Abstract
We have shown that five days of mild hypoxia has significant effects on fetal ECoG activity, heart rate and blood pressure. We now studied if mild prolonged hypoxemia had an adverse effect on the fetal cardiovascular and neural responses to repeated cord occlusion and on the magnitude of neuronal damage. Fetal and maternal catheters were placed at 120 days’ gestation and animals allocated at random to receive intratracheal maternal administration of nitrogen (n=8) or compressed air in controls (n=7). Five days after surgery, nitrogen infusion was adjusted to reduce fetal brachial artery pO2 by 25%. After 5 days of chronic hypoxemia the umbilical cord was completely occluded for 5 minutes every 30 minutes for a total of four occlusions. Data are presented as mean ± SEM and were analyzed by Two Way ANOVA or two sample t test. Nitrogen infusion decreased fetal pO2 by 26% (20.5±1.7 vs. 14.3±0.8 mmHg) without changing fetal pCO2 or pH. Pre-existing hypoxia fetuses had a greater terminal fall in heart rate in occlusions II, III and IV, and also had a more severe terminal hypotension in the final occlusion. Pre-existing hypoxia was associated with a greater fall in spectral edge frequency during occlussions from 14.4±0.9 Hz to 6.9±0.4 Hz vs. 13.6±1.64 Hz to 10.6±0.77 Hz in controls, p<0.05. In addition, during the three-day post-occlusion period the contribution of theta and alpha band frequencies to total ECoG activity was significantly lower in the pre-existing hypoxia fetuses (p<0.05). These effects were associated with increased neuronal loss in the striatum (p<0.05). In summary, the cardiovascular and neural response indicate a detrimental effect of pre-existing mild hypoxia on fetal outcome following repeated umbilical cord occlusions.
INTRODUCTION
Hypoxic-ischemic brain injury is the most important cause of neurological morbidity and mortality in preterm and term infants. Perinatal asphyxia remains a significant cause of infant death and impaired neurodevelopmental outcome and it is considered to be the cause of at least 20% of cerebral palsy cases (Patel and Edwards, 1997). Placental insufficiency and intrauterine growth restriction are chronic hypoxic states of many different etiologies which are commonly associated with increased neurological morbidity and mortality (Rees et al., 1998; Uvebrant and Hagberg, 1992; Williams and O’Brien, 1997). Selective neuronal loss is one of the most common brain injuries associated with hypoxic-ischemic insults in the term fetus. This type of brain damage is characterized by neuronal loss in discrete anatomic regions of the brain (Mallard et al., 1992; Mallard et al., 1995a; Martin et al., 1997). The areas most commonly affected are the sensory-motor cortex, hippocampus, striatum, and cerebellum. However, there is paucity of data regarding the effects of mild chronic hypoxia on the fetal brain response to a subsequent acute hypoxic-ischemic episode or the mechanisms that underlie the increased risk for impaired neuronal functional status. The functional impact of severe hypoxia/ischemia on neuronal function has been assessed by many authors using different approaches such as raw electrocorticogram (ECoG) signals (Gunn et al., 1991; Richardson et al., 1985), spectral analysis (Williams et al., 1992), cortical impedance (De Haan et al., 1997), sensory and auditory evoked potentials (Gunn et al., 1991; Richardson et al., 1992). Also important is the effect hypoxia has on cardiovascular performance. Neuronal loss following a severe acute hypoxic ischemic insult is dependent to a great extent on cardiovascular stability. The more severe the hypotension the greater the number of neurons lost (Mallard et al., 1995b). Similarly, a recent report shows that spontaneously hypoxic term fetuses submitted to brief repeated cord occlusions develop a more rapid cardiovascular deterioration (Westgate et al., 2005).
We have previously shown that five days of mild hypoxia has significant effects on Type I NOS gene and protein expression in fetal brain (Pryor et al., 2002), fetal ECoG activity, blood pressure and heart rate (Pulgar et al., 2006). Therefore, the aim of this study was to determine the effects of mild chronic hypoxia on the fetal neural and cardiovascular responses to a superimposed acute hypoxic-ischemic episode.
RESULTS
Animal preparation
Fetal weight did not differ significantly between the control and pre-existing hypoxia animals at the time of necropsy (3.8 kg ± 0.34 vs. 3.6 ± 0.17 kg, p>0.05). The effects of 5 days mild hypoxemia on fetal metabolic and cardiovascular responses have been reported elsewhere (Pulgar et al., 2006). Briefly, on the day previous to the cord occlusion (Day -1) pre-hypoxic fetuses displayed increased heart rate (171±5 vs. 158±3 bpm, p<0.05) and arterial blood pressure (55±2 vs. 49±2 mmHg, p<0.05). With the exception of fetal arterial pO2, hemoglobin saturation and oxygen content (Table 1, p<0.05) all other arterial blood gas variables were not different in pre-existing hypoxia animals compared to control animals. Maternal arterial oxygen content in control animals was 13.1 ± 0.81 ml/dL compared to 10.8 ± 0.52 ml/dL in pre-existing hypoxia animals (p<0.05). During the three post-occlusion days, maternal pH and pCO2 remained stable in both groups (pre-existing hypoxia Day-1 7.48±0.01 and 39.4±0.9 mm Hg; average post-occlusion days 7.46±0.01 and 35.6±0.7 mmHg; control Day -1 7.49±0.01 and 36.7±0.7 mmHg; average post-occlusion days 7.48±0.01 and 37.1±0.6 mmHg). Table 1 shows fetal arterial blood gases, pH, lactate and oxygen content throughout the occlusion experiment and at 24 (Day +1) and 72 (Day+3) hours after occlusions. During the occlusion period a significant decrease in arterial pO2, hemoglobin saturation and O2 content was observed in both groups. The pre-existing hypoxia group had significantly lower pH and higher base deficit (BD) values in occlusions III and IV (Table 1; p<0.05). After the occlusion period all blood gas and metabolic variables returned to pre-occlusion values in both groups. The 25% reduction in fetal arterial pO2 was maintained throughout the three-days post occlusion period. During the occlusion and on Day 1 and 3 after the occlusion, plasma lactate concentration was not different between groups (Table 1).
Table 1.
Fetal arterial blood gases, pH, oxygen content and lactate levels in control (A) and pre-hypoxic (B) fetuses during the period of repeated complete cord occlusion and 24 (Day +1) and 72 h (Day +2) after the last occlusion.
| A | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Occ I | Pre | Occ II | Pre | Occ III | Pre | Occ IV | Day +1 | Day +3 | |
| PH | 7.35±0.01 | 7.11±0.04 | 7.26±0.01 | 7.03±0.03 | 7.23±0.01 | 7.02±0.04 | 7.21±0.02 | 7.05±0.05 | 7.35±0.01 | 7.34±0.01 |
| PCO2 mmHg | 55±1 | 90±8 | 55±1 | 98±6 | 55±1 | 94±6 | 55±1 | 86±8 | 55±1 | 55±1 |
| PO2 mmHg | 22±2 | 7±2 | 21±1 | 7±1 | 22±2 | 7±1 | 23±2 | 8±2 | 20±1 | 19±1 |
| SatHb % | 53±4 | 16±7 | 51±3 | 10±1 | 52±3 | 11±2 | 51±4 | 15±4 | 50±3 | 49±3 |
| O2 ct mL/dL | 7.7±1 | 2.6±1 | 7.4±0.7 | 2.9±1.1 | 7.6±0.8 | 2.9±1 | 7.4±0.8 | 2.5±0.9 | 6.9±1 | 7.4±0.8 |
| BD mEq/L | −3.3±0.6 | 5.7±1 | 3.8±0.7 | 10.2±1.4 | 4.8±0.4 | 11.3±0.9 | 6.5±1 | 11±1.7 | −3.3±0.2 | −2±0.4 |
| [Lactate] mM | 1.4±0.2 | 3.7±0.7 | nd | 4.8±1 | nd | 5±1 | nd | 5.1±1 | 2.1±0.5 | 2±0.4 |
|
| ||||||||||
| B | ||||||||||
| PH | 7.36±0.00 | 7.07±0.05 | 7.27±0.02 | 6.97±0.02* | 7.22±0.01 | 6.96±0.02* | 7.19±0.01 | 6.95±0.02* | 7.36±0.01 | 7.35±0.01 |
| PCO2 mmHg | 53±2 | 98±8 | 55±3 | 105±4 | 54±3 | 97±3 | 54±3 | 94±4 | 53±1 | 52±1 |
| PO2 mmHg | 14±1* | 5±1 | 16±1* | 5±1 | 17±1* | 6±1 | 17±1* | 5±1 | 16±1* | 15±1* |
| SatHb % | 34±3* | 11±2 | 33±5* | 9±1 | 34±5* | 10±1 | 36±4* | 10±1 | 40±3* | 37±2* |
| O2 ct mL/dL | 5.1±0.3* | 1.7±0.4 | 5±0.6* | 3.5±1.8 | 4.6±0.6* | 1.5±0.1 | 5.4±0.6* | 1.6±0.1 | 5.1±0.5* | 5.7±0.5* |
| BD mEq/L | −2.4±0.4 | 7.2±1.8 | 2.8±1.1 | 13.8±1.1 | 7.2±0.5 | 16.2±0.3* | 9±0.5 | 16.8±0.7* | −2.7±0.7 | −2±0.5 |
| [Lactate] mM | 1.7±0.2 | 3.7±0.5 | nd | 4.9±0.7 | nd | 6.3±0.5 | nd | 5.5±0.5 | 1.7±0.4 | 1.7±0.4 |
p<0.05 when compared to control group; nd: not measured.
Acute hemodynamic responses to repeated cord occlusion
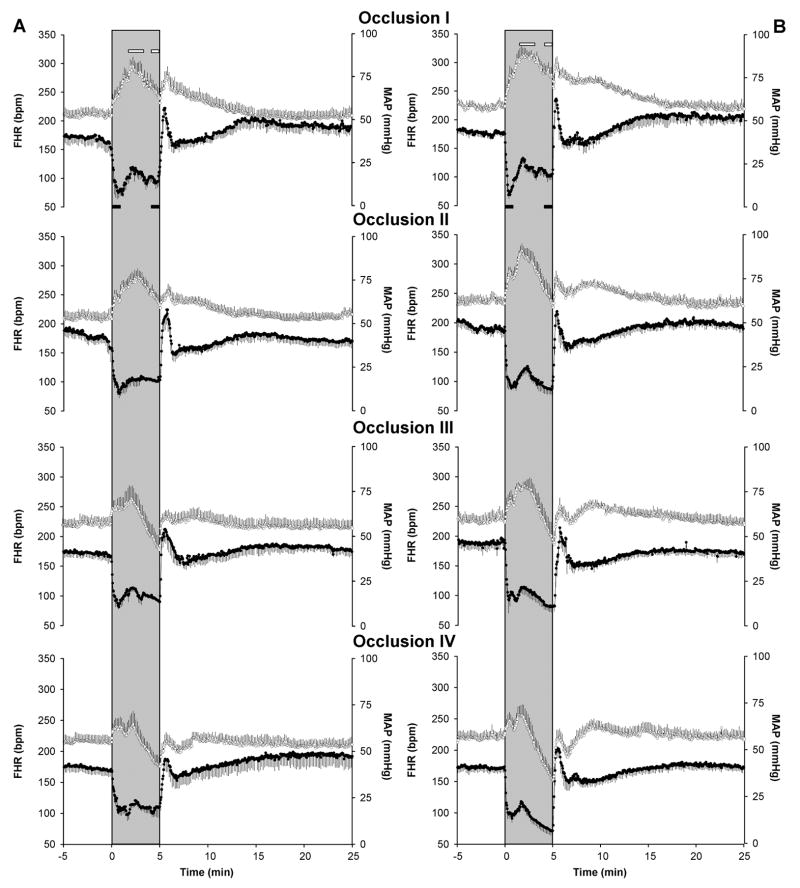
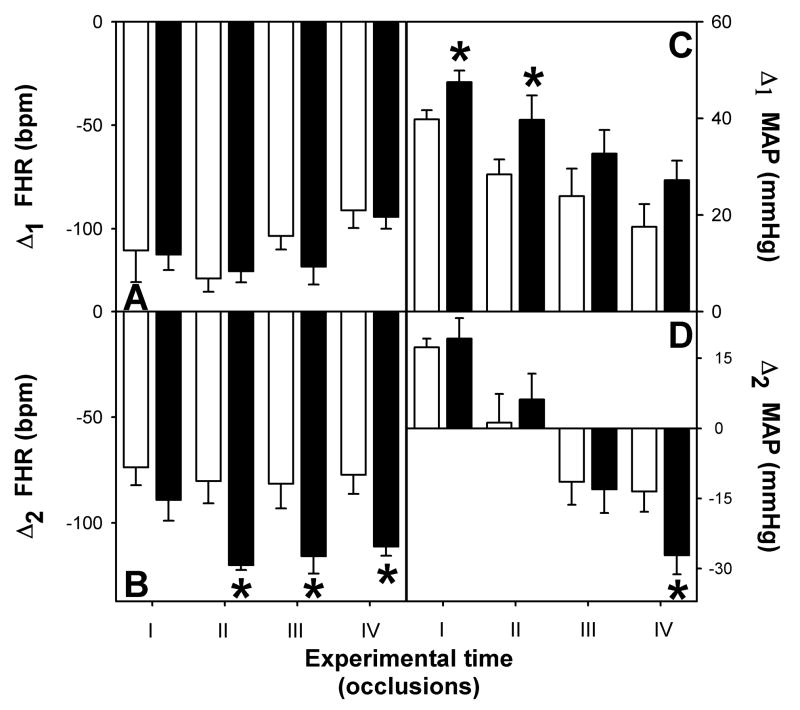
As shown in Figure 1, complete umbilical cord occlusion induced a pronounced bradycardia and hypertension in both control (A) and pre-existing hypoxia (B) fetuses. During the first minute of the five-minute cord occlusion, the magnitude of the decrease in fetal heart (Δ1 FHR) was not affected by the preexistent hypoxia (Figure 2, Panel A). However, during the last minute of the cord occlusion (Δ2 FHR), pre-existing hypoxia fetuses had a greater fall in heart rate in the last three occlusions (Figure 2, Panel B; p<0.05). The changes in arterial blood pressure were also analyzed at specific points during occlusions. As shown in Figure 1, there was an initial hypertensive response, that peaked in both groups between 1.5 and 3 minutes, which attenuated with each subsequent occlusion (Δ1 MAP). In the first two occlusions, fetal arterial blood pressure remained elevated throughout the occlusion, whereas in the last two occlusions it fell below pre-occlusion levels during the last minute of occlusion (Δ2 MAP). Peak fetal arterial blood pressure was greater in the pre-existing hypoxia group in the first two occlusions (Figure 2, Panel C; p<0.05). In both groups, values for Δ2 MAP were negative on the last two occlusions. However, while the decrease in arterial blood pressure was of approximately 15 mmHg in control fetuses it fell by 30 mmHg in the pre-existing hypoxia group (Figure 2, Panel D; p<0.05).
Figure 1.
Effect of repeated total umbilical cord occlusions on fetal heart rate (FHR;●) and mean arterial blood pressure (MAP;○) in fetal sheep. Four five-min occlusions (I, II, III, IV) in control (A, n=7) and pre-existing hypoxia group (B, n=8). Shaded area represents the occlusion period, the periods where maxima and minima values were determined for MAP (open bars) and for FHR (solid bars) are also depicted.
Figure 2.
Magnitude of the fetal heart rate changes during the first minute (A) and during the last minute (B) and magnitude of the arterial blood pressure changes during the hypertensive phase (C) and during the last minute (D) of umbilical cord occlusion. Control (□, n=7) and pre-existing hypoxia (■, n=8) group. Values are mean±SEM, * p<0.05.
Hemodynamic status following repeated cord occlusion
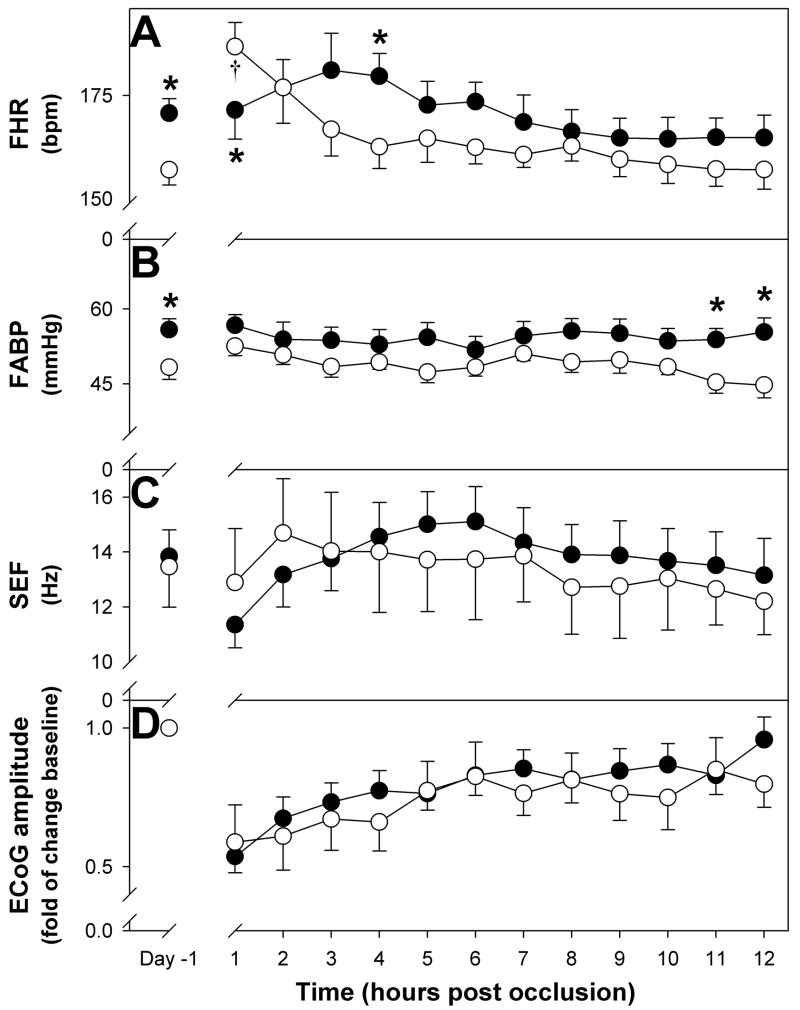
During the first twelve hours post-occlusion fetal heart rate remained unchanged in the pre-hypoxic group, in contrast in the control group there was a significant tachycardia during the first hour (Figure 3A; p<0.05). During this 12-hour-period arterial blood pressure remained unchanged in both groups (Figure 3B). For these two variables umbilical cord occlusion did not modify the effect of pre-existing hypoxia thus both fetal heart rate and arterial blood pressure were significantly higher in the pre-hypoxic group (FHR, F=8.2, p<0.01; ABP, F=30.7, p<0.001).
Figure 3.
Fetal responses during the first twelve hours after umbilical cord occlusions. Changes in fetal heart rate (FHR), arterial blood pressure (FABP), spectral edge frequency (SEF) and ECoG amplitude are shown. † p<0.05 vs Day -1; * p<0.05 vs control.
Long-term hemodynamic effects of repeated cord occlusion
Throughout the three days post-occlusion, FHR and arterial blood pressure showed no differences compared to pre-occlusion values in each group. As a consequence the effect of preexisting hypoxia on these variables was not modified thus both fetal heart rate and arterial blood pressure were significantly higher in the pre-hypoxic group (FHR pre-hypoxic 169±3.8 bpm; control 161±2.1 bpm, F=6.7, p=0.012; ABP pre-hypoxic 55.3±2.3 mmHg; control 48.5±1.6 mmHg, F=19.3, p<0.001).
Acute ECoG responses to repeated cord occlusion
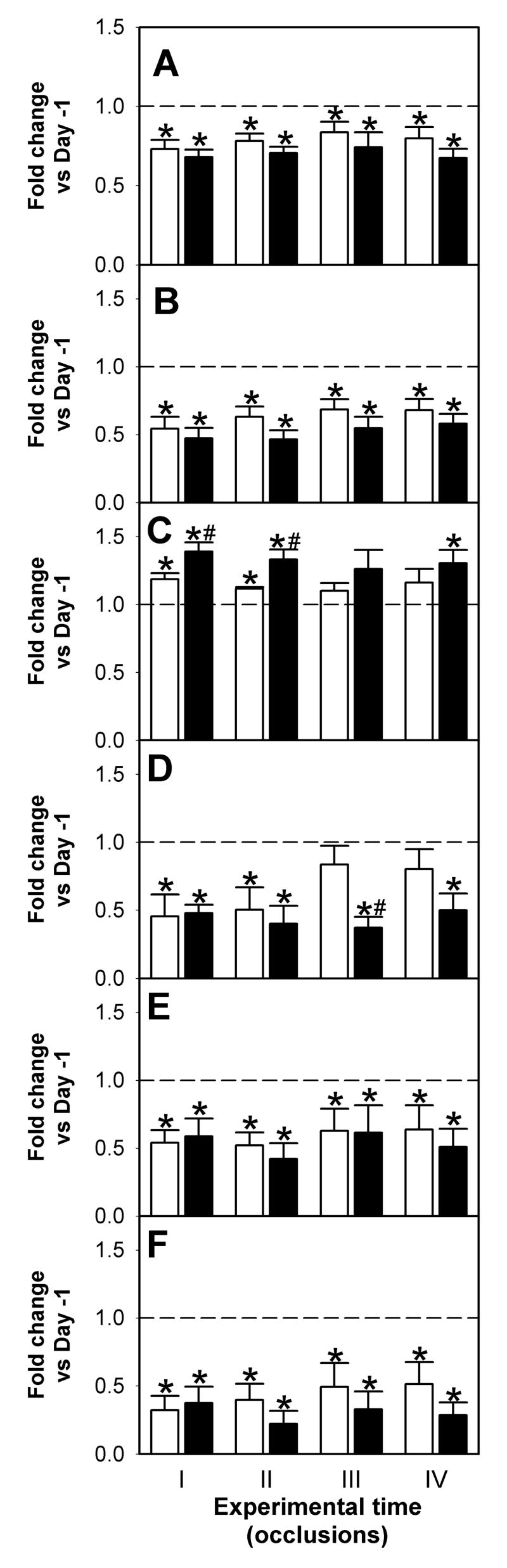
We have previously reported that chronic hypoxemia alters fetal ECoG sleep state by increasing the time spent in low voltage (LV) state and the spectral edge frequency of this state. The increase in SEF is consistent with a decrease in the contribution of delta band and an increase in the contribution of beta1 activities to the total ECoG power. No differences were observed in total EcoG amplitude or the contribution of the different frequencies in high voltage (HV) state (Pulgar et al., 2006). During umbilical cord occlusions, total ECoG amplitude was significantly reduced in both groups (pre-hypoxic 44±9; control 54±1 % of previous day, p<0.05) with no differences between groups. The analysis of the average effect across all four occlusions showed a decrease in the spectral edge frequency of the ECoG in both groups; with the decrease in the pre-hypoxic group being significantly larger (pre-hypoxic; 14.4±0.9 Hz vs. 6.9±0.4 Hz; control 13.6±1.64 Hz vs. 10.6±0.77 Hz, p<0.05). Also, the contribution of delta band activity to total power, expressed as fold change of the previous day, was significantly higher in the pre-hypoxic group (1.3±0.05 vs. 1.1±0.04, p<0.05). Similarly, the reduction in the contribution of theta band activity was more pronounced in the pre-hypoxic group (0.4±0.05 vs. 0.67±0.1, p<0.05). There were no differences in the contribution of alpha and beta1 band activities during occlusions (0.53±0.07 vs. 0.58±0.06 and 0.3±0.05 vs. 0.4±0.07 respectively, p>0.05). When the effect of each occlusion was analyzed individually we observed the median frequency and SEF values were significantly lower than Day -1 (Figure 4A and B; p<0.05) in both groups during the four occlusions. The contribution of delta band frequencies to the total power increased compared to pre-occlusion values, whereas the contribution of faster frequencies decreased. While in occlusions I and II a significant increase in the contribution of delta band frequencies to total ECoG power was evident in both groups, the increase in the pre-hypoxic group was significantly higher (Figure 4C; pre-hypoxic vs. control, F= 7.021, p<0.02). The contribution of theta band (4–8 Hz) frequencies was diminished in the pre-hypoxic group during the four occlusions (Figure 4D; pre-hypoxic vs. control, F = 6.19, p<0.02). The contribution of frequency bands greater than 8 Hz (alpha and beta1) was lower in both groups (Figure 4E and F; p<0.05), however, no treatment effect was observed.
Figure 4.
Effects of four 5-min umbilical cord occlusions on ECoG spectral frequencies. Changes in median frequency (A), spectral edge frequency (SEF, B) and frequency bands (Delta, C; Theta, D; Alpha, E and Beta1, F)relative to pre-occlusion values (Day -1; dotted line) in control (□, n=5) and pre-hypoxic (■, n=6) fetuses. * p<0.05 vs Day -1; # p<0.05 vs control.
ECoG activity following repeated cord occlusion
During the first twelve hours post-occlusion, SEF and ECoG amplitude were not statistically different between groups (Figure 3C and D). Similarly, no statistically significant differences were observed during the three-day post occlusion period in SEF (pre-hypoxic 12.8±1.2 Hz; control 13.4±1.4 Hz), the time spent in HV (pre-hypoxic 40±1%; control 43±2%) and LV state (pre-hypoxic 50±1%; control 48±2%) and ECoG amplitude (data not shown). There were, however, differences in the contribution of the different frequencies to total ECoG power between the two groups. As shown on Figure 5, the analysis of total ECoG showed a significantly lower contribution of theta band (Panel A) to the total power in pre-hypoxic fetuses throughout the three-day post-occlusion period (F=16.89, p<0.001). The contribution of alpha band (Panel B) was lower in the pre-hypoxic group during the three days (F=5.063, p<0.05). The contribution of the different frequencies to the HV state was not different between groups (data not shown). In contrast, during LV state the contribution of alpha bands in the pre-hypoxic group was lower than the control group (Panel D, F= 7.795, p<0.05).
Figure 5.
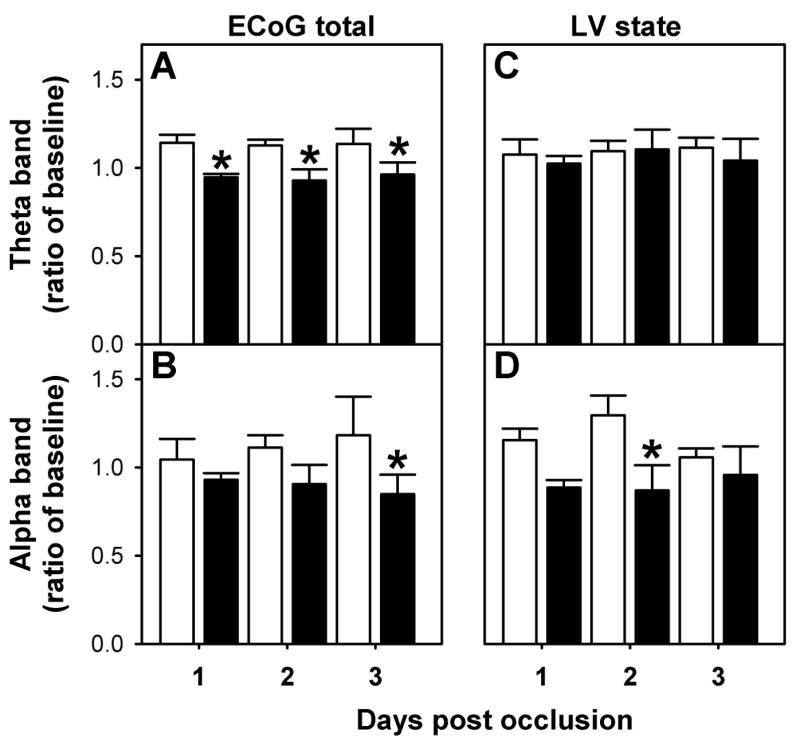
Power Spectrum Analysis of fetal ECoG in the days following the occlusion period. Relative spectral power of Theta (A) and Alpha (B) bands for total ECoG and in the low voltage state Theta (C) and Alpha (D). Control (□, n=5) and pre-existing hypoxia (■, n=6) group, *p<0.05 by ANOVA.
Neuronal survival following repeated cord occlusion
Dying neurons were identified in lateral cortex and striatum in control and pre-existing hypoxia animals using Fluoro-Jade B staining. The proportion of positively stained cells ranged between 10–19% in cortex and 9–44% in striatum. While the number of Fluoro-Jade B positive cells indexed to the number of DAPI stained cell nuclei (total number of cells) was the same for both groups in the cortex (Table 2), a statistically significant increase in the number of dying cells was observed in the striatum of pre-existing hypoxia animals (Figure 6 and Table 2, p<0.05).
Table 2.
Number of cells stained with Fluoro Jade B (FJ-B) and number of cell nuclei stained with DAPI in fetal sheep basal ganglia and lateral cortex three days after repeated cord occlusion.
| Control | Pre-existing hypoxia | |||||||
|---|---|---|---|---|---|---|---|---|
| FJ-B | DAPI | FJ-B/DAPI | n | FJ-B | DAPI | FJ-B/DAPI | n | |
| Basal Ganglia | 32±6 | 221±38 | 0.16±0.02 | 5 | 71±15 | 243±13 | 0.29±0.05* | 5 |
| Cortex | 36±4 | 263±14 | 0.14±0.01 | 5 | 37±4 | 285±16 | 0.15±0.02 | 5 |
p<0.05 pre-existing hypoxia vs control.
Figure 6.
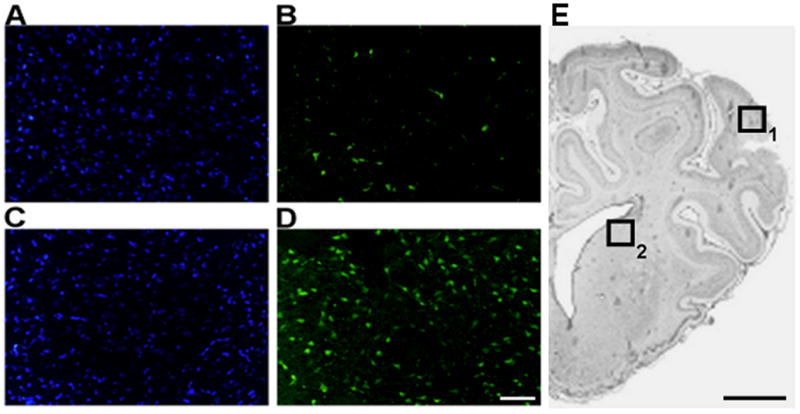
Representative microphotographs of DAPI (A, B) and Fluoro Jade-B (C, D) staining in basal ganglia sections of control (A, C) and pre-existing hypoxia (B, D) fetuses brain. Bar = 50 μm. Low resolution microphotograph showing the areas (boxes) used to measure cortical (1) and striatal (2) neuronal damage (E). Bar= 5 mm
DISCUSSION
The main finding of the present study is that prolonged mild hypoxemia has an adverse effect on the cardiovascular and neural responses to repeated umbilical cord occlusion and subsequent neuronal loss in chronically instrumented late gestation fetal sheep. Fetuses with pre-existing mild hypoxia had a greater terminal fall in heart rate in occlusions II, III and IV, and a greater terminal hypotension in occlusion IV. Pre-existing hypoxia also enhanced the depression of the fetal ECoG during and after the occlusions with a greater fall in the spectral edge frequency during occlusions and a lower contribution of theta and alpha bands to the total power in the after occlusion period. These effects were associated with an enhanced neuronal loss in the striatum.
The magnitude of the reduction in fetal oxygenation during the pre-existing hypoxia period was selected to ensure that there was no associated fetal acidosis. Consistent with our previous observations (Pryor et al., 2002; Pulgar et al., 2006), while fetal plasma pH and lactate levels were not statistically different between groups, a significant tachycardia throughout the entire 5 days experimental period and an elevation in arterial blood pressure during the last two days of hypoxemia were observed in pre-existing hypoxia fetuses (Pulgar et al., 2006).
We applied a focused approach to the second-to-second analysis of the complex temporal profile of the fetal cardiovascular responses (Giussani et al., 1997; Matthews et al., 1990). During the first minute of occlusion the expected bradycardic response was present in both groups and no differences in the magnitude of the fall in heart rate were observed. This initial bradycardia is thought to represent a chemoreceptor response that is vagally mediated (Bartelds et al., 1993). Consistent with our data, in chronically hypoxic high altitude-exposed fetal sheep with a comparable reduction in fetal arterial pO2 and without acidosis, the bradycardic response to umbilical cord occlusion was shown not to be affected (Imamura et al., 2004). As reported by others for partial cord occlusions (Giussani et al., 1997), we observed that following the initial bradycardia fetal heart rate stabilized or even increased slightly only to fall again during the last minute of occlusion. This transient increase in heart rate parallels the increase in arterial pressure and thus may also be the result of increased sympathetic drive (Lewis et al., 1984). In contrast to the bradycardia of the first minute, fetal heart rate in pre-existing hypoxia fetuses was significantly lower than in normoxic fetuses during the last minute only in the last three occlusions. The mechanism of this late fall is less clear. Among the possible mechanisms are recruitment of other peripheral chemoreceptor input (Bartelds et al., 1993), a synergistic effect of severe hypercarbia on carotid chemoreceptors (Blanco et al., 1984), a direct effect of ischemia on cardiac pacemaker cells (Gryshchenko et al., 2002), and an activation of the cardioinhibitory Bezold-Jarisch reflex (Aviado and Guevara, 2001; Dawes and Comroe, Jr., 1954). This latter reflex originates from the stimulation of both mechanically and chemically sensitive receptors in the ventricle which can be elicited by coronary ischemia and asphyxia (Thoren, 1972).
The initial hypertensive response (Δ1 MAP) peaked at the same time in both groups, but it was greater in the pre-existing hypoxia group. The enhanced blood pressure observed in the pre-existing hypoxia during the last two days of the five-day hypoxia period most likely reflects an increase in circulating catecholamines (Kitanaka et al., 1989). An elevation in plasma catecholamines elicited by chemoreceptor mechanisms is also thought to be responsible for the initial hypertension (Boekkooi et al., 1992; Cohn et al., 1974; Giussani et al., 1997). Thus, we suggest that it is possible that the difference in the initial blood pressure between control and pre-existing hypoxia group is determined by enhanced circulating catecholamines. During the last two occlusions terminal fetal arterial blood pressure fell below pre-occlusion levels (Δ2 MAP) in both groups. However, in the last occlusion pre-existing hypoxia fetuses had a much more severe hypotension. The mechanism for the late hypotension is commonly thought to represent the effect of hypoxia on myocardial contractility. Severe hypoxia is associated with glycogen and ATP depletion and the degree of depletion has been shown to correlate with poor cardiovascular performance (Hokegard et al., 1981). In the presence of severe acidosis reversible subendocardial injury is a major contributor to poor cardiac performance (Gunn et al., 2000).
Consistent with previous reports (Mallard et al., 1995b), repeated cord occlusion had profound effects on ECoG activity in the fetal sheep. Although the reduction in amplitude of the electrical signal observed during occlusions was similar between groups, analysis of the frequency components reveled a more pronounced reduction of the spectral edge frequency (SEF) in the hypoxic group. The decrease in SEF during the occlusion period can be accounted for by an increase in the proportion of frequencies in the delta band (1–4 Hz) and a concomitant decrease in the proportion of frequencies greater than 4 Hz in both groups. The increased contribution of delta band in the pre-existing hypoxia group during occlusions suggests that, in addition to the effects on cardiovascular performance, mild hypoxemia sensitizes the fetal brain to a subsequent asphyctic insult. We also observed a significant difference in the pattern of ECoG activity between the two groups in the days following the occlusion, with a lower proportion of theta and alpha band in the total ECoG signal of hypoxic fetuses. This pattern of reduced theta and alpha band contribution to total power was also observed in the low voltage state. Collectively, these observations indicate that chronic mild hypoxemia alters the brain response to a super-imposed acute hypoxic-ischemic insult. A clear relationship between the changes in ECoG and neuronal outcome has not been clearly established. The effect of asphyxia, induced by umbilical cord occlusion, on neuronal survival is dependent of the duration and frequency of the insult. In keeping with Mallard’s data (Mallard et al., 1995b), we also found that this paradigm of repeated cord occlusion results in greater neuronal damage in the striatum. These authors concluded that both the degree of hypotension and the increase in the ratio T/QRS have an impact on the extent of striatal neuronal loss. Although we used the relatively novel fluorescent indicator Fluoro Jade-B to evaluate neuronal death in cortex and striatum, the proportion of dead cells (30%) in our study is in the same range as previously reported (Mallard et al., 1995b).
In summary, we have found that mild chronic hypoxia sensitizes the fetal sheep to repeated umbilical cord occlusion and the effect is observed in the cardiovascular response, the electrocorticographic pattern and it is associated with a greater degree of neuronal loss. These recent findings together with our previous observations (Pryor et al., 2002) strongly suggest that preexisting mild hypoxemia has a detrimental effect on the fetal cardiovascular and neurological outcome following umbilical cord occlusion.
EXPERIMENTAL PROCEDURES
Animal preparation and postoperative care
All procedures for housing, handling, surgical implantation of catheters and postoperative management were approved by Wake Forest University’s Institutional Animal Care and Use Committee. Fifteen date-mated sheep at 118–120 days gestational age (term 144 ± 5 days, 0.83 gestation) were brought to the laboratory and placed in metabolism cages with free access to food and water. Twenty-four hours before surgery, food and water were withheld. Ewes were operated on under halothane general anesthesia (1.5% to 2.0% in 2L/min oxygen) as previously described (Pryor et al., 2002). Briefly, maternal vascular catheters (carotid artery, jugular vein, and femoral artery) and a tracheal catheter were inserted. Fetal catheters (femoral artery, brachial artery and amniotic) were placed together with stainless steel electrodes (N13-50F-360, New England Electric Wire Corp.; Lisbon, NH) through holes drilled over the parietal bone. A 24 mm vascular occluder was placed at the base of the umbilical cord. The volume (6ml) needed to produce a complete cord occlusion was confirmed to be adequate for each occluder during surgery. Catheters, occluder and electrodes were exteriorized through the flank of the ewe. Amnioinfusion was performed with 500cc normal saline immediately postoperatively to replenish amniotic fluid losses. All vascular catheters were infused with heparinized saline (4U/ml). Animals were allowed to eat and drink ad libitum.
Animal studies
All studies began at least four days after surgery in animals in which fetal arterial pO2, pH, pCO2, and bicarbonate were within normal range for our laboratory (21±0.7mmHg, 7.35±0.01, 52.3±0.8mmHg, 27.3±0.5 mEq/L respectively). The fetal ages at surgery were 120 ± 0.5 days for the control animals and 119± 0.7 days for the hypoxic group. Throughout the experiment, fetal and maternal hemodynamic variables as well as ECoG activity were continuously recorded. Maternal intratracheal administration of nitrogen gas (PGS45, purity-99.96%, National Welders, Charlotte, NC) was utilized to induce maternal hypoxemia and reduce fetal brachial artery pO2 by 25% (n=8). Compressed air (medical grade, National Welders, Charlotte, NC) was administered in the control animals (n=7). Maternal and fetal pCO2 were maintained at normal levels by using intratracheal infusion of CO2 [0.5–1 L/min, purity 99.5%, National Welders, Charlotte, NC] (Pryor et al., 2002; Pulgar et al., 2006).
After 5 days of hypoxic (pre-existing hypoxia group) or normoxic (control group) conditions, four 5-minute occlusions (1 every 30 minutes) were applied to both groups. In the case of twins, cord occlusion was performed just in one fetus. Blood samples were obtained during the last minute of the occlusion (blood draw started four minutes into the occlusion). Three days after the occlusion fetuses were delivered by cesarean section and euthanized by exanguination under halothane general anesthesia. The brain was bisected through the midline and the left hemisphere placed in 4% buffered paraformaldehyde for 24 hours at 4°C. The fixed tissue was cryopreserved by sequential sucrose dehydration (10–30%) over three days and stored at −80°C embedded in OCT compound (Sakura Tissue-Tek. San Marcos, CA).
Data acquisition
Maternal and fetal blood pressure were recorded using solid state pressure transducers (World Precision Instruments, Sarasota, FL) connected to preamplifiers of a custom-built data acquisition system (CWE Inc, Ardmore, PA). Biophysical signals were analog-filtered and sampled at 150 Hz before processed by the 12 bit A/D converter. Blood pressure signals were low-pass filtered at 50 Hz and the fetal ECoG signal was band-pass filtered between 1–50 Hz.
Pressure recorded from a fluid-filled catheter tied to the back of the animal and from the amniotic catheter was electronically subtracted from the maternal and fetal arterial pressure catheters to remove differences determined by the sheep changing position and uterine contraction respectively.
Data analysis
Data reduction for the cardiovascular variables was performed by generating 1-second averages of systolic, diastolic and mean arterial blood pressure (MAP) and heart rate (FHR) of the 5-minute period that preceded each occlusion, the 5 minute occlusion and the 25-minute period following each occlusion using custom-written software (Cruncher, Instrument Concepts Inc. Great Village, Nova Scotia). For clarity of presentation, Figure 1 displays 3-second averages. Data were analyzed according to (Matthews et al., 1990) as described by (Giussani et al., 1997). For each animal, we derived the minimum FHR for the first and last minute of each occlusion and we also obtained the MAP value during the hypertensive phase and during the hypotensive phase for each occlusion. MAP maxima were determined in the time interval between 1.5 and 3 min and MAP minima determined during the last minute within the 5-min occlusion period shown by the open bars in Figure 1. FHR minima were determined in the first and last minute of occlusion (solid bars in Figure 1). These values were compared to the 5 min previous to each occlusion (baseline) and are expressed as deltas: Δ1MAP=highest MAP between 1.5 and 3 minutes - baseline; Δ2MAP= lowest MAP at minute 5 - baseline; Δ1FHR=lowest FHR at minute 1- baseline; Δ2FHR= lowest FHR at minute 5 - baseline.
For the ECoG signal, the program (Cruncher, Instruments Concepts Inc., Great Village, Nova Scotia) identifies epochs of high (HV) or low voltage (LV) and performs power spectral analysis using the Fast Fourier Transformation for LV and HV epochs. The software provides the total spectral power between 1–30 Hz and power for 1–4 Hz (delta), 4–8 Hz (theta), 8–13 Hz (alpha), 13–20 Hz (beta1) bands, the spectral edge frequency (frequency below which 95% of the spectral power resides) and the median frequency. The software also provides the total electrical activity present in a particular analysis period (Pulgar et al., 2006). For each occlusion and for the days post occlusion, we analyzed the total ECoG activity and the frequency components of the ECoG by comparing the average over each particular interval, i.e., 5-min or 20 hours, to the values of the average of a 20-hour period of the pre-occlusion day. Power spectrum analysis was performed on the total ECoG signal and also for HV and LV states. Unfortunately, a complete series of ECoG data for the entire experiment was available only for 5 animals in control and 6 animals in the pre-hypoxic group.
Differentiation of HV and LV states
The software uses a threshold derived from the digitized ECoG signal to segregate the electrical signal into periods of high voltage and low voltage. The signal is full wave-rectified, smoothed at 0.01 Hz and the threshold applied. Once the software has determined the presence of a particular event type (HV or LV), it calculates the FFT frequency power spectrum using the raw data. The threshold level was defined for each animal on the first day of analysis and applied to the entire analysis period (Pulgar et al., 2006).
Fluoro Jade B staining
Fluoro Jade B, a relatively specific indicator of neuronal damage (Rocha et al., 2004; Schmued and Hopkins, 2000), was used to quantify neuronal damage in basal ganglia, the most affected brain region for this paradigm of cord occlusion (Mallard et al., 1995b) and lateral cortex to complement our previous work on Type I NOS expression in these regions following identical hypoxic conditions (Pryor et al., 2002). Fixed-frozen tissues were sectioned and equivalent sections were identified following the sheep stereotaxic atlas (Gluckman and Parsons, 1983). 10 μm-sections were placed on gelatin-coated glass slides were sequentially immersed in 80% ethanol/1% NaOH in (5 min), 70% ethanol (2 min), H2O (2 min), 0.06% KMnO4 (15 min), H2O (2 min), 0.001% Fluoro-Jade B staining solution (30 min), H2O (2 min), xylene (2 min) and coverslipped with DAPI- containing mounting media. Three fields from each selected brain area were used for the analysis. Images were taken at 20x magnification and analyzed using Sigma Scan Pro software. The software was calibrated for 340 pixels to represent 100 μm and objects between 100 and 999 pixels were selected for counting.
Statistical Analysis
Data are expressed as mean±SEM. Comparisons for all variables between the pre-existing hypoxia and control groups were analyzed by two-way analysis of variance, followed by post hoc two sample t test using the Bonferroni correction. Relative comparisons vs. Day-1, expressed as fold change, were analyzed by one-sample t-test (GraphPad PRISM 4 software). Statistical significance was accepted at a p value <0.05.
Acknowledgments
Funded by NIH HD37885.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aviado DM, Guevara AD. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann N Y Acad Sci. 2001;940:48–58. [PubMed] [Google Scholar]
- Bartelds B, van BF, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res. 1993;34:51–55. doi: 10.1203/00006450-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekkooi PF, Baan J, Jr, Teitel D, Rudolph AM. Chemoreceptor responsiveness in fetal sheep. Am J Physiol. 1992;263:H162–H167. doi: 10.1152/ajpheart.1992.263.1.H162. [DOI] [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Comroe JH., Jr Chemoreflexes from the heart and lungs. Physiol Rev. 1954;34:167–201. doi: 10.1152/physrev.1954.34.2.167. [DOI] [PubMed] [Google Scholar]
- De Haan HH, Gunn AJ, Williams CE, Gluckman PD. Brief repeated umbilical cord occlusions cause sustained cytotoxic cerebral edema and focal infarcts in near-term fetal lambs. Pediatr Res. 1997;41:96–104. doi: 10.1203/00006450-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Unno N, Jenkins SL, Wentworth RA, Derks JB, Collins JH, Nathanielsz PW. Dynamics of cardiovascular responses to repeated partial umbilical cord compression in late-gestation sheep fetus. AJP - Heart Circ Phys. 1997;273:H2351–H2360. doi: 10.1152/ajpheart.1997.273.5.H2351. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Parsons Y. Stereotaxic method and atlas for the ovine fetal forebrain. J Dev Physiol. 1983;5:101–128. [PubMed] [Google Scholar]
- Gryshchenko O, Qu J, Nathan RD. Ischemia alters the electrical activity of pacemaker cells isolated from the rabbit sinoatrial node. Am J Physiol Heart Circ Physiol. 2002;282:H2284–H2295. doi: 10.1152/ajpheart.00833.2001. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Cook CJ, Williams CE, Johnston BM, Gluckman PD. Electrophysiological responses of the fetus to hypoxia and asphyxia. J Dev Physiol. 1991;16:147–153. [PubMed] [Google Scholar]
- Gunn AJ, Maxwell L, De Haan HH, Bennet L, Williams CE, Gluckman PD, Gunn TR. Delayed hypotension and subendocardial injury after repeated umbilical cord occlusion in near-term fetal lambs. Am J Obstet Gynecol. 2000;183:1564–1572. doi: 10.1067/mob.2000.108084. [DOI] [PubMed] [Google Scholar]
- Hokegard KH, Eriksson BO, Kjellmer I, Magno R, Rosen KG. Myocardial metabolism in relation to electrocardiographic changes and cardiac function during graded hypoxia in the fetal lamb. Acta Physiol Scand. 1981;113:1–7. doi: 10.1111/j.1748-1716.1981.tb06853.x. [DOI] [PubMed] [Google Scholar]
- Imamura T, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters endocrine and physiologic responses to umbilical cord occlusion in the ovine fetus. J Soc Gynecol Invest. 2004;11:131–140. doi: 10.1016/j.jsgi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol. 1989;256:R1348–R1354. doi: 10.1152/ajpregu.1989.256.6.R1348. [DOI] [PubMed] [Google Scholar]
- Lewis AB, Wolf WJ, Sischo W. Cardiovascular and catecholamine responses to successive episodes of hypoxemia in the fetus. Biol Neonate. 1984;45:105–111. doi: 10.1159/000241883. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Gunn AJ, Williams CE, Johnston BM, Gluckman PD. Transient umbilical cord occlusion causes hippocampal damage in the fetal sheep. Am J Obstet Gynecol. 1992;167:1423–1430. doi: 10.1016/s0002-9378(11)91728-1. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Williams CE, Johnston BM, Gluckman PD. Neuronal damage in the developing brain following intrauterine asphyxia. Reprod Fertil and Dev. 1995a;7:647–653. doi: 10.1071/rd9950647. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Williams CE, Johnston BM, Gunning MI, Davis S, Gluckman PD. Repeated episodes of umbilical cord occlusion in fetal sheep lead to preferential damage to the striatum and sensitize the heart to further insults. Pediatr Res. 1995b;37:707–713. doi: 10.1203/00006450-199506000-00006. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Edwards AD. Prediction of outcome after perinatal asphyxia. Current Opinion in Pediatrics. 1997;9:128–132. doi: 10.1097/00008480-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Pryor E, Zhang J, Massmann G, Figueroa J. Prolonged mild fetal hypoxia up-regulates type I nitric oxide synthase expression in discrete areas of the late-gestation fetal sheep brain. Am J Obstet Gynecol. 2002;187:164–170. doi: 10.1067/mob.2002.122403. [DOI] [PubMed] [Google Scholar]
- Pulgar VM, Zhang J, Massmann GA, Figueroa JP. Prolonged mild hypoxia alters fetal sheep electrocorticogram activity. J Soc Gynecol Invest. 2006;13:404–411. doi: 10.1016/j.jsgi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Rees S, Mallard EC, Breen S, Stringer M, Cock M, Harding R. Fetal brain injury following prolonged hypoxemia and placental insufficiency: a review. Comp Biochem Physiol A Mol Integ Physiol. 1998;119:653–660. doi: 10.1016/s1095-6433(98)01001-0. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Carmichael L, Homan J, Patrick JE. Electrocortical activity, electroocular activity, and breathing movements in fetal sheep with prolonged and graded hypoxemia. Am J Obstet Gynecol. 1992;167:553–558. doi: 10.1016/s0002-9378(11)91452-5. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Patrick JE, Abduljabbar H. Cerebral oxidative metabolism in the fetal lamb: relationship to electrocortical state. Am J Obstet Gynecol. 1985;153:426–431. doi: 10.1016/0002-9378(85)90081-x. [DOI] [PubMed] [Google Scholar]
- Rocha E, Hammond R, Richardson B. Necrotic cell injury in the preterm and near-term ovine fetal brain after intermittent umbilical cord occlusion. Am J Obstet Gynecol. 2004;191:488–496. doi: 10.1016/j.ajog.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Thoren P. Left ventricular receptors activated by severe asphyxia and by coronary artery occlusion. Acta Physiol Scand. 1972;85:455–463. doi: 10.1111/j.1748-1716.1971.tb05283.x. [DOI] [PubMed] [Google Scholar]
- Uvebrant P, Hagberg G. Intrauterine growth in children with cerebral palsy. Acta Paediatrica. 1992;81:407–412. doi: 10.1111/j.1651-2227.1992.tb12259.x. [DOI] [PubMed] [Google Scholar]
- Westgate JA, Wassink G, Bennet L, Gunn AJ. Spontaneous hypoxia in multiple pregnancies is associated with early fetal decompensation and enhanced T-wave elevation during brief repeated cord occlusion in near-term fetal sheep. Am J Obstet Gynecol. 2005;193:1526–1533. doi: 10.1016/j.ajog.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Williams CE, Gunn AJ, Mallard EC, Gluckman PD. Outcome after ischemia in the developing sheep brain: an electroencephalographic and histological study. Ann Neurol. 1992;31:14–21. doi: 10.1002/ana.410310104. [DOI] [PubMed] [Google Scholar]
- Williams MC, O’Brien WF. A comparison of birth weight and weight/length ratio for gestation as correlates of perinatal morbidity. J Perinatol. 1997;17:346–350. [PubMed] [Google Scholar]