Abstract
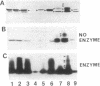
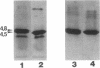
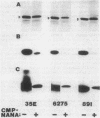
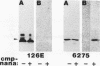
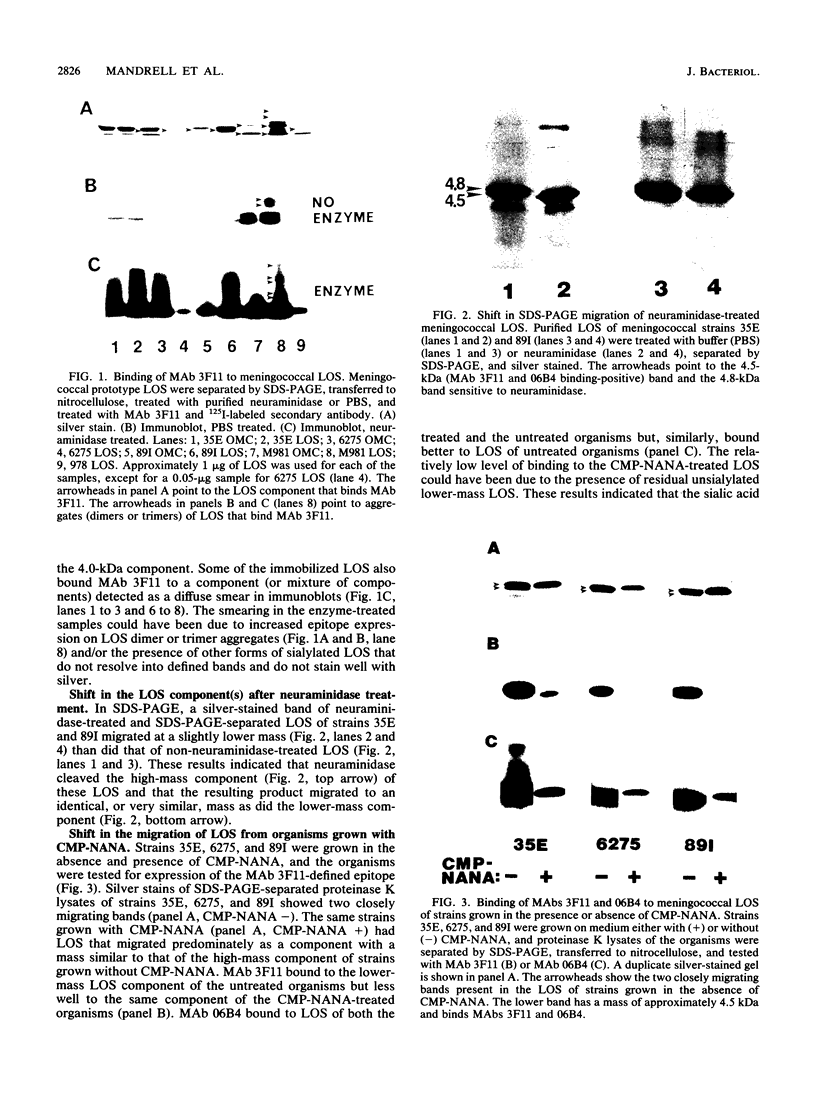
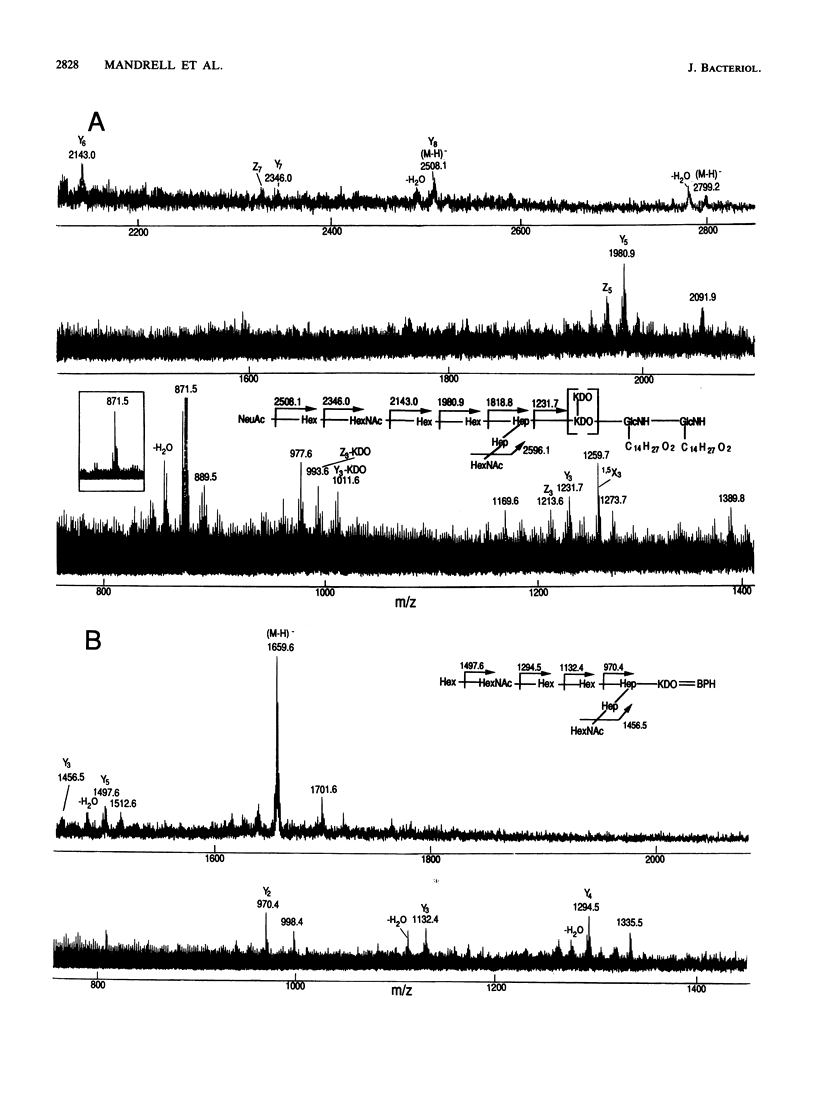
Monoclonal antibodies (MAb) 3F11 and 06B4 recognize epitopes that are conserved on gonococcal lipooligosaccharides (LOS), present on some meningococcal LOS, and conserved on human erythrocytes. LOS of some group B and C prototype meningococcal LOS strains (LOS serotypes L1 to L8) treated with neuraminidase showed increased expression of the 3F11 and 06B4 MAb-defined epitopes. Neuraminidase-treated LOS separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver stained showed a shift in migration from a component with a mass of approximately 4.8 kDa to a component with a mass of between 4.5 and 4.6 kDa. The same strains grown in medium with excess CMP-N-acetylneuraminic acid had LOS that shifted in migration to a slightly higher component (mass, approximately 4.8 kDa). Chemical analysis of the neuraminidase-digested products from one LOS indicated it contained approximately 1.5% sialic acid. Covalent linkage between sialic acid and the LOS was confirmed by analysis of de-O-acylated and dephosphorylated LOS by liquid secondary ion mass spectrometry. Three studies show that some meningococci contain sialic acid in their LOS, that the sialic acid is cleaved and lost in conventional acetic acid hydrolysis, and that the sialic acid alters the expression of MAb-defined epitopes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed H., Gabius H. J. Purification and properties of a Ca2+-independent sialic acid-binding lectin from human placenta with preferential affinity to O-acetylsialic acids. J Biol Chem. 1989 Nov 5;264(31):18673–18678. [PubMed] [Google Scholar]
- Apicella M. A., Bennett K. M., Hermerath C. A., Roberts D. E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981 Dec;34(3):751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Mandrell R. E., Shero M., Wilson M. E., Griffiss J. M., Brooks G. F., Lammel C., Breen J. F., Rice P. A. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis. 1990 Aug;162(2):506–512. doi: 10.1093/infdis/162.2.506. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Shero M., Jarvis G. A., Griffiss J. M., Mandrell R. E., Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987 Aug;55(8):1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKLOW R. S., WARREN L. Biosynthesis of sialic acids by Neisseria meningitidis. J Biol Chem. 1962 Nov;237:3520–3526. [PubMed] [Google Scholar]
- Barondes S. H. Soluble lectins: a new class of extracellular proteins. Science. 1984 Mar 23;223(4642):1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- Brackmann T., Geldmeyer S., Jahn R., Söling H. D. Radioenzymatic determination of CMP-N-acetylsialic acid and free N-acetylsialic acid in biological material. Anal Biochem. 1983 Apr 15;130(2):369–375. doi: 10.1016/0003-2697(83)90601-2. [DOI] [PubMed] [Google Scholar]
- Campagnari A. A., Spinola S. M., Lesse A. J., Kwaik Y. A., Mandrell R. E., Apicella M. A. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990 May;8(5):353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Dell A., Azadi P., Tiller P., Thomas-Oates J., Jennings H. J., Beurret M., Michon F. Analysis of oligosaccharide epitopes of meningococcal lipopolysaccharides by fast-atom-bombardment mass spectrometry. Carbohydr Res. 1990 Apr 25;200:59–76. doi: 10.1016/0008-6215(90)84182-t. [DOI] [PubMed] [Google Scholar]
- Dell A., Carman N. H., Tiller P. R., Thomas-Oates J. E. Fast atom bombardment mass spectrometric strategies for characterizing carbohydrate-containing biopolymers. Biomed Environ Mass Spectrom. 1988 Oct;16(1-12):19–24. doi: 10.1002/bms.1200160104. [DOI] [PubMed] [Google Scholar]
- Falick A. M., Wang G. H., Walls F. C. Ion source for liquid matrix secondary ionization mass spectrometry. Anal Chem. 1986 Jun;58(7):1308–1311. doi: 10.1021/ac00298a009. [DOI] [PubMed] [Google Scholar]
- Fox A. J., Jones D. M., Scotland S. M., Rowe B., Smith A., Brown M. R., Fitzgeorge R. G., Baskerville A., Parsons N. J., Cole J. A. Serum killing of meningococci and several other gram-negative bacterial species is not decreased by incubating them with cytidine 5'-monophospho-N-acetyl neuraminic acid. Microb Pathog. 1989 Oct;7(4):317–318. doi: 10.1016/0882-4010(89)90050-8. [DOI] [PubMed] [Google Scholar]
- Frosch M., Weisgerber C., Meyer T. F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. N., Dell A., Oates J. E., Wu P., Klock J. C., Fukuda M. Structures of glycosphingolipids isolated from human granulocytes. The presence of a series of linear poly-N-acetyllactosaminylceramide and its significance in glycolipids of whole blood cells. J Biol Chem. 1985 Jan 25;260(2):1067–1082. [PubMed] [Google Scholar]
- Gibson B. W., Webb J. W., Yamasaki R., Fisher S. J., Burlingame A. L., Mandrell R. E., Schneider H., Griffiss J. M. Structure and heterogeneity of the oligosaccharides from the lipopolysaccharides of a pyocin-resistant Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1989 Jan;86(1):17–21. doi: 10.1073/pnas.86.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D., Goroff D. K., Baker C. J. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J Infect Dis. 1984 Jul;150(1):71–79. doi: 10.1093/infdis/150.1.71. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Schneider H., Mandrell R. E., Yamasaki R., Jarvis G. A., Kim J. J., Gibson B. W., Hamadeh R., Apicella M. A. Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S287–S295. doi: 10.1093/cid/10.supplement_2.s287. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Blood group ABH and Ii antigens of human erythrocytes: chemistry, polymorphism, and their developmental change. Semin Hematol. 1981 Jan;18(1):39–62. [PubMed] [Google Scholar]
- Hammond B. W., Kingsbury D. T., Weiss E. Modification of meningococcal polysaccharide antigens for use in passive hemagglutination tests. J Immunol. 1968 Oct;101(4):808–809. [PubMed] [Google Scholar]
- Helander I. M., Lindner B., Brade H., Altmann K., Lindberg A. A., Rietschel E. T., Zähringer U. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd-/b+. Description of a novel deep-rough chemotype. Eur J Biochem. 1988 Nov 15;177(3):483–492. doi: 10.1111/j.1432-1033.1988.tb14398.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Bhattacharjee A. K., Kenne L., Kenny C. P., Calver G. The R-type lipopolysaccharides of Neisseria meningitidis. Can J Biochem. 1980 Feb;58(2):128–136. doi: 10.1139/o80-018. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Hawes G. B., Adams G. A., Kenny C. P. The chemical composition and serological reactions of lipopolysaccharides from serogroups A,B,X, and Y Neisseria meningitidis. Can J Biochem. 1973 Oct;51(10):1347–1354. doi: 10.1139/o73-178. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Johnson K. G., Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983 Sep 16;121:233–241. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Griffiss J. M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989 Feb;57(2):602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MERGENHAGEN S. E., MARTIN G. R., SCHIFFMANN E. Studies on an endotoxin of a group C Neisseria meningitidis. J Immunol. 1963 Feb;90:312–317. [PubMed] [Google Scholar]
- Macher B. A., Klock J. C., Fukuda M. N., Fukuda M. Isolation and structural characterization of human lymphocyte and neutrophil gangliosides. J Biol Chem. 1981 Feb 25;256(4):1968–1974. [PubMed] [Google Scholar]
- Maloney P. C., Schneider H., Brandt B. L. Production and degradation of serogroup B Neisseria meningitidis polysaccharide. Infect Immun. 1972 Nov;6(5):657–658. doi: 10.1128/iai.6.5.657-661.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977 May;16(2):471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J Immunol. 1982 Nov;129(5):2172–2178. [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F., Beurret M., Gamian A., Brisson J. R., Jennings H. J. Structure of the L5 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem. 1990 May 5;265(13):7243–7247. [PubMed] [Google Scholar]
- Nairn C. A., Cole J. A., Patel P. V., Parsons N. J., Fox J. E., Smith H. Cytidine 5'-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988 Dec;134(12):3295–3306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Patel P. V., Tan E. L., Andrade J. R., Nairn C. A., Goldner M., Cole J. A., Smith H. Cytidine 5'-monophospho-N-acetyl neuraminic acid and a low molecular weight factor from human blood cells induce lipopolysaccharide alteration in gonococci when conferring resistance to killing by human serum. Microb Pathog. 1988 Oct;5(4):303–309. doi: 10.1016/0882-4010(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Phillips N. J., John C. M., Reinders L. G., Gibson B. W., Apicella M. A., Griffiss J. M. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed Environ Mass Spectrom. 1990 Nov;19(11):731–745. doi: 10.1002/bms.1200191112. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead A., Main J. S., Ward M. E., Watt P. J. Studies on lipopolysaccharides isolated from strains of Neisseria gonorrhoeae. J Gen Microbiol. 1975 May;88(1):123–131. doi: 10.1099/00221287-88-1-123. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Hyver K., Honovich J., Cotter R. J., Mascagni P., Schneider H. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. Combined laser desorption and fast atom bombardment mass spectral analysis of high performance liquid chromatography-purified dimethyl derivatives. J Biol Chem. 1986 Aug 15;261(23):10624–10631. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WARREN L., BLACKLOW R. S. The biosynthesis of cytidine 5'-monophospho-n-acetylneuraminic acid by an enzyme from Neisseria meningitidis. J Biol Chem. 1962 Nov;237:3527–3534. [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman G. M., Caird J. D. Composition of the lipopolysaccharide of Neisseria gonorrhoeae. Infect Immun. 1977 May;16(2):550–556. doi: 10.1128/iai.16.2.550-556.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R., Schneider H., Griffiss J. M., Mandrell R. Epitope expression of gonococcal lipooligosaccharide (LOS). Importance of the lipoidal moiety for expression of an epitope that exists in the oligosaccharide moiety of LOS. Mol Immunol. 1988 Aug;25(8):799–809. doi: 10.1016/0161-5890(88)90116-2. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]