | $14.40 | $1,729.50 | $1,729.50 |
| Total treatment cost | $33,580 | $88.205 | $330,335 |
| Relative treatment cost | 38% | 100% | 375% |
| Malaria prevalence: cost-benefit policy breakpoint§ | 1.0% | NA | NA |
NA = not applicable.
Theoretical.
If overseas presumptive therapy had not started in 1999, an expected 191 cases of malaria would have occurred among 2,332 refugees. This is derived from the preimplementation refugee malaria rate of 8.2%.
Prevalence of malaria above which it is more cost-beneficial to use an overseas presumptive treatment strategy.
FIGURE 4.
Cost-benefits analysis of pre-departure presumptive anti-malarial treatment.
When we compared 1996–1998 to the post-treatment-initiation years of 2004–2005, the number needed to treat with overseas presumptive treatment to prevent one symptomatic malaria case is estimated to be 13.9 persons (95% CI = 9.8–24.0). The estimated overseas cost to prevent one clinical malaria case in the U.S. is between $141 and $346. This compares favorably to HCMC billing charges that average $1,730 per treatment. This strategy becomes cumulatively more attractive as a higher percentage of refugees are treated (Figure 4). Strategies after arrival in the United States, whether presumptive treatment or screen and treat, are more expensive than overseas therapy (Figure 4). Given the aforementioned costs and billing charges, the breakpoint malaria prevalence at which the cost-benefits of presumptive pre-departure therapy using AL become favorable is a malaria prevalence >1%.
DISCUSSION
The goals of this study were two-fold. The first goal was to assess whether presumptive treatment of refugees from West Africa for malaria is effective in decreasing the incidence of symptomatic malaria after arrival in the United States. The second goal was to determine the conditions under which this strategy results in cost-benefits for the future. A significant decrease in malaria among newly arrived West African refugees was documented after the implementation of presumptive pre-departure treatment. As more refugees and a higher percentage were treated starting in 2003, continued significant decreases in malaria rates were observed despite increased numbers of West African refugee arrivals. Presumptive pre-departure treatment of West African refugees was associated with decreasing malaria rates in West African refugees in Hennepin County.
When compared with other refugees, the prevalence of symptomatic malaria cases in West African refugees is disproportionately high.10 Malaria prevalence in this region undoubtedly plays a major role. Significant numbers of malarial cases in West African refugees were seen even before large-scale immigration from West Africa began (Figure 2). A recent study in a pediatric population of Liberian refugees found that 60% (34 of 57) were infected, most of them asymptomatically.4 This is probably an underestimate because only one set of thick and thin smears was used in addition to clinical criteria. Liberia, Côte d’Ivoire, and Ghana frequently reach holoendemic status for malaria with at least 80% of children 2–12 of age infected.26
The cost of symptomatic treatment of malaria in West African refugees at HCMC from 1998 to 2005 was $88,205. Presumptive treatment using blood-phase anti-malarial drugs, such as AL, may artificially increase the proportion of P. ovale observed. Both P. ovale and P. vivax may persist as hepatic hypnozoites, unaffected by blood-phase anti-malarial drugs only to relapse later. Plasmodium vivax is rare in indigenous West Africans because of the genetic protection afforded by the frequent absence of Duffy-group antigens in this population.
The medical charges for malaria treatment in the U.S. in this study are similar to those of a 1995 study in which the mean charge of P. falciparum treatment adjusted to constant 2005 dollars was $4,280 (range = $300–$124,500), with mild cases averaging $730, moderate cases $4,215, and severe cases $19,530.26 This is comparable to our charges of $6,290 (range = $1,345–$18,175) for hospitalized patients and $475 for outpatient management. Severe malaria did not occur in the semi-immune refugee population we studied.
Estimated costs of presumptive treatment of all West African refugees (n = 2,332) who resettled in Hennepin County from 1998 to 2004 with a hypothetical adult course of AL ($14.40 per refugee) would have been $33,580. The breakpoint prevalence rate of malaria at which presumptive pre-departure therapy results in cost-savings (1%) is far lower than malarial prevalence rates in most of sub-Saharan Africa. If presumptive overseas therapy had not started in 1999, an estimated 191 cases of malaria would have been expected with $330,335 in charges.
Evaluating the cost-benefits of malaria screening in refugees is beyond the scope of this paper but a brief note is warranted. Preliminary data suggests that a single blood smear and rapid diagnostic testing are relatively insensitive in detecting malaria in asymptomatic individuals.27 Therefore, the gold standard for testing remains three sets of smears ($144) separated over time. If additional visits and treatment of positive cases are excluded, the charges for screening the 2,332 West African refugees who arrived in Hennepin County in 1998–2004 are estimated to be approximately $335,800 or 1,000% of the costs of presumptive therapy. A screen and treat strategy with just one blood smear is more expensive than empirical atovaquone-proguanil therapy for all refugees arriving in the United States.
The direct comparison between presumptive treatment of all refugees and U.S. case-management charges in this retrospective study is affected by several factors. The actual costs associated with implementing this pre-departure initiative have not been published. We have used estimates based on twice the published costs of delivering anti-malarial therapy in Zambia ($5.05) and Tanzania ($6.01) plus drug cost to remain conservative.18,19 Actual costs may be lower. Since 1999, an increasing percentage of West African refugees received treatment prior to departure. The U.S. medical charges would have undoubtedly been higher had the pre-departure treatment not been undertaken, as shown by the significant reduction in malaria cases after 1999. Refugees may also have been treated for malaria in other health care facilities and therefore would be undetected by this study. Refugee screening in Hennepin County occurs in affiliated clinics and 88% of diagnosed Hennepin County malaria was treated in our system prior to incremental implementation of overseas treatment. Any additional cases treated in the United States prior to full implementation of the overseas program would increase the cost-savings of presumptive treatment. Furthermore, one-third of refugees received pediatric doses, which would decrease the cost estimates that were based on adult dosages. Additionally, prior studies have shown that most West African refugees had malaria on arrival before implementation of presumptive overseas treatment.4,27 The success of presumptive treatment may be used to support the discontinuation of malaria screening post-arrival, increasing cost-benefits. Lastly, it has been shown by other investigators that approximately 60% of malaria is misdiagnosed at presentation and multiple visits are often made prior to diagnosis.28 The costs of extra visits due to misdiagnosis were not quantified in this study. These considerations would all increase the cost-savings of overseas presumptive treatment.
There are confounders limiting the precision of the cost-benefits analysis. Calculating the West African refugee population potentially seeking care at HCMC is difficult. An unknown number of West African refugees with a port of entry elsewhere in Minnesota or the United States have secondarily re-settled in Hennepin County. This secondary migration into Minnesota after primary resettlement elsewhere in the United States accounts for approximately 36% of Minnesota’s refugees and 37% of West African refugee malaria cases at HCMC.29 Secondary migration has not markedly changed, averaging 1,900 ± 850 net migrants into Minnesota during 1997–2004.29 Thus, decreased secondary migration patterns do not account for the reduction in malaria. It is also possible that not all ill refugees presented for health care in the United States, but instead obtained anti-malarial medication via refugee social networks.
The conclusions of this study could be verified by repeating this investigation in other states with centers like HCMC where most refugees seek health care. During 2000–2004, Hennepin County accepted 8,577 primary refugees and approximately 4,000 secondary refugees, more than 44 other statewide totals.29 Prior to overseas presumptive anti-malarial treatment, the 34 cases of malaria (59% were West African refugees) diagnosed at HCMC in 1998 were greater than the malaria cases reported statewide in 43 states.30 Thus, although this is a single-center study, the study population is large.
Despite the limitations of an observational design, this study supports the continuation of the current overseas presumptive anti-malarial treatment program for West African refugees. This program has dramatically reduced clinical malaria in this population. Shifting the malaria burden from welcoming communities in the United States to the presumptive treatment of refugees in West Africa is feasible and results in cost savings. The development of inexpensive, effective, and well-tolerated short-course treatments for hypnozoites could result in further cost savings for refugee resettlement programs. This study also suggests that presumptively treating future refugee populations prior to relocation in the United States will result in cost savings when the malaria prevalence exceeds 1%.
Acknowledgments
We thank the HCMC microbiology laboratory and Blain Mamo (Minnesota Department of Health) for their assistance; and Julie Boulware for graphic design.
Financial support: William M. Stauffner and David R. Boulware received support from National Institute of Allergy and Infectious Diseases/National Institutes of Health grant T32-AI055433.
REFERENCES
- 1.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 2.Skarbinski J, James EM, Causer LM, Barber AM, Mali S, Nguyen-Dinh P, Roberts JM, Parise ME, Slutsker L, Newman RD. Malaria surveillance–United States, 2004. MMWR Surveill Summ. 2006;55:23–37. [PubMed] [Google Scholar]
- 3.Eliades MJ, Shah S, Nguyen-Dinh P, Newman RD, Barber AM, Nguyen-Dinh P, Roberts JM, Mali S, Parise ME, Barber AM, Steketee R. Malaria surveillance–United States, 2003. MMWR Surveill Summ. 2005;54:25–39. [PubMed] [Google Scholar]
- 4.Maroushek SR, Aguilar EF, Stauffer W, Abd-Alla MD. Malaria among refugee children at arrival in the United States. Pediatr Infect Dis J. 2005;24:450–452. doi: 10.1097/01.inf.0000160948.22407.0d. [DOI] [PubMed] [Google Scholar]
- 5.Daggy RH, Muegge OJ, Riley WA. A preliminary survey of the anopheline mosquito fauna of southeastern Minnesota and adjacent Wisconsin areas. Minn Med. 1998;81:41–44. [Google Scholar]
- 6.Zucker J. Changing patterns of autochthonous malaria transmission in the United States: a review of recent outbreaks. Emerg Infect Dis. 1996;2:37–43. doi: 10.3201/eid0201.960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Filler SJ, Mac-Arthur JR, Parise M, Wirtz R, Eliades MJ, Dasilva A, Steketee R. Locally acquired mosquito-transmitted malaria: a guide for investigations in the United States epidemiological investigation. MMWR Morb Mortal Wkly Rep. 2006;55:1–9. [PubMed] [Google Scholar]
- 8.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Minnesota Department of Health. [Accessed October 24, 2006];Refugee Health Statistics. Available from http://www.health.state.mn.us/divs/idepc/refugee/stats/index.html.
- 10.Seys SA, Bender JB. The changing epidemiology of malaria in Minnesota. Emerg Infect Dis. 2001;7:993–995. doi: 10.3201/eid0706.010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Organization for Migration; Migrant Health Department; United States Refugee Program; Medical Program for Sub-Saharan Africa. October 1, 2003–September 30, 2004, Fiscal Year 2004 Report, and October 1, 2001–June 2002, Fiscal Year 2002 Report. Pre-Departure Anti-Malarial and Anti-Intestinal Parasitosis Treatment Report
- 12.Centers for Medicare and Medicaid Services. [Accessed March 7, 2007];100% MEDPAR Inpatient Hospital National Data for Fiscal Year 2005. Available from http://www.cms.hhs.gov/MedicareFeeforSvcPartsAB/03_MEDPAR.asp.
- 13.Collins WE, Jeffery GM. A retrospective examination of sporozoite-induced and trophozoite-induced infections with Plasmodium ovale: development of parasitologic and clinical immunity during primary infection. Am J Trop Med Hyg. 2002;66:492–502. doi: 10.4269/ajtmh.2002.66.492. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. [Accessed November 6, 2006];Procurement of Artemether-Lumefantrine (Coartem®) through WHO. Available from http://www.emro.who.int/rbm/background%20documents/egy04/CoA.pdf.
- 15.Arrow KJ, Gelband H, Jamison DT. Making antimalarial agents available in Africa. N Engl J Med. 2005;352:333–335. doi: 10.1056/NEJMp058168. [DOI] [PubMed] [Google Scholar]
- 16.Arrow KJ, Panosian C, Gelband H. Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 17.Pan American Health Organization. [Accessed October 24, 2006];Access to Antimalarial Medicines: Improving the Affordability and Financing of Artemisinin-Based Combination Therapies. Available from http://www.paho.org/English/AD/DPC/CD/mal-afford.htm.
- 18.Wiseman V, Kim M, Mutabingwa T, Whitty CJ. Cost-effectiveness study of three antimalarial drug combinations in Tanzania. PLoS Med. 2006;3:e373. doi: 10.1371/journal.pmed.0030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanda P, Masiye F, Chitah B, Sipilanyambe N, Hawela M, Banda P, Okorosobo T. A cost-effectiveness analysis of artemether lumefantrine for treatment of uncomplicated malaria in Zambia. Malar J. 2007;6:21. doi: 10.1186/1475-2875-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Geneva: World Health Organization; Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated falciparum Malaria. 2003
- 21.Piola P, Fogg C, Bajunirwe F, Biraro S, Grabdesso F, Ruzagira E, BAbigumira J, Kigozi I, Kiguli J, Kyomuhendo J, Ferradini L, Taylor W, Checchi F, Guthmann JP. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet. 2005;365:1467–1473. doi: 10.1016/S0140-6736(05)66416-1. [DOI] [PubMed] [Google Scholar]
- 22.Chanda P, Hawela M, Kango M, Sipilanyambe N. Assessment of the therapeutic efficacy of a paediatric formulation of artemether-lumefantrine (Coartesiane) for the treatment of uncomplicated Plasmodium falciparum in children in Zambia. Malar J. 2006;5:75. doi: 10.1186/1475-2875-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bukirwa H, Yeka A, Kamya MR, Talisuna A, Banek K, Bakyaita N, Rwakimari JB, Rosenthal PJ, Wabwire-Mangen F, Dorsey G, Staedke SG. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin Trials. 2006;1:e7. doi: 10.1371/journal.pctr.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jima D, Tesfaye G, Medhin A, Kebede A, Argaw D, Babaniyi O. Safety and efficacy of artemether-lumefantrine in the treatment of uncomplicated falciparum malaria in Ethiopia. East Afr Med J. 2005;82:387–390. doi: 10.4314/eamj.v82i8.9321. [DOI] [PubMed] [Google Scholar]
- 25.Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, Greenwood BM, Whitty CJ. Amodiaquine alone, amodiaquine + sulfadoxine-pyrimethamine, amodiaquine + artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four arm randomized effectiveness trial. Lancet. 2005;365:1474–1480. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- 26.White NJ. Malaria. In: Cook GC, Zumla AL, editors. Manson’s Tropical Diseases. 21st edition. London: W. B. Saunders; 2003. pp. 1206–1210. [Google Scholar]
- 27.Stauffer WM, Newberry AM, Cartwright CP, Rosenblatt JE, Hanson K, Sloan L, Tsukayama D, Taylor C, Juni B. Evaluation of malaria screening in newly arrived refugees to the United States by microscopy and rapid antigen capture enzyme assay (Binax-Now™) Pediatr Infect Dis J. 2006;25:948–950. doi: 10.1097/01.inf.0000235747.28644.6f. [DOI] [PubMed] [Google Scholar]
- 28.Kain KC, Harrington MA, Tennyson S, Keystone JS. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis. 1998;27:142–149. doi: 10.1086/514616. [DOI] [PubMed] [Google Scholar]
- 29.Office of Refugee Resettlement Annual Reports to Congress 1997–2004. [Accessed December 11, 2006]; Available from http://www.acf.hhs.gov/programs/orr/policy.
- 30.Holtz TH, Kachur SP, MacArthur JR, Roberts JM, Barber AM, Steketee RW, Parise ME. Malaria surveillance–United States, 1998. MMWR CDC Surveill Summ. 2001;50:1–20. [PubMed] [Google Scholar]