Abstract
Cranial nerves VII, IX and X provide both gustatory (taste) and non-gustatory (touch, pain, temperature) innervation to the oral cavity of vertebrates. Gustatory neurons innervate taste buds and project centrally to the rostral nucleus of the solitary tract (NTS), while neurons providing general epithelial innervation to the oropharynx project to non-gustatory hindbrain regions, i.e., spinal trigeminal nucleus. In addition to this dichotomy in function, cranial ganglia VII, IX and X have dual embryonic origins, comprising sensory neurons derived from both cranial neural crest and epibranchial placodes. We used a fate mapping approach to test the hypothesis that epibranchial placodes give rise to gustatory neurons, while the neural crest generates non-gustatory cells. Placodal ectoderm or neural crest was grafted from Green Fluorescent Protein (GFP) expressing salamander embryos into unlabeled hosts, allowing us to discern the postembryonic central and peripheral projections of each embryonic neuronal population. Neurites that innervate taste buds are exclusively placodal in origin, and their central processes project to the NTS, consistent with a gustatory fate. In contrast, neural crest-derived neurons do not innervate taste buds; instead, neurites of these sensory neurons terminate as free nerve endings within the oral epithelium. Further, the majority of centrally directed fibers of neural crest neurons terminate outside the NTS, in regions that receive general epithelial afferents. Our data provide empirical evidence that embryonic origin dictates mature neuron function within cranial sensory ganglia: specifically, gustatory neurons derive from epibranchial placodes while neural crest-derived neurons provide general epithelial innervation to the oral cavity.
Keywords: Cranial sensory ganglia, gustatory neurons, taste buds, axolotl, amphibian, GFP, neural crest, placodes, gangliogenesis
Introduction
Innervation of the oral cavity is provided by branchiomeric nerves, V, VII, IX and X, whose cell bodies are housed in cranial ganglia adjacent to the hindbrain (Gross et al., 2003; Hatini et al., 1999; Landacre, 1910; Landacre, 1912; Northcutt and Brändle, 1995). Three broad types of sensory neurons are found in these ganglia, i.e., somatosensory, general viscerosensory, and special viscerosensory, which together provide non-gustatory and gustatory innervation to the oral and pharyngeal regions. Non-gustatory neurons include both somatosensory fibers, which terminate as free nerve endings in the non-taste bud-bearing regions of the oropharyngeal epithelia and project centrally to the nucleus of the spinal tract of V (SpV, Halsell et al., 1993; SpV, Martin and Mason, 1977; Phelan and Falls, 1991), and general visceral fibers which innervate sensory receptors in the cardiovascular and gastric epithelia and project to the caudal solitary nucleus (NTS, Cechetto, 1987; NTS, Roth and Wake, 1985; Zhang and Ashwell, 2001). Gustatory neurons, which are also termed special visceral (see Finger, 1993), innervate specialized peripheral receptor organs, the taste buds, embedded in the epithelial surfaces of the mouth and pharynx, and project to the rostral solitary nucleus within the hindbrain (Herrick, 1944; Martin and Mason, 1977; Opdam and Nieuwenhuys, 1976; Smith and Davis, 2000).
In addition to their diverse sensory functions, neurons of cranial sensory ganglia VII, IX and X are derived from two distinct embryonic cell populations: the cranial neural crest, and neurogenic epibranchial placodes (Adelmann, 1925; Coghill, 1916; D'Amico-Martel and Noden, 1983; His, 1868; Landacre, 1910; Landacre, 1912; Narayanan and Narayanan, 1980; Northcutt and Brändle, 1995). Neural crest cells arise at the dorsal-most region of the developing neural fold, migrate ventrolaterally, and a subset differentiate into sensory neurons within the ganglia of the branchiomeric nerves (Hall, 1989; Hörstadius, 1950; Le Douarin and Dupin, 1993). Neural crest cells also contribute glial cells to the cranial ganglia (D'Amico-Martel and Noden, 1983). The epibranchial placodes appear slightly later in development, after the cranial neural crest has begun to migrate, as a series of thickenings in the ectoderm just dorsal to each of the branchial arches. These placodes generate neurons which then coalesce with the neural crest-derived cells to form ganglia VII, IX and X (Baker and Bronner-Fraser, 2001; D'Amico-Martel and Noden, 1983; Gross et al., 2003; Landacre, 1933).
One ambiguity of cranial nerve development is how the different sensory identities of the neuronal subtypes of ganglia VII, IX and X are established. One possibility is that the embryonic origin restricts neuronal developmental potential to a particular sensory subtype. Many have speculated that the epibranchial placodes contribute gustatory neurons to these ganglia, while the neural crest supplies general epithelial afferents (Ayer-Le Lievre and Le Douarin, 1982; D'Amico-Martel and Noden, 1983; Davies and Lindsay, 1985; Gross et al., 2003; Herrick, 1901; Landacre, 1910; Landacre, 1912; Landacre, 1921; Narayanan and Narayanan, 1980; Northcutt and Brändle, 1995; Stone, 1922; Yntema, 1937; Yntema, 1943; Yntema, 1944). Several descriptive studies support the idea that gustatory neurons arise from the epibranchial placodes. For example, there is a reliable correlation between the number of gustatory fibers exiting the ganglion and the size of the epibranchial placode neuronal contribution, although the proportion of placodal versus crest-derived neurons to individual ganglia varies among species (Ayer-Le Lievre and Le Douarin, 1982; D'Amico-Martel and Noden, 1983; Herrick, 1901; Landacre, 1910; Landacre, 1912; Narayanan and Narayanan, 1980; Northcutt and Brändle, 1995). Further, gustatory neurons are only present in cranial ganglia that have a neuronal contribution from the epibranchial placodes, again leading many investigators to assume that gustatory neurons are descendent from epibranchial placodes (Coghill, 1916; Gross et al., 2003; Landacre, 1910; Landacre, 1933; Yntema, 1943). However, the inability to specifically label the neurites of placodal versus neural crest neurons has precluded attempts to discern the embryonic origins of the three subtypes of sensory neurons within the ganglia (somatosensory, general visceral, and special visceral), and none of the previous work conclusively defines a role for embryonic origin in mature neuron fate.
The present study focuses on resolving the embryonic origin (epibranchial placode or neural crest) of the sensory neurons within cranial ganglia VII, IX and X, which innervate taste buds. If placodal neurons are exclusively gustatory, these neurons will innervate only taste buds in the periphery, and project centrally to the gustatory hindbrain nucleus, the rostral NTS. Conversely, if neural crest-derived neurons are non-gustatory, providing solely general epithelial innervation, they will terminate as free nerve endings in the oral epithelium and project axons to the appropriate hindbrain region, i.e. SpV. To test these hypotheses, we used embryos of Ambystoma mexicanum, the axolotl. Taste buds in this aquatic salamander are easily recognized at early postembryonic or larval stages (Barlow et al., 1996), and the development and neuroanatomy of cranial ganglia VII, IX and X, including central termination fields, have been described in great detail in this genus (Coghill, 1916; Herrick, 1901; Herrick, 1948; Landacre, 1910; Landacre, 1921; Landacre, 1931; Landacre, 1933; Northcutt et al., 2000; Northcutt and Brändle, 1995; Northcutt et al., 1994; Roth et al., 1993; Roth and Wake, 1985; Stone, 1922). By grafting epibranchial placode or neural crest precursors labeled cytoplasmically with GFP into unlabeled host embryos, we mapped the ganglionic location, and peripheral and central projection pattern of each population of descendant sensory neurons. We found a complete segregation of cell fate depending on embryonic origin as assessed by neuroanatomical criteria. Our results demonstrate that, with respect to the oropharyngeal region, gustatory neurons are indeed exclusively placodal in origin, while those derived from neural crest are entirely non-gustatory.
Materials and methods
Animal housing and care
Wild type pigmented, albino, and transgenic-GFP (Sobkow et al., 2006) embryos of the axolotl, Ambystoma mexicanum, were obtained from the Ambystoma Genetic Stock Center at the University of Kentucky (Lexington, KY). Embryos and larvae from stages 2 to 41 (Bordzilovskaya et al., 1989) were maintained in 20% Holtfreter's Solution (HF) at 22°C unless otherwise noted. Animal procedures were approved by University of Colorado IACUC.
GFP microinjection
Prior to availability of GFP transgenic axolotl embryos (Sobkow et al., 2006), embryos were injected with GFP mRNA at the 1-2 cell stage. GFP mRNA was made in vitro using the SP6 promoter of the pCS2mt-GFP plasmid, gift of Dr. Mike Klymkowsky, University of Colorado Boulder (Klymkowsky, 1999), and the mMessage mMachine kit (Ambion, Texas, USA). For injections, pigmented embryos were immersed in 0.1% formalin in 20% HF as an antifungal treatment, rinsed twice in 20% HF, and manually removed from their egg jellies. Dejellied embryos were stabilized in trenches cut into Sylgard-lined (Dow Corning, Michigan, USA) dishes in 100% HF supplemented with penicillin and streptomycin (400μg/ml each) and gentamycin (25mg/ml). Two to 5 nl of GFP mRNA (225pg/nl in dH20) were injected into each blastomere at the two-cell stage. Injected embryos were kept in 10% HF to prevent exogastrulation and returned to 100% HF once gastrulation was complete.
Transplant microsurgery
Microsurgical procedures were adapted from those described previously (Barlow and Northcutt, 1995; Gross et al., 2003). Briefly, neural crest grafts were performed at stages 17-18 (mid-neurula), prior to crest migration (Falck et al., 2002; Northcutt and Brändle, 1995), while grafts of ectoderm containing the presumptive epibranchial placodes were performed at stage 19 (late neurula, Gross et al., 2003). In the case of pigmented donor embryos labeled via GFP mRNA microinjection, albino host embryos of the same stage were used. When transgenic GFP donor embryos were utilized, wild type pigmented embryos were used as hosts. In preparation for surgery, embryos were immersed in 100% HF and held in position in beds made in Permoplast® Clay (AMACO, Illinois, USA). The subregion of the anterior neural fold (NC: Barlow and Northcutt, 1995) or the presumptive epibranchial placode ectoderm (EP: Gross et al., 2003), was removed cleanly from GFP-labeled donor embryos with flame-etched tungsten microneedles and transplanted isochronically and homotopically into host embryos whose own neural fold (n=16) or presumptive epibranchial placode-containing ectoderm (n=33) had been removed (Fig. 1B-F). All grafts were unilateral with care taken to maintain the correct orientation of the transplanted tissue. Grafts were held in place with mild pressure by a clay bridge for 2 hours at room temperature. The bridges were removed and host embryos were transferred to agarose-lined dishes containing 100% HF and kept at 10°C overnight to allow healing. The next day embryos were returned to room temperature and allowed to develop normally until stage 41 (∼8 days) when mature taste buds are present and innervated (Barlow et al., 1996; Barlow and Northcutt, 1995). Larvae were anesthetized in 0.01% tricaine methansulfonate (MS-222, Sigma-Aldrich, Missouri, USA) in 100% HF, decapitated and fixed in 4% paraformaldehyde in 0.1M phosphate buffer (pH=7.3) overnight at 4°C.
Figure 1. Experimental approach for fate mapping of epibranchial placode and neural crest cells.
(A) Grafts were taken from GFP mRNA microinjected or transgenic GFP-expressing donor embryos. (B) Schematic diagram of transplantation of epibranchial placodal ectoderm (EP) from stage 19 GFP-labeled donor into unlabeled host embryo. (C) Diagram of GFP-labeled neural crest (NC) transplantation in stage 17 embryos. A small piece of the dorsal neural fold containing premigratory neural crest cells of the cranial ganglia (area between dashed white line) as well as cells destined to give rise to dorsal hindbrain were transplanted. (D) GFP-labeled placodal ectoderm or (F) neural fold graft recipients one-hour post-surgery. The embryo and the dorsal neural fold are outlined in white. Orientation is identical to B&C. (E) In an ectoderm graft recipient, 8 days later (stage 41), GFP-labeled nerves (arrows) in the jaw are visible in lateral view. The eye (“e”) is autofluorescent and is not labeled with GFP. (G) In a stage 41 neural fold graft recipient, GFP-labeled cartilage and muscle (asterisks) as well as GFP-labeled nerves (arrows) are evident in the jaw. In all images, anterior is right, and dorsal is up. GFP = green. Scale bars: 2mm for A-D, F; 500 μm for E and G.
Immunofluorescence
Immunofluorescence was carried out using standard immunohistochemical methods (Barlow et al., 1996 1997, Gross, 2003; Parker et al., 2004). For sections, fixed heads were rinsed in phosphate buffered saline, cryoprotected in sucrose, embedded in OCT (Tissue-Tex, Indiana, USA) and sectioned in the transverse plane at 16μm. For whole mount staining, heads were bisected at the jaw on the unoperated side and treated with Proteinase K (10μg/ml) for 25 minutes at 50°C prior to immunostaining. Primary antisera used were: mouse anti-acetylated-alpha-tubulin (AT: 1:1000, Clone 6-11b-1, Sigma-Aldrich), which labels nerve fibers in axolotls (Barlow et al., 1996; Northcutt and Brändle, 1995); rabbit anti-calretinin (CR: 1:2000, Swant, Switzerland), a marker of taste buds in axolotls (Barlow et al., 1996); and rabbit anti-GFP (GFP: 1:2000, ab290, Abcam, Cambridge, UK). Secondary antisera were used at 1:1000 and include goat anti-mouse Alexa 546 for anti-AT, goat anti-rabbit Alexa 488 for anti-GFP and goat anti-rabbit Alexa 546 for anti-CR (Molecular Probes, California, USA). Sections were nuclear counterstained with Hoechst 33258 (Molecular Probes) and coverslipped with Fluoromount G (SouthernBiotech, Alabama, USA). To assess the extent of GFP expression in fine terminal nerve fibers labeled with anti-AT within taste buds and the oral epithelium, tyramide amplification of anti-GFP or anti-acetylated-alpha-tubulin (AT) was performed. Both primary anti-sera were diluted at 1:10,000, and biotinylated Fab fragments were used at 1:1000 (Jackson ImmunoResearch, Pennsylvania, USA). Sections were then incubated with strepavidin conjugated with horseradish peroxidase at 1:500 concentration (Vectrastain ABC kit, Vector Laboratories, California, USA) and incubated in tyramide-Alexa 488 (TSA Kit#22, Molecular Probes) for amplification of GFP or tyramide-Alexa 568 (TSA Kit#24) for amplification of AT.
Image acquisition
Multi-channel digital images were obtained using a Zeiss Axioplan fluorescence microscope and a Zeiss high-resolution Axiocam CCD camera with Axiovision software (Zeiss, Germany). Z-stack confocal images were acquired at 1μm intervals through 16 μm cryosections on a laser-scanning Olympus Fluoview confocal microscope and Fluoview software or an Olympus IX81 inverted microscope with spinning disk attachment (Olympus, Pennsylvania, USA) and Slidebook software (Intelligent Imaging, Colorado, USA). Images were saved as TIFF files, and contrast adjusted and cropped in Adobe Photoshop CS for Macintosh.
Results
Pre-placodal ectoderm and pre-migratory neural crest grafts label sensory neurons in cranial ganglia V, VII, IX and X
To map the peripheral and central targets of placode-derived neurons, the ectoderm adjacent to the lateral neural folds was removed from late neurula (stage 19) GFP-labeled embryos (Figure 1A) and grafted homotopically into unlabeled hosts of the same stage (Figure 1B, D). This region of ectoderm contains the presumptive epibranchial placodes (Gross et al., 2003), although the placodes are not evident as regions of columnar epithelium until twelve hours later at stage 26 (Northcutt and Brändle, 1995). To label neural crest-derived neurons, small regions of the dorsal neural tube (area between white dashed lines, Figure 1C) from GFP-labeled neurulae at stage 17 were grafted homotopically and isochronically into unlabeled hosts (Figure 1C, F). At this stage, cranial neural crest cells have not yet left the neural folds (Falck et al., 2002; Northcutt and Brändle, 1995), thus the graft comprises both premigratory neural crest cells, as well as a portion of the dorsal neural tube destined to give rise to hindbrain neurons (Jacob and Guthrie, 2000). Neural fold grafts from the center of the experimental region produced labeled cells in ganglion VII. More anterior grafts resulted in labeled cells in ganglion V, while more posterior grafts produced labeled cells in ganglia IX and X.
For analysis of the peripheral and central innervation targets of GFP-labeled neurons, only those graft recipients with normal head morphology were analyzed. GFP-positive nerve fibers were seen clearly in the upper and lower jaws of intact placodal (arrows, Figure 1E) and neural crest (arrows, Figure 1G) graft recipients. In sections of larvae with epibranchial placode grafts, neural crest-derived structures, such as cranial cartilage, odontoblasts, melanocytes and peripheral glia were devoid of GFP (Table 1), verifying that the GFP-labeled pre-placodal region was transplanted without unintended inclusion of early migrating neural crest cells. In neural crest graft recipients, by contrast, GFP-labeled cells populated expected cephalic neural crest derivatives (Table 1), including peripheral Schwann cells (Figure 4A), jaw cartilage (Figures 1G, asterisk; 4B), odontoblasts (Figure 4C), and melanocytes (not shown).
Table 1.
Distribution of GFP-labeled cells descendent from epibranchial placodes (EP) or neural crest (NC)
| Donor Tissue | Percentage of experimental larvae with GFP-labeled cells in each tissue (# of larvae) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fibers in taste buds | Free nerve endings in oral epithelium | Fibers in rostral fasciculus solitarius | Ganglion V | Ganglion VII | Ganglion IX | Ganglion X | Cartilage, Melanocytes, Odontoblasts | Peripheral glia | |
| EP grafts
(n=33) |
100% (33) | 0% (0) | 76% (25) | 6% (2)‡ | 88% (29) | 79% (26) | 61% (20) | 0% (0) | 0% (0) |
|
| |||||||||
| NC grafts*
(n=16) |
0% (0) | 100% (16) | 0% (0) | 75% (12) | 81% (13) | 31% (5) | 31% (5) | 100% (16) | 100% (16) |
from trigeminal placode
only larvae receiving micrografts of neural crest were included
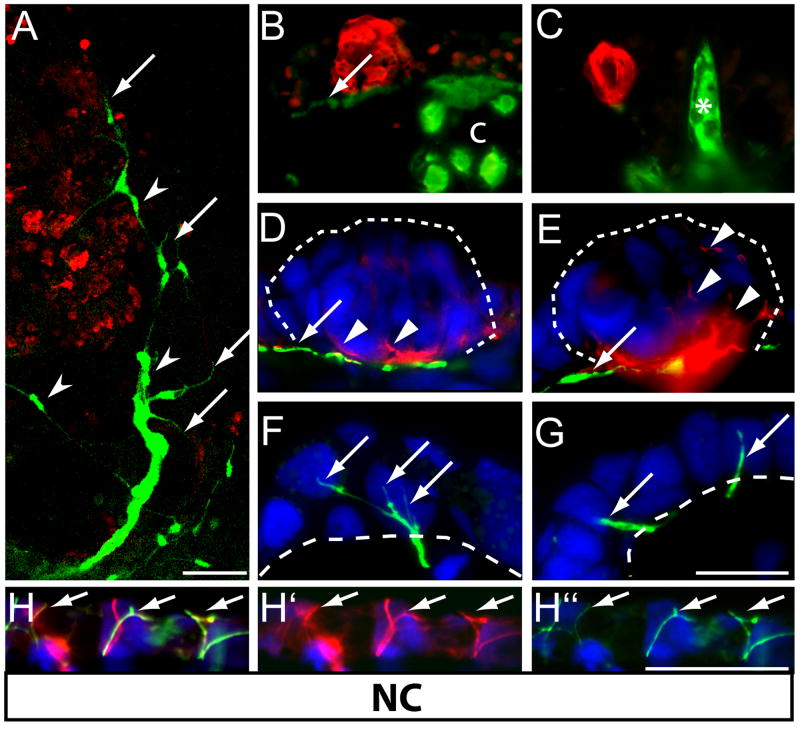
Figure 4. Sensory neurons derived from neural crest innervate non-taste bud-bearing epithelium in the oral cavity.
(A) Confocal z-stack of the interior of upper jaw of neural crest GFP-graft recipient at stage 41, reveals GFP-positive nerve fibers (arrows) terminating in oral epithelium devoid of taste buds. GFP-positive Schwann cells (arrowheads) ensheath these peripheral nerves. (B-C) In transverse sections, several expected neural crest derivatives are labeled with GFP, including nerve fibers (arrow in B), cartilage (‘c’ in B) and odontoblasts (asterisk in C). No GFP-labeled nerve fibers were present in calretinin (red) positive taste buds. (D-E) GFP-labeled crest-derived nerve fibers (arrows) travel beneath taste buds (outlined with dashed line), but do not project into taste buds. AT-positive fibers (red) within taste buds (arrowheads) are not labeled with GFP. (F-G). Instead, GFP-positive fibers of neural crest neurons terminate as free nerve endings (arrows in F, G) within the non-taste-bud-bearing oropharyngeal epithelium. Dashed line indicates basement membrane. (H) Merged image shows that some of the AT-positive (red, H‘) nerve fibers in the skin above the otic vesicle are also neural crest derived as evident by double-labeling with GFP (green, H“). Anti-GFP=green. Nuclei are stained blue with Hoechst in D-H. Scale bar = 100 μm for A; 50 for B-C, and 25 μm for D-H.
GFP-labeled cells were prevalent within cranial ganglia V, VII, IX and X, concordant with the axial level of each initial graft, either of epibranchial placodes or neural tube (see Hörstadius, 1950; Hall, 1989). Neurons were identified by immunostaining for acetylated alpha-tubulin (AT: Northcutt, 1995, Barlow et al., 1996). GFP-labeled, AT-positive neurons derived from epibranchial placodes were restricted to ganglia VII, IX and X (arrowheads, Figure 2A-D), and were generally not detected in the trigeminal (Vth) ganglion (Figure 2A), as these sensory neurons are not derived from epibranchial placodes (Baker and Bronner-Fraser, 2001; Brugmann and Moody, 2005; Gross et al., 2003; Schlosser, 2006). However, in two cases, the more anterior region of ectoderm containing the trigeminal placode (Northcutt and Brändle, 1995) was inadvertently included in the epibranchial placode graft, resulting in some labeled neurons within the Vth ganglion (Table 1).
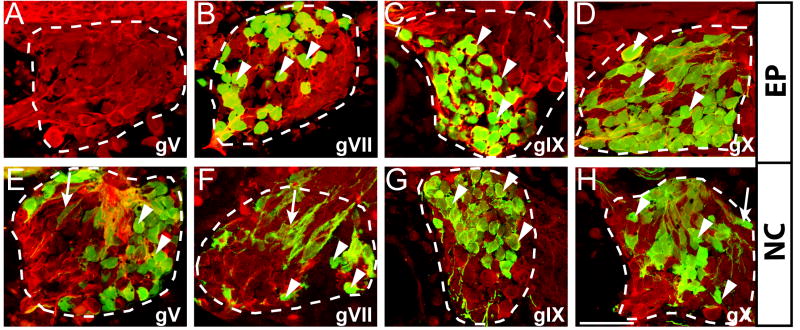
Figure 2. Epibranchial placodes contribute neurons to ganglia VII, IX, and X, while the neural crest contributes both neurons and glia.
Confocal z-stacks through sagittal sections of ganglia V (gV), VII (gVII), IX (gIX) and X (gX) reveal the presence of GFP-labeled cells. (A-D) In sections from larvae with epibranchial placode grafts, GFP labels neurons (arrowheads) within gVII (B), gVII (C) and gX (D), but does not label neurons in ganglion V. (E-H) In larvae with NC grafts, neural crest derivatives within the ganglia include both neurons (arrowheads) and glial cells (arrows) in gV (E), gVII (F), gIX (G) and gX (H). Labeled neurons (arrowheads) have characteristic large round nuclei and are immunoreactive for acetylated tubulin and GFP. GFP-labeled satellite glial cells (arrows) can be seen ensheathing sensory neurons. Ganglia are outlined with a dashed line. Dorsal is up, anterior is to the left. Anti-GFP = green; anti-acetylated tubulin = red. Scale bar = 50 μm.
GFP-labeled, AT-immunopositive neurons derived from the neural crest were also found in ganglia V, VII, IX and X (arrowheads, Figure 2E-H), and additional GFP-labeled neural crest-derived cells appeared to ensheath neurons within the ganglia (arrows, Figure 2E, F, H), characteristic of ganglionic satellite glia, cells known to arise from neural crest (Ayer-Le Lievre and Le Douarin, 1982; Hanani; Kelsh et al., 2000). Although in some ganglia, epibranchial placode-derived neurons were concentrated in to the distal portions (gIX: Figure 2C) and neural crest-derived neurons were predominantly located in the proximal region (gV and gIX: Figure 2E, G), in other ganglia there was substantial co-mingling of epibranchial placode-derived and neural crest-derived neurons (gVII: Figure 2B, F; gX: Figure 2D, H). As mentioned, the relative contributions of placodal versus crest-derived neurons, as well as the degree to which the placodal and crest-derived portions of the ganglia are anatomically segregated vary across species. While in amniotes, the distal (placodal) and proximal (crest-derived) post-otic ganglia are separate (D'Amico-Martel and Noden, 1983; Kious et al., 2002), in anamniotes, including axolotls, the proximal and distal components of ganglia IX and X are fused into a large post-otic complex (Landacre, 1933; Northcutt and Brändle, 1995).
Thus, as reported by others (Barlow and Northcutt, 1995; D'Amico-Martel and Noden, 1983; Gross et al., 2003; Landacre, 1910; Landacre, 1912; Landacre, 1931; Landacre, 1933; Narayanan and Narayanan, 1980; Yntema, 1937), both epibranchial placodes and cranial neural crest contribute sensory neurons to the VIIth IXth and Xth cranial ganglia.
Peripheral nerve fibers of placodal neurons innervate taste buds
In axolotls, a substantial subset of taste cells within each taste bud is immunopositive for calretinin (CR: Barlow et al., 1996), thus we used this immunomarker to determine if taste buds are innervated by nerve fibers of GFP-labeled placodal neurons. GFP-positive fibers of neurons descendent from the placodal graft were evident in the mouth and pharynx in all EP graft recipients (Table 1). Placode-derived peripheral neurites traveled along the basement membrane of the oral epithelium and the epithelium of the internal gill arches, turning apically to invade numerous CR-positive taste buds throughout the lower and upper jaws (Figure 3A-G), as well as along the surface of the internal gills ipsilateral to the graft (data not shown). GFP-positive fibers typically formed a dense nerve plexus beneath taste buds from which fine fibers extended into taste buds (arrows, Figure 3B-D). The proportion of taste buds contacted by GFP-labeled neurites varied with experimental animal. On average, 38% of the taste buds on the operated side of each graft recipient were contacted by GFP positive fibers, with a range of 11-67% across individuals.
Figure 3. Sensory neurons derived from epibranchial placodes innervate taste buds.
(A) Confocal z-stack of the interior of the upper jaw of an epibranchial placode GFP-graft recipient at stage 41. GFP-positive rami of the VIIth nerve (arrows) branch to contact taste buds labeled with anti-calretinin (red). Anterior is to the top. (B-D) Transverse sections through calretinin-positive taste buds reveal that GFP-labeled fibers (green) form a large nerve plexus beneath each taste bud and extend fine processes (arrows) apically into the buds. (E) GFP-positive fibers (arrows) within taste buds (outlined by white dashed lines) are also positive for anti-acetylated tubulin (AT, red) in (F). (G) Merged image shows that GFP label co-localizes with AT. Nuclei are stained blue with Hoechst. Anti-GFP = green. Scale bar in A = 100μm; B-G = 25 μm.
In some cases, graft recipients were processed with antibodies against both GFP and acetylated tubulin to examine if GFP-labeled processes in the oral cavity were indeed axons, and to examine the extent of GFP labeling in the distal tips of placodal nerve fibers. GFP-expressing fibers were always AT-immunopositive, including those fine fibers within taste buds (arrows, Figure 3E-G). Although the majority of fibers contacting taste buds are known to serve a gustatory function, there have been reports that a small number of mechanosensory fibers also innervate the base of taste buds (Nagai, 1993a; Nagai, 1993b). In two cases, the trigeminal placode was inadvertently included in the epibranchial graft, yet no free nerve endings were observed in the oral epithelium. This result is in agreement with previous reports that there is no trigeminal innervation of the oral epithelium of axolotls (Northcutt et al., 2000), in contrast with the well documented general sensory innervation of the lingual and palatal epithelium by the trigeminal nerve in mammals (Arvidsson et al., 1995; Chan and Byers, 1985; Farbman and Mbiene, 1991). In a small subset of cases, the lateral line placodes were inadvertently included in the graft as evidenced by GFP-positive neuromasts in the skin (Northcutt, 1992; Northcutt and Brändle, 1995; Northcutt et al., 1994), which have strictly surface innervation.
Importantly, although GFP-labeled nerve fibers emerged from the subepithelial nerve plexus to enter taste buds, no GFP expression was seen in free nerve endings in the surrounding non-taste bud-bearing lingual epithelium, indicating that within the oral cavity, neurons derived from the epibranchial placodes serve a gustatory function exclusively. We also noticed GFP-labeled fibers in the visceral epithelium of the gut (data not shown) of animals that had received epibranchial placode grafts, indicating that both special visceral (gustatory) and general visceral neurons are derived from the epibranchial placodes.
Neural crest neurons do not innervate taste buds, but innervate adjacent oral and pharyngeal epithelia
GFP-labeled nerve fibers (arrows, Figure 4A) and Schwann cells (arrowheads, Figure 4A) were present in the oral cavity of neural crest graft recipients. The peripheral neurites of GFP-positive neural crest neurons were evident throughout the oral epithelium and underlying mesenchyme. Similar to placodal fibers, neurites of crest-derived neurons traveled along the basement membrane of the oral and gill epithelia (arrows, Figure 4B, D-E); however, crest-derived fibers never invaded taste buds despite their close proximity. For example, GFP-labeled fibers were found traveling through and beyond the AT-immunoreactive basal nerve plexus of a taste bud, yet none of the AT-positive fibers within taste cells (arrowheads, Figure 4D-E) were double labeled with GFP. Fine neurites of neural crest neurons only invaded the non-taste bud-bearing epithelium, where they terminated as GFP-labeled free nerve endings (Figure 4F-G). The lack of innervation of taste buds by crest-derived neurons was not a result of insufficient GFP in the peripheral processes. Tyramide amplification of both anti-AT and anti-GFP immunofluorescent staining consistently revealed that neural crest-derived fibers were double labeled, including free nerve endings in the epithelium. In addition to the oral epithelium, very fine AT-immunopositive and GFP-labeled cutaneous nerve fibers also innervated the skin above the otic vesicle (Figure 4H), as has been reported for the facial ganglion in mammals (Martin and Mason, 1977). In sum, these data suggest that the crest-derived neurons provide only non-gustatory, general epithelial innervation to the oral and pharyngeal surfaces.
Placodal neurons project to the gustatory hindbrain nucleus
Next, we examined the central projections of GFP-labeled placodal neurons, as these afferents, if gustatory in function, should project to the solitary nucleus of the hindbrain, and specifically to its most rostral domain. In salamanders, as in other vertebrates, the afferent fibers of the cranial nerves VII, IX and X enter the brainstem and divide to join distinct ascending and descending tracts of the fasciculated functional columns of the medulla (Nagai and Matsushima, 1990; Opdam and Nieuwenhuys, 1976; Smith and Davis, 2000). Gustatory, or special visceral, afferents converge into a single bundle of axons, collectively referred to as the fasciculus solitarius. Gustatory fibers within the fasciculus solitarius then synapse on neurons of the rostral nucleus of the solitary tract (NTS).
In epibranchial placodal graft recipients, at the levels of cranial nerve roots VII, IX and X, a large number of GFP-labeled fibers were evident in the fasciculus solitarius, located in the dorsomedial region of the hindbrain (Figure 5A-C). A high density of GFP-positive fibers were present in the more rostral portion of the tract where gustatory fibers terminate (Figure 5A). In larvae with GFP-labeled fibers in the gut (data not shown), GFP-labeled afferents of IX and X were also present within the more caudal regions of the solitary tract nucleus (Figure 5B,C), where non-gustatory, general visceral afferents terminate (Cechetto, 1987; Herrick, 1948; Nagai and Matsushima, 1990; Opdam and Nieuwenhuys, 1976; Smith and Davis, 2000). In animals in which lateral line placodes were inadvertently included in the graft, a small number of GFP-positive fibers was also occasionally seen in the known central target of the lateral line nerve, the lateralis column of the hindbrain, dorsal to the fasciculus solitarius (Figure 5C; Altman and Dawes, 1983; Kishida et al., 1987; Northcutt, 1992). Importantly, however, GFP-labeled fibers were never seen in the more ventrolateral spinal trigeminal tract (Figure 5B-C), which receives general epithelial afferents from the head and oral cavity, i.e., those fibers providing mechano-, thermo- and nociceptive innervation. Thus, in addition to exclusively innervating taste buds in the oral and pharyngeal epithelia, the GFP-labeled axons of epibranchial placodal neurons project to gustatory hindbrain regions.
Figure 5. Placode-derived sensory neurons project to the solitary tract nucleus via the fasciculus solitarius, while neural crest-derived sensory neurons do not.
Top panel is a diagram of a lateral view of a larval axolotl brain, anterior to the right, with lines indicating the level of the transverse sections shown in A-F below. (A-C) Central projections of GFP-labeled placodal neurons (green) are only seen in the fasciculus solitarius (f.sol.) shown here at the level of the (A) VIIth, (B) IXth, and (C) Xth nerve roots on the operated side of placodal ectoderm larval graft recipients. (D-F) Central projections of GFP-labeled neural crest neurons were not present in (D) the fasciculus solitarius rostrally at the level of the VIIth root or more caudally at the level of the (E) IXth or (F) Xth roots, but rather were present in other specific hindbrain regions, including the spinal trigeminal tract (SpV). Central afferents of coincidentaly labeled lateral line neurons (r.l.l.) were sometimes seen in both placode (C) and crest (F) graft recipeints. Anterior is up. Nuclei are stained blue with Hoechst. Anti-GFP = green. f.sol. = fasciulus solitarius; r.l.l. = roots of the lateral line nerves; r.IX & rX = roots of IX and X; SpV = spinal trigeminal tract. Scale bar=20μm.
Neural crest neurons project centrally to the spinal trigeminal tract
Neural fold grafts contained both premigratory neural crest, as well as cells destined to contribute to the hindbrain (Jacob and Guthrie, 2000). In our initial experiments, the neural tube grafts were large, extending from the presumptive midbrain anteriorly, to the caudal extent of the presumptive hindbrain region (white dashed lines in Figure 1C; GFP label in 1F). In these initial grafts, large numbers of hindbrain neurons and their processes were labeled with GFP, obscuring the central projections of the concomitantly labeled neural crest-derived sensory neurons. Subsequent grafting of smaller pieces of neural tube within the same region (Figure 1C) labeled many fewer hindbrain cells in each embryo, and allowed better mapping of the central terminations of crest-derived ganglion neurons.
In contrast to the centrally directed afferents of placodal neurons, no GFP-labeled fibers were found in the rostral fasciculus solitarius in neural crest graft recipients (Figure 5D, E). Rather, GFP-labeled fibers of crest-derived neurons were present in the ventrolateral spinal trigeminal tract (SpV, Figure 5E, F), which carries pain, temperature and touch sensation (Smith and Davis, 2000). In animals that received more posterior neural tube micrografts in which the trigeminal ganglion was not labeled (n=4), GFP-positive fibers of nerves IX and X were consistently seen in the SpV. In animals in which the dorsolateral placodes were inadvertently included in a neural tube graft, as evidenced by GFP-positive neuromasts in the skin above the eye or along the trunk, GFP-positive fibers were present in the dorsal lateralis column (r.l.l.; Figure 5F). Occasionally, in some animals, GFP-positive fibers were seen in the motor columns and invading peripheral jaw musculature (data not shown), indicating the incorporation of presumptive motor neurons in the original neural fold graft (Barbas-Henry, 1982; Deban et al., 2001). Incidentally, we also did not see fibers terminating in the caudal NTS (Figure 5F) supporting a non-neural crest origin for general visceral afferents.
In sum, based on central projections and peripheral targets, we conclude that cranial neural crest-derived neurons of ganglia V, VII, IX and X are entirely non-gustatory in function, and contribute only general epithelial innervation to the oral and pharyngeal cavity.
Discussion
Embryonic origin defines gustatory versus general epithelial neuronal fate in cranial nerve ganglia
During embryonic development, cephalic sensory neurons arise from 2 sources, the ectodermal placodes and rhombomeric neural crest, and combine to form the cranial ganglia of the trigeminal (V), facial (VII), glossopharyngeal (IX) and vagal (X) nerves. In particular, the epibranchial placodes contribute neurons specifically to ganglia VII, IX and X, which house at least 2 general functional classes of neurons with respect to the oral and pharyngeal cavities, i.e., gustatory and non-gustatory, or general epithelial innervation (Adelmann, 1925; Barlow et al., 1996; Barlow and Northcutt, 1997; Coghill, 1916; Falck et al., 2002; Landacre, 1910; Landacre, 1912; Landacre, 1921; Northcutt and Brändle, 1995), raising the question of whether embryonic origin dictates the mature function of sensory ganglion neurons. Earlier studies have examined the contributions of these embryonic tissues to cranial nerve ganglia via extirpation of either the neural crest or epibranchial placode precursors (Stone, 1922; Yntema, 1943; Yntema, 1944). In chicks, where the proximal and distal postotic ganglia are anatomically separate (Ayer-Le Lievre and Le Douarin, 1982; Davies and Lindsay, 1985), ablation of neural crest cells resulted in a loss of sensory neurons in the proximal ganglia while ablation of the epibranchial placodes resulted in reduced distal ganglion neurons (Yntema, 1944). In amphibians, proximal and distal portions of the ganglia comprising the postotic ganglionic complex are less clear post-embryonically (Northcutt and Brändle, 1995). Nonetheless, ablation of the epibranchial placodes in early embryos resulted in fewer neurons within the more distal regions of these ganglia.
Numerous careful fate mapping studies have reinforced the dual origins of sensory neurons in these ganglia (Artinger et al., 1998; Ayer-Le Lievre and Le Douarin, 1982; D'Amico-Martel and Noden, 1983; Le Douarin, 1984; Narayanan and Narayanan, 1980; Yntema, 1937; Yntema, 1943). Both the vital dye used to stain the surface of donor cells in Ambystoma punctatum embryos (Yntema, 1937; Yntema, 1943) and the nuclear marker used to fate map donor quail cells in chick embryos (Le Douarin, 1984; Le Douarin and Teillet, 1974) have previously shown both neural crest and epibranchial placodes contribute sensory neurons to cranial ganglia VII, IX and X. In all of these studies, however, only neuronal cell bodies were labeled while the axonal processes of the two neuronal populations, crest-derived and placodal, were not labeled. Thus, while key in leading to our understanding of the roles of neural crest and epibranchial placodes in cranial ganglion cell development, these studies did now allow correlation of embryonic origin with specific sensory function at the cellular level.
Here we employ a cytoplasmic marker (GFP) to follow the fates, and, importantly, the ultimate central and peripheral projection patterns of neural crest versus epibranchial placode derived sensory neurons. We find that gustatory neurons derive exclusively from the epibranchial placodes, while the neural crest contributes only general innervation of the oral and pharyngeal epithelia, concisely linking embryonic origin to mature neuron function. In addition, although not systematically examined in this study, we found that epibranchial placode grafts also resulted in the labeling of general visceral sensory neurons; GFP-labeled fibers innervated elements of the gut, and in these same graft recipients, GFP-labeled fibers projected to the caudal domain of the solitary tract nucleus, the known central target for general visceral afferents (Cechetto, 1987; Herrick, 1948; Nagai and Matsushima, 1990; Opdam and Nieuwenhuys, 1976; Smith and Davis, 2000). These data are thus consistent with the presumed epibranchial placodal origin of general visceral sensory neurons of gIX and X, which innervate receptors in the cardiovascular system and gut (Harrison et al., 1995; Kirby, 1987; Kirby, 1988).
Our criteria for identifying gustatory neurons are straightforward: These neurons innervate taste buds in the periphery, and project to the rostral nucleus of the solitary tract (NTS) in the hindbrain (Cechetto, 1987; Herrick, 1948; Nagai and Matsushima, 1990; Opdam and Nieuwenhuys, 1976; Smith and Davis, 2000). Our criteria for identifying non-gustatory neurons providing general epithelial innervation to the oral cavity are similarly clear-cut, and are based on peripheral and central anatomy. General epithelial neurons of ganglia VII, IX and X terminate as free nerve endings in the oral epithelium, and not in taste buds, and centrally, they project to the spinal trigeminal tract (SpV), and not to the NTS. The SpV tract carries mechanoreceptive, thermoreceptive and nocioceptive innervation from the surface of the head, limbs and trunk (Halsell et al., 1993; Martin and Mason, 1977; Phelan and Falls, 1991). We thus assume that the general epithelial innervation from neural crest-derived neurons provides similar information from the oral and pharyngeal epithelia.
In our fate mapping studies, a number of other placode and neural crest derived cells were also labeled; some of these inadvertently. Specifically, on a small number of occasions, the dorsolateral placodes were included in the grafts. These placodes give rise to both the sensory neurons and the peripheral sensory organs, the neuromasts and ampullary organs, of the lateral line system (Northcutt et al., 1995; Northcutt et al., 1994). Although these placodes are first evident as ectodermal thickenings immediately dorsal to the epibranchial placodes at early tailbud stages (Northcutt and Brändle, 1995), the presumptive dorsolateral placodes are located in the lateral wall of the neural fold at late neurula stages (Northcutt et al., 1996). This location places the dorsolateral placodes precisely between the neural crest (dorsal neural fold) and the presumptive epibranchial placodes (immediately ventral to the lateral neural fold; Gross et al., 2003) at the time of our grafting experiments. Thus, the infrequent inclusion of presumptive lateral line primordia in both our NC and EP grafts simply indicates: 1) the close proximity of these cells to EP and NC; and 2) the precision with which we must perform these microsurgeries.
In another small subset of placodal grafts, we encountered labeled cells in the trigeminal ganglion. The trigeminal (gV) comprises both neural crest-derived and placode derived sensory neurons (D'Amico-Martel and Noden, 1983; Hamburger, 1961; Lwigale, 2001; Northcutt and Brändle, 1995; Stainier and Gilbert, 1990). In these placodal grafts, the extent of the transplanted ectoderm likely extended rostrally and slightly ventrally to include the presumptive trigeminal placode, thus giving rise to a subset of GFP labeled sensory neurons in gV. Despite the presence of GFP-labeled trigeminal neurons, we did not encounter labeled free nerve endings within the oral epithelium. This is consistent with the known lack of trigeminal innervation of the oral cavity of axolotls (Northcutt et al., 2000), yet is distinct from the pattern in mammals and birds, where the rostral oral cavity, including much of the lingual and palatal epithelia, is innervated by branches of the trigeminal nerve (Arvidsson et al., 1995; Chan and Byers, 1985; Farbman and Mbiene, 1991). In axolotls, however, the general innervation of the oral cavity is instead provided by ganglia VII, IX and X, consistent again with our finding that the neural crest-derived neurons of these ganglia project to the SpV.
This difference in innervation pattern between mammals and amphibians may be due to the germ layer of origin of the oral epithelium, i.e., ectoderm versus endoderm. The oral cavity is where the endodermal gut epithelium meets the ectodermal skin during embryonic development in all vertebrates (Barlow, 2000; Barlow and Northcutt, 1995; Dickinson and Sive, 2006; Takahama et al., 1988; Waterman, 1977; Waterman and Schoenwolf, 1980). Importantly, the location of this abutment is not conserved across vertebrates. In axolotls, ectoderm covers only the interior surface of the anterior portion of the mandibular arch (Barlow, 2000), while the majority of the oral cavity is derived from endoderm (Barlow and Northcutt, 1995; Barlow, 2000). In contrast, in chick, the endoderm/ectoderm border is quite posterior; the majority of the oral cavity epithelium is derived from ectoderm, extending posteriorly well beyond the territory of the internal surface of the mandible (Fontaine and Le Douarin, 1977). The distribution of ectoderm versus endoderm in mammals, although it has not been directly mapped, is thought to be similar to chick (see Fontaine and Le Douarin, 1977), in that the lingual and palatal epithelia are ectodermal, while in the posterior third of the oral cavity, the epithelia are derived from endoderm (Tam, 1989). The extent of ectoderm within the oral cavity appears to thus correlate with the pattern of trigeminal innervation. In axolotls, which possess ectoderm only at the very anterior region (Barlow, 2000), trigeminal innervation is likewise restricted to this domain (Northcutt et al., 2000), while in mammals, with a much greater ectodermal contribution, a large portion of the lingual surface is innervated by the lingual branch of the trigeminal nerve (Marfurt and Turner, 1983; Suemune et al., 1992). In both cases, the trigeminal nerve carries general epithelial information to the appropriate hindbrain tract, but in axolotls, the trigeminal receptive field is much reduced in the oral cavity. Yet, the endodermal epithelium must receive innervation that allows this somatosensory type of information to travel to the brain. This requirement is filled by the neural crest-derived cells in ganglia VII, IX and X.
Epibranchial placodes and neural crest have distinct developmental trajectories
Epibranchial placodes and rhombomeric neural crest have disparate origins, yet both generate migratory neuroblasts that converge to populate the same cranial sensory ganglia. The epibranchial placodes form as thickenings in the ectoderm above the clefts between the branchial arches, and their formation, indeed their ability to generate immature neurons, is completely independent of neural crest cells (Begbie et al., 1999; Gross et al., 2003). Induction of the placodes occurs through a complex multistep process involving Wnt, Fgf, and Bmp signaling from surrounding tissues (Holzschuh et al., 2005; Litsiou et al., 2005; Macatee et al., 2003; Nechiporuk et al., 2007; Nechiporuk et al., 2005; Nikaido et al., 2007; Phillips et al., 2004; Sun et al., 2007). Prior to the induction of specific placodes, Fgf signaling from the mesoderm induces a pre-placodal region at the anterior edge of the neural plate border (Litsiou et al., 2005; Nechiporuk et al., 2007; Nikaido et al., 2007) causing the simple epithelial cells of the cranial ectoderm to take on a columnar morphology. Wnt signaling restricts placodal formation to the cranial region (Litsiou et al., 2005). Later, neurogenesis in the epibranchial placodes is induced by Fgf signaling from the pharyngeal endoderm (Nechiporuk et al., 2005; Sun et al., 2007) in conjunction with Bmp signaling (Holzschuh et al., 2005; Begbie et al., 1999). Neural crest cells, by contrast, are induced by Bmp signaling from the dorsal neural tube in conjunction with Fgf, and Wnt signaling from non-neural ectoderm and adjacent mesoderm (Baker and Bronner-Fraser, 1997; Basch and Bronner-Fraser, 2006; Garcia-Castro and Bronner-Fraser, 1999; Selleck et al., 1998), and arise at the border of the neural and non-neural ectoderm (Epperlein and Lofberg, 1993; Falck et al., 2002). Importantly, neural crest derived neurons undergo neurogenesis and ganglion formation entirely independently of placodes (Harrison et al., 1995; Stone, 1922).
It appears that in neuroblasts derived from both epibranchial placodes and neural crest, decisions regarding sensory fate are made very early. Following ablation of either the neural crest or the placodes, the remaining population of neuroblasts will migrate and form smaller, functional ganglia (Stone, 1922; Yntema, 1943; Yntema, 1944), though these ganglia do not always form in the proper sites (Begbie and Graham, 2001; Kuratani et al., 1991). Further, when epibranchial placodes are cultured in the absence of neural crest, the resultant placodal sensory neurons appear to acquire a gustatory fate, in that their axons grow toward the appropriate target, pharyngeal epithelium (Gross et al., 2003). Similarly, the fate of neurons derived from the otic placode is also determined before target innervation (Fritzsch et al., 2005). While interactions between placodal and neural crest neurons are not required for the adoption of specific sensory fates, the presence of both populations within the ganglia is critical for proper innervation of both central (Begbie and Graham, 2001; Kuratani et al., 1991) and peripheral (Hamburger, 1961; Lwigale, 2001) targets.
Epibranchial placode and neural crest derived neurons have different neurotrophic requirements
Neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and neurotrophin 4 (NT-4) play demonstrated roles in neuronal survival, axon pathfinding and target innervation for a wide variety of neuronal subtypes, such that different types of neurons are often supported by different neurotrophins (Agerman et al., 2003; Ernfors, 2001; Hamburger and Levi-Montalcini, 1949; Krimm et al., 2006; LeMaster et al., 1999). This is indeed the case for placodal versus neural crest-derived neurons of the trigeminal ganglion. Placodal neurons within ganglion V are not responsive to NGF but are responsive to BDNF, while neural crest neurons are responsive to both NGF and BDNF (Davies and Lindsay, 1985; Lindsay et al., 1985a; Lindsay et al., 1985b).
Within the taste system, studies in neurotrophin knockout mice have revealed that the survival of gustatory neurons is BDNF-dependent, while that of non-gustatory neurons relies on NT-3 (Liebl et al., 1997; Nosrat et al., 1997). Differential expression during development by placodal versus neural crest neurons of specific neurotrophin (trk) receptors likely underlies these specific sensitivities. Certainly in adult ganglia, trkB (the receptor for BDNF) is expressed in many geniculate (gVII) and petrosal (gIX) neurons, while trkC (the NT-3 receptor) expression is detected in only small neuronal subsets within these same ganglia (Matsumoto et al., 2001). Consistent with these expression data, knockout of trkB in mice leads to loss of gustatory innervation and death of sensory neurons within ganglion VII (Fritzsch et al., 1997). Recent studies have established that expression of both BDNF and NT4 in the taste periphery also plays an important role in axonal targeting of taste buds by gustatory neurons during development (Lopez and Krimm, 2006a; Lopez and Krimm, 2006b). Because we have now demonstrated that the epibranchial placodes give rise to gustatory neurons, we predict that these placodal neurons are likewise BDNF dependent, and are now testing this hypothesis.
Acknowledgments
The authors thank the Ambystoma Genetic Stock Center (AGSC) at the University of Kentucky for axolotl embryos; Dr. Mike Klymkowsky, CU Boulder, for GFP plasmid; Dr. Thomas Finger for thoughtful discussions and critical input; Josh Gross and Dianna Bartel for suggestions on microsurgical and immunohistochemical techniques; and the Rocky Mountain Taste and Smell Center Imaging Core (P30 DC004657 to Dr. Diego Restrepo). This work was supported by NSF support to AGSC by NSF-DBI-0443496, as well as NIDCD Grants DC003947 to LAB and DC007796 to DEH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adelmann HB. The development of the neural folds and cranial ganglia of the rat. Journal of Comparative Neurology. 1925;39:19–171. [Google Scholar]
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–91. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- Altman JS, Dawes EA. A cobalt study of medullary sensory projections from lateral line nerves, associated cutaneous nerves, and the VIIIth nerve in adult Xenopus. J Comp Neurol. 1983;213:310–26. doi: 10.1002/cne.902130307. [DOI] [PubMed] [Google Scholar]
- Artinger KB, Fedtsova N, Rhee JM, Bronner-Fraser M, Turner E. Placodal origin of Brn-3-expressing cranial sensory neurons. Journal of Neurobiology. 1998;36:572–85. doi: 10.1002/(sici)1097-4695(19980915)36:4<572::aid-neu10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Arvidsson J, Fundin BT, Pfaller K. Innervation of the hard palate in the rat studied by anterograde transport of horseradish peroxidase conjugates. The Journal of Comparative Neurology. 1995;351:489–498. doi: 10.1002/cne.903510402. [DOI] [PubMed] [Google Scholar]
- Ayer-Le Lievre CS, Le Douarin NM. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Dev Biol. 1982;94:291–310. doi: 10.1016/0012-1606(82)90349-9. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. The origins of the neural crest. Part I: embryonic induction. Mech Dev. 1997;69:3–11. doi: 10.1016/s0925-4773(97)00132-9. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Barbas-Henry HA. The motor nuclei and primary projections of the facial nerve in the monitor lizard Varanus exanthematicus. Journal of Comparative Neurology. 1982;207:105–13. doi: 10.1002/cne.902070202. [DOI] [PubMed] [Google Scholar]
- Barlow LA. Taste buds in ectoderm are induced by endoderm: implications for mechanisms governing taste bud development. In: Jacobson KO, Olsson L, editors. Regulatory Processes in Development. Portland Press Ltd.; London: 2000. pp. 185–190. [Google Scholar]
- Barlow LA, Chien CB, Northcutt RG. Embryonic taste buds develop in the absence of innervation. Development. 1996;122:1103–11. doi: 10.1242/dev.122.4.1103. [DOI] [PubMed] [Google Scholar]
- Barlow LA, Northcutt RG. Embryonic origin of amphibian taste buds. Dev Biol. 1995;169:273–85. doi: 10.1006/dbio.1995.1143. [DOI] [PubMed] [Google Scholar]
- Barlow LA, Northcutt RG. Taste buds develop autonomously from endoderm without induction by cephalic neural crest or paraxial mesoderm. Development. 1997;124:949–57. doi: 10.1242/dev.124.5.949. [DOI] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M. Neural crest inducing signals. Adv Exp Med Biol. 2006;589:24–31. doi: 10.1007/978-0-387-46954-6_2. [DOI] [PubMed] [Google Scholar]
- Begbie J, Brunet JF, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001;294:595–8. doi: 10.1126/science.1062028. [DOI] [PubMed] [Google Scholar]
- Bordzilovskaya NP, Dettlaff TA, Duhon ST, Malacinski GM. Developmental-stage series of axolotl embryos. In: Armstong JB, Malacinski GM, editors. Developmental Biology of the Axolotl. Oxford University Press; New York: 1989. pp. 201–219. [Google Scholar]
- Brugmann SA, Moody SA. Induction and specification of the vertebrate ectodermal placodes: precursors of the cranial sensory organs. Biology of the Cell. 2005;97:303–19. doi: 10.1042/BC20040515. [DOI] [PubMed] [Google Scholar]
- Cechetto DF. Central representation of visceral function. Federation proceedings. 1987;46:17–23. [PubMed] [Google Scholar]
- Chan KY, Byers MR. Sensory nerve endings of the incisive papilla of rat hard palate studied by peroxidase cytochemical methods. The Journal of Comparative Neurology. 1985;234:192–200. doi: 10.1002/cne.902340206. [DOI] [PubMed] [Google Scholar]
- Coghill GE. Correlated Anatomical and Physiological Studies of the Growth of the Nervous System of Amphibia. II. The afferent system of the head of Amblystoma. Journal of Comparative Neurology. 1916;26:247–340. [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. American Journal of Anatomy. 1983;166:445–68. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Davies AM, Lindsay RM. The Cranial Sensory Ganglia in Culture: Differences in the Response of Placode-Derived and Neural Crest-Derived Neurons to Nerve Growth Factor. Dev Biol. 1985;111:62–72. [Google Scholar]
- Deban SM, O'Reilly JC, Nishikawa KC. The Evolution of the Motor Control of Feeding in Amphibians. American Zoologist. 2001;41:1280–1298. [Google Scholar]
- Dickinson AJ, Sive H. Development of the primary mouth in Xenopus laevis. Dev Biol. 2006;295:700–13. doi: 10.1016/j.ydbio.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Epperlein HH, Lofberg J. The development of the neural crest in amphibians. Annals of Anatomy. 1993;175:483–99. doi: 10.1016/s0940-9602(11)80207-4. [DOI] [PubMed] [Google Scholar]
- Ernfors P. Local and target-derived actions of neurotrophins during peripheral nervous system development. Cell Mol Life Sci. 2001;58:1036–44. doi: 10.1007/PL00000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck P, Hanken J, Olsson L. Cranial neural crest emergence and migration in the Mexican axolotl (Ambystoma mexicanum) Zoology. 2002;105:195–202. doi: 10.1078/0944-2006-00079. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Mbiene JP. Early development and innervation of taste bud-bearing papillae on the rat tongue. The Journal of Comparative Neurology. 1991;304:172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- Finger TE. What's so special about special visceral? Acta Anatomica. 1993;148:132–8. doi: 10.1159/000147532. [DOI] [PubMed] [Google Scholar]
- Fontaine J, Le Douarin NM. Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J Embryol Exp Morphol. 1977;41:209–22. [PubMed] [Google Scholar]
- Fritzsch B, Gregory D, Rosa-Molinar E. The development of the hindbrain afferent projections in the axolotl: evidence for timing as a specific mechanism of afferent fiber sorting. Zoology (Jena) 2005;108:297–306. doi: 10.1016/j.zool.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. International Journal of Developmental Biology. 1997;15:563–76. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro M, Bronner-Fraser M. Induction and differentiation of the neural crest. Current Opinion in Cell Biology. 1999;11:695–8. doi: 10.1016/s0955-0674(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Gross JB, Gottlieb AA, Barlow LA. Gustatory neurons derived from epibranchial placodes are attracted to, and trophically supported by, taste bud-bearing endoderm in vitro. Dev Biol. 2003;264:467–81. doi: 10.1016/j.ydbio.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Hall BK. The Neural Crest: Including a facsimile reprint of ‘The Neural Crest’ by Sven Hörstadius. Oxford University Press; USA: 1989. [Google Scholar]
- Halsell CB, Travers JB, Travers SP. Gustatory and tactile stimulation of the posterior tongue activate overlapping but distinctive regions within the nucleus of the solitary tract. Brain Res. 1993;632:161–73. doi: 10.1016/0006-8993(93)91151-h. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Experimental analysis of the dual origin of the trigeminal ganglion in the chick embryo. Journal of Experimental Zoology. 1961;148:91–123. doi: 10.1002/jez.1401480202. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Levi-Montalcini RJ. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. Journal of Experimental Zoology. 1949;111:457–502. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Research Reviews. 2005;48:457–76. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Harrison TA, Stadt HA, Kumiski D, Kirby ML. Compensatory responses and development of the nodose ganglion following ablation of placodal precursors in the embryonic chick (Gallus domesticus) Cell Tissue Research. 1995;281:379–85. doi: 10.1007/BF00583407. [DOI] [PubMed] [Google Scholar]
- Hatini V, Ye X, Balas G, Lai E. Dynamics of placodal lineage development revealed by targeted transgene expression. Developmental Dynamics. 1999;215:332–43. doi: 10.1002/(SICI)1097-0177(199908)215:4<332::AID-AJA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Herrick CJ. The cranial nerves and cutaneous sense organs of the North American siluroid fishes. Journal of Comparative Neurology. 1901;11:177–249. [Google Scholar]
- Herrick CJ. The Fasciculus Solitarius and Its Connections in Amphibians and Fishes. Journal of Comparative Neurology. 1944;81:307–331. [Google Scholar]
- Herrick CJ. The Brain of the Tiger Salamander, Ambystoma tigerinum. The University of Chicago Press; Chicago, Illinois: 1948. [Google Scholar]
- His W. Untersuchungen über die erste Anlage des Wirbeltierleibes. Leipzig 1868 [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Hörstadius S. The Neural Crest: Its properties and derivatives in the light of experimental research. Oxford University Press; New York: 1950. [Google Scholar]
- Jacob J, Guthrie S. Facial visceral motor neurons display specific rhombomere origin and axon pathfinding behavior in the chick. J Neurosci. 2000;20:7664–71. doi: 10.1523/JNEUROSCI.20-20-07664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsh RN, Dutton K, Medlin J, Eisen JS. Expression of zebrafish fkd6 in neural crest-derived glia. Mech Dev. 2000;93:161–4. doi: 10.1016/s0925-4773(00)00250-1. [DOI] [PubMed] [Google Scholar]
- Kious BM, Baker CV, Bronner-Fraser M, Knecht AK. Identification and characterization of a calcium channel gamma subunit expressed in differentiating neurons and myoblasts. Dev Biol. 2002;243:249–59. doi: 10.1006/dbio.2001.0570. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Nodose placode contributes autonomic neurons to the heart in the absence of cardiac neural crest. J Neurosci. 1987;8:1089–95. doi: 10.1523/JNEUROSCI.08-04-01089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML. Nodose placode provides ectomesenchyme to the developing chick heart in the absence of cardiac neural crest. Cell Tissue Research. 1988;252:17–22. doi: 10.1007/BF00213821. [DOI] [PubMed] [Google Scholar]
- Kishida R, Goris RC, Nishizawa H, Koyama H, Kadota T, Amemiya F. Primary neurons of the lateral line nerves and their central projections in hagfishes. J Comp Neurol. 1987;264:303–10. doi: 10.1002/cne.902640303. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW. Plakophilin, armadillo repeats, and nuclear localization. Microscopy Research and Technique. 1999;45:43–54. doi: 10.1002/(SICI)1097-0029(19990401)45:1<43::AID-JEMT4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Davis BM, Noel T, Albers KM. Overexpression of neurotrophin 4 in skin enhances myelinated sensory endings but does not influence sensory neuron number. Journal of Comparative Neurology. 2006;498:455–65. doi: 10.1002/cne.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani SC, Miyagawa-Tomita S, Kirby ML. Development of cranial nerves in the chick embryo with special reference to the alterations of cardiac branches after ablation of the cardiac neural crest. Anat Embryol (Berl) 1991;183:501–14. doi: 10.1007/BF00186439. [DOI] [PubMed] [Google Scholar]
- Landacre FL. The origin of the cranial ganglia in Ameiurus. Journal of Comparative Neurology. 1910;20:309–411. [Google Scholar]
- Landacre FL. The epibranchial placodes of Lepidosteus osseus and their relation to the cerebral ganglia. Journal of Comparative Neurology. 1912;22:1–69. [Google Scholar]
- Landacre FL. The fate of the neural crest in the head of urodeles. Journal of Comparative Neurology. 1921;33:1–44. [Google Scholar]
- Landacre FL. Data on the relative time of formation of the cerebral ganglia of Amblystoma jeffersonianum. Journal of Comparative Neurology. 1931;53 [Google Scholar]
- Landacre FL. The epibranchial placode of the facial nerve in Amblystroma jeffersonianum. Journal of Comparative Neurology. 1933;58:289–309. [Google Scholar]
- Le Douarin NM. Ontogeny of the peripheral nervous system from the neural crest and the placodes. A developmental model studied on the basis of the quail-chick chimaera system. Harvey Lectures. 1984;80:137–86. [PubMed] [Google Scholar]
- Le Douarin NM, Dupin E. Cell lineage analysis in neural crest ontogeny. Journal of Neurobiology. 1993;24:146–61. doi: 10.1002/neu.480240203. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41:162–84. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci. 1999;19:5919–31. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–21. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Barde YA, Davies AM, Rohrer H. Differences and similarities in the neurotrophic growth factor requirements of sensory neurons derived from neural crest and neural placode. Journal of Cell Science Supplement. 1985a;3:115–29. doi: 10.1242/jcs.1985.supplement_3.12. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Thoenen H, Barde YA. Placode and neural crest-derived sensory neurons are responsive at early developmental stages to brain-derived neurotrophic factor. Dev Biol. 1985b;112:319–28. doi: 10.1016/0012-1606(85)90402-6. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005 doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Dev Biol. 2006a;292:457–68. doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Refinement of innervation accuracy following initial targeting of peripheral gustatory fibers. J Neurobiol. 2006b;66:1033–43. doi: 10.1002/neu.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwigale PY. Embryonic origin of avian corneal sensory nerves. Dev Biol. 2001;239:323–37. doi: 10.1006/dbio.2001.0450. [DOI] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130:6361–74. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Turner DF. Sensory nerve endings in the rat oro-facial region labeled by the anterograde and transganglionic transport of horseradish peroxidase: a new method for tracing peripheral nerve fibers. Brain Res. 1983;261:1–12. doi: 10.1016/0006-8993(83)91277-5. [DOI] [PubMed] [Google Scholar]
- Martin MR, Mason CA. The seventh cranial nerve of the rat. Visualization of efferent and afferent pathways by cobalt precipitation. Brain Res. 1977;121:21–41. doi: 10.1016/0006-8993(77)90436-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Emori Y, Ninomiya Y, Abe K. A comparative study of three cranial sensory ganglia projecting into the oral cavity: in situ hybridization analyses of neurotrophin receptors and thermosensitive cation channels. Brain Research. Molecular Brain Research. 2001;93:105–12. doi: 10.1016/s0169-328x(01)00129-2. [DOI] [PubMed] [Google Scholar]
- Nagai T. Fluorescent dye (DiI) reveals the sensory cells in the lingual epithelium: a confocal laser scanning microscopic study. Jpn J Physiol. 1993a;43 1:S179–81. [PubMed] [Google Scholar]
- Nagai T. Transcellular labeling by DiI demonstrates the glossopharyngeal innervation of taste buds in the lingual epithelium of the axolotl. J Comp Neurol. 1993b;331:122–33. doi: 10.1002/cne.903310108. [DOI] [PubMed] [Google Scholar]
- Nagai T, Matsushima T. Morphology and distribution of the glossopharyngeal nerve afferent and efferent neurons in the Mexican salamander, axolotl: a cobaltic-lysine study. Journal of Comparative Neurology. 1990;302:473–84. doi: 10.1002/cne.903020305. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Narayanan Y. Neural crest and placodal contributions in the development of the glossopharyngeal-vagal complex in the chick. Anat Rec. 1980;196:71–82. doi: 10.1002/ar.1091960108. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–23. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–30. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2007;236:564–71. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Distribution and innervation of lateral line organs in the axolotl. Journal of Comparative Neurology. 1992;325:95–123. doi: 10.1002/cne.903250109. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Barlow LA, Braun CB, Catania KC. Distribution and innervation of taste buds in the axolotl. Brain Behavior and Evolution. 2000;56:123–45. doi: 10.1159/000047200. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Barlow LA, Catania KC, Braun CB. Developmental fate of the lateral and medial walls of the neural folds in axolotls. American Zoologist. 1996;36:74A. [Google Scholar]
- Northcutt RG, Brandle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev Biol. 1995;168:358–73. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brändle K. Development of branchiomeric and lateral line nerves in the axolotl. Journal of Comparative Neurology. 1995;355:427–54. doi: 10.1002/cne.903550309. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Catania KC, Criley BB. Development of lateral line organs in the axolotl. Journal of Comparative Neurology. 1994;340:480–514. doi: 10.1002/cne.903400404. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–42. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Opdam P, Nieuwenhuys R. Topological Analysis of the Brain Stem of the Axolotl Ambystoma mexicanum. Journal of Comparative Neurology. 1976;165:285–306. doi: 10.1002/cne.901650303. [DOI] [PubMed] [Google Scholar]
- Parker MA, Bell ML, Barlow LA. Cell contact-dependent mechanisms specify taste bud pattern during a critical period early in embryonic development. Developmental Dynamics. 2004;230:630–42. doi: 10.1002/dvdy.20086. [DOI] [PubMed] [Google Scholar]
- Phelan KD, Falls WM. The spinotrigeminal pathway and its spatial relationship to the origin of trigeminospinal projections in the rat. Neuroscience. 1991;40:477–96. doi: 10.1016/0306-4522(91)90135-b. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Storch EM, Lekven AC, Riley BB. A direct role for Fgf but not Wnt in otic placode induction. Development. 2004;131:923–31. doi: 10.1242/dev.00978. [DOI] [PubMed] [Google Scholar]
- Roth G, Nishikawa KC, Naujoks-Manteuffel C, Schmidt A, Wake DB. Paedomorphosis and simplification in the nervous system of salamanders. Brain Behavior and Evolution. 1993;42:137–70. doi: 10.1159/000114147. [DOI] [PubMed] [Google Scholar]
- Roth G, Wake DB. The structure of the brainstem and cervical spinal cord in lungless salamanders (family plethodontidae) and its relation to feeding. Journal of Comparative Neurology. 1985;241:99–110. doi: 10.1002/cne.902410108. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–51. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Garcia-Castro MI, Artinger KB, Bronner-Fraser M. Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development. 1998;125:4919–30. doi: 10.1242/dev.125.24.4919. [DOI] [PubMed] [Google Scholar]
- Smith DV, Davis BJ. Chapter 14: Neural Representation of Taste. In: Finger TE, Silver WL, Restrepo D, editors. The Neurobiology of Taste and Smell. 2nd. Wiley-Liss, Inc.; New York: 2000. pp. 353–392. [Google Scholar]
- Sobkow L, Epperlein HH, Herklotz S, Straube WL, Tanaka EM. A germline GFP transgenic axolotl and its use to track cell fate: dual origin of the fin mesenchyme during development and the fate of blood cells during regeneration. Dev Biol. 2006;290:386–97. doi: 10.1016/j.ydbio.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci U S A. 1990;87:923–7. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS. Experiments on the Development of the Cranial Ganglia and the Lateral Line Sense Organs in Amblystoma Punctatum. Journal Experimental Zoology. 1922;35:421–96. [Google Scholar]
- Suemune S, Nishimori T, Hosoi M, Suzuki Y, Tsuru H, Kawata T, Yamauchi K, Maeda N. Trigeminal nerve endings of lingual mucosa and musculature of the rat. Brain Res. 1992;586:162–5. doi: 10.1016/0006-8993(92)91389-v. [DOI] [PubMed] [Google Scholar]
- Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–86. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Takahama H, Sasaki F, Watanabe K. Morphological changes in the oral (buccopharyngeal) membrane in urodelan embryos: development of the mouth opening. J Morphol. 1988;195:59–69. doi: 10.1002/jmor.1051950106. [DOI] [PubMed] [Google Scholar]
- Tam PP. Regionalisation of the mouse embryonic ectoderm: allocation of prospective ectodermal tissues during gastrulation. Development. 1989;107:55–67. doi: 10.1242/dev.107.1.55. [DOI] [PubMed] [Google Scholar]
- Waterman RE. Ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the hamster embryo. Dev Biol. 1977;58:219–29. doi: 10.1016/0012-1606(77)90088-4. [DOI] [PubMed] [Google Scholar]
- Waterman RE, Schoenwolf GC. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the chick embryo. Anat Rec. 1980;197:441–70. doi: 10.1002/ar.1091970408. [DOI] [PubMed] [Google Scholar]
- Yntema CL. An experimental study of the origin of the cells which constitute the VIIth and VIIIth cranial ganglia and nerves in the embryo of Amblystoma punctatum. Journal of Experimental Zoology. 1937;75:75–101. [Google Scholar]
- Yntema CL. An experimental study of the origin of sensory neurons and sheath cells of the IXth and Xth cranial nerves in Amblystoma punctatum. Journal of Experimental Zoology. 1943;92:93–119. [Google Scholar]
- Yntema CL. Experiments on the origin of the sensory ganglia of the facial nerve in the chick. Journal of Comparative Neurology. 1944;81:147–164. [Google Scholar]
- Zhang LL, Ashwell KW. The development of cranial nerve and visceral afferents to the nucleus of the solitary tract in the rat. Anat Embryol (Berl) 2001;204:135–51. doi: 10.1007/s004290100185. [DOI] [PubMed] [Google Scholar]