Abstract
Rationale: Several occupational exposures adversely affect lung function.
Objectives: This study reports the influence of continued occupational dust and fume exposures on the rate of decline of lung function in participants with early chronic obstructive pulmonary disease (COPD) studied in a population-based study.
Methods: Subjects consisted of 5,724 participants in the Lung Health Study, a multicenter study of smoking cessation and anticholinergic bronchodilator administration in smokers with early COPD (3,592 men; 2,132 women). Average post-bronchodilator FEV1 at entry was 78.4% predicted for men and 78.2% predicted for women; all participants had an FEV1/FVC ratio less than 0.70.
Measurements and Main Results: Participants underwent a baseline evaluation and five annual follow-up assessments, including questionnaires and spirometry. The effect of ongoing dust or fume exposure on FEV1 in each follow-up year was statistically evaluated with a mixed-effects regression model, which was adjusted for FEV1 at entry, age, airway responsiveness to methacholine, baseline smoking intensity, and time-varying (yearly) smoking status during each follow-up year. In men with early COPD, each year of continued fume exposure was associated with a 0.25% predicted reduction in post-bronchodilator FEV1% predicted. Continued smoking and airway hyperresponsiveness were also associated with reduction in FEV1 during each year of follow-up in both men and women. Statistically significant effects of dust exposure on the rate of decline were not found, nor were effects of fume exposure noted in women.
Conclusions: These results suggest a need for secondary prevention by controlling occupational fume exposures.
Keywords: forced expiratory volume; longitudinal studies; pulmonary disease, chronic obstructive
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Many studies of the influence of occupational exposure on chronic obstructive pulmonary disease (COPD) have been cross sectional. Few were based on groups of subjects with early COPD.
What This Study Adds to the Field
This longitudinal study demonstrates that ongoing occupational exposure to fumes is associated with an increased rate of decline of lung function in persons with COPD.
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of mortality and morbidity (1–3). Although the primary cause of COPD is tobacco smoking, increasing evidence implicates occupational and environmental exposures as additional etiologic factors (4, 5). Occupational exposures may influence the course of COPD in several ways: (1) causing COPD; (2) modifying the effect of tobacco smoke in causing COPD, such as amplifying its adverse impact; (3) creating greater disability by adding exposure-related impairment to that due to tobacco smoking; and (4) accelerating the rate of decline of respiratory function in persons with established COPD.
In this study, we investigated the impact of occupational exposures upon the course of lung function change over 5 years in persons with early COPD enrolled in the Lung Health Study (LHS). The LHS is a large, multicenter trial of smoking cessation and bronchodilator administration in smokers with early COPD (6).
Both tobacco smoking and occupational exposures have been associated with accelerated decline of lung function (7–10). The unusually detailed and careful annual follow-up of smoking, occupational exposures, and lung function assessments of the LHS facilitate distinguishing the effects of both smoking and occupational exposure.
It is generally believed that the effect of tobacco smoking is greater than that of most occupational exposures, and because of the overwhelming effect of tobacco smoking, it is often difficult to discern additional effects of occupational exposure. Despite this, many studies have shown that occupation has a significant contributory role (4, 5, 11). In this article, we report analyses of the impact of ongoing fume and dust exposure on the rate of progression of COPD. Some of the results of these studies have been previously reported in the form of an abstract (12).
METHODS
Participants
All participants had early COPD, which was defined by a post-bronchodilator FEV1/FVC ratio of less than 0.70 and an FEV1 between 55 and 90% of predicted. All participants were current smokers at the time of entry. Participants were required to provide written informed consent, approved by each clinical center's human subjects review board, before entry into the study. Participants were recruited in 10 study centers in the United States and Canada. The study participants were 95.8% white, 3.8% African American, 0.1% Asian; 0.6% were Hispanic, and are included in the white group (13). After screening to determine eligibility, each participant underwent a baseline evaluation, including spirometry, interview, and measurement of airway responsiveness by methacholine challenge testing (14–17). Participants were then randomly assigned to usual care or to a special smoking cessation intervention group (1,964 and 3,923 participants, respectively). In addition, those in the special intervention group were also randomly assigned to receive an inhaled anticholinergic bronchodilator or a placebo inhaler (n = 1961 and 1962, respectively) (18, 19). Participants underwent follow-up evaluation, including repeated questionnaires and spirometry, on an annual basis for 5 years.
Interview Data
Several questions concerning employment were incorporated into the baseline interview. These included: (1) employment status (not working, employed full time, employed part time); (2) current/most recent job (title, industry, and field); (3) usual job (job held longest: title, industry, and field); (4) self-reported “dust exposure,” “fume exposure,” or “mask use” for current job. Tobacco use questions included intensity of smoking at entry (expressed as cigarettes/d) and age at which smoking began.
During the five annual follow-up visits, the questions about employment status and self-reported exposures (dust exposure, fume exposure, and mask use) were repeated. In addition, smoking status was ascertained by questionnaire and verified by end-expired CO and salivary cotinine measurements. Spirometry testing was performed at each of the annual follow-up visits.
Spirometry, using standardized techniques, was performed before and after inhalation of a short-acting bronchodilator (200 μg isoproterenol) (14). Results are expressed as percentage of the predicted value using the equations of Crapo and colleagues (20) to be consistent with prior publications concerning the LHS. Predicted values for African Americans were calculated by multiplying the corresponding predicted value for white participants by 0.88. Spirometry results are expressed as percentage of the predicted value, rather than as an absolute value, to scale for body size and to track an individual's course over time, even if height changed. Intensity of smoking at baseline (cigarettes/d) was selected as the major baseline measure of smoking, because earlier analyses of the LHS have shown this to be an important predictor of subsequent rate of decline of FEV1 (21).
Airway responsiveness was determined by methacholine challenge using a modification of the Chai protocol (22). Airway responsiveness was expressed by the slope of methacholine dose–FEV1 relationship (methacholine challenge test slope [SMCT]). The SMCT was calculated as: (final FEV1 − baseline FEV1)/(cumulative concentration of methacholine attained). An increasingly negative SMCT implies greater airway responsiveness.
Using a general, linear, mixed-effects model, post-bronchodilator FEV1% predicted at each follow-up year was regressed on both baseline characteristics and time-varying characteristics. Baseline characteristics included age, airway responsiveness determined from methacholine challenge testing at entry (as SMCT), baseline FEV1% predicted, and a measure of baseline occupational exposure. Time-dependent variables included self-reported occupational exposure and self-reported smoking (smoking status, cigarettes/d, or smoking intensity as a categorical variable). Self-reported occupational exposure to dust and fume at baseline and each follow-up year were used as indicators of occupational exposure. Dust and fume exposure were used in separate models. Persons who were not working were considered not occupationally exposed. The model also included year of study and incorporated a first-order autoregressive covariance structure (23).
Smoking intensity at baseline was expressed as cigarettes per day. Smoking during the follow-up years was expressed in several alternative ways: smoking status (smoking, not smoking); smoking category (using six categories defined by cigarettes/d: 0, 1–15, 16–25, 26–35, 36–45, ⩾ 45); and cigarettes/day.
Separate models were used for dust exposure and for fume exposure; all analyses were stratified by gender. Several combinations of methods expressing exposure and smoking were used. Similar analyses were conducted for FVC% predicted (pre- and post-bronchodilator) and for prebronchodilator FEV1% predicted.
Hypothesis testing was conducted assuming that each regression model was independent. There was no explicit adjustment of hypothesis testing P values for the multiple comparisons consequent to the use of multiple models; each model's p value was calculated independently.
RESULTS
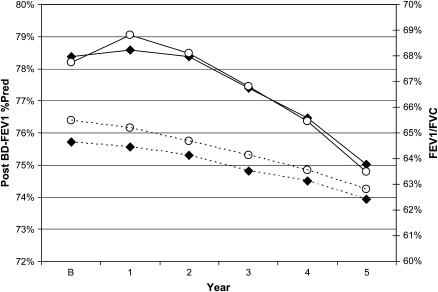
The study included 3,592 men and 2,132 women who participated in the LHS and who did not have missing data. Recruitment began in October 1986. Table 1 summarizes the characteristics of the participants. The average age at baseline was 48.4 and 48.5 years for men and women, respectively (age range, 34–66 yr for men and 35–67 yr for women). Participants had an average of 5.6 annual visits, including the initial survey (78.4% had all five follow-up visits); 5,334 were still participating at Year 5. All were smokers at the initiation of the study. The average prebronchodilator FEV1 for men at baseline was 75.2% of predicted, with a range of 48.2–92.4%, whereas average prebronchodilator FEV1 for women at baseline was 75.1% of predicted, with a range of 51.8–95.7%. The average airway responsiveness was −9.5 ml/mg (SD = 19.4) in men and −18.2 ml/mg (SD = 28.1) in women at baseline. Figure 1 shows the progression of the post-bronchodilator FEV1 and the FEV1/FVC ratio over the course of the study.
TABLE 1.
CHARACTERISTICS OF PARTICIPANTS
| Characteristics | Baseline | Year 5 |
|---|---|---|
| Men | ||
| No. of participants | 3,592 | 3,343 |
| Spirometry, pre-BD, mean (SD) | ||
| FEV1% predicted | 75.2 (8.8) | 71.1 (12.3) |
| FVC% predicted | 96.6 (10.6) | 93.3 (12.0) |
| FEV1/FVC ratio | 62.7 (5.7) | 60.7 (7.8) |
| Spirometry, post-BD, mean (SD) | ||
| FEV1% predicted | 78.4 (9.2) | 75.0 (12.0) |
| FVC% predicted | 97.6 (10.6) | 95.9 (11.7) |
| FEV1/FVC ratio | 64.6 (6.2) | 62.4 (8.0) |
| Women | ||
| No. of participants | 2,132 | 1,992 |
| Spirometry, pre-BD, mean (SD) | ||
| FEV1% predicted | 75.1 (8.8) | 70.6 (12.7) |
| FVC% predicted | 97.3 (10.3) | 93.3 (12.6) |
| FEV1/FVC ratio | 63.3 (5.3) | 61.0 (7.4) |
| Spirometry, post-BD, mean (SD) | ||
| FEV1% predicted | 78.2 (9.0) | 74.8 (12.3) |
| FVC% predicted | 98.1 (10.4) | 96.3 (11.8) |
| FEV1/FVC ratio | 65.5 (5.9) | 62.8 (7.9) |
Definition of abbreviation: BD = bronchodilator.
Figure 1.
Post-bronchodilator (BD) FEV1 and FEV1/FVC ratio by year. The figure shows the post-BD FEV1 (% predicted) and FEV1/FVC ratio at baseline (B) and each follow-up year (men, solid diamonds; women, open circles; FEV1, solid lines; FEV1/FVC ratio, dashed lines).
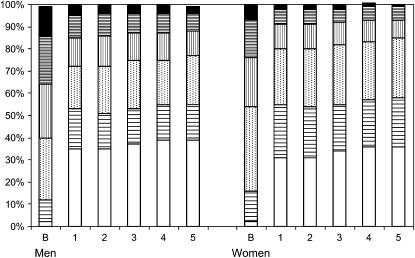
Figure 2 shows the smoking status of the participants at baseline and during follow-up: 50.7% continued to smoke throughout the follow-up visits; 19.8% were sustained quitters (persons who did not report smoking at any of the follow-up visits); and 29.5% were intermittent smokers, according to LHS criteria. Figure 2 summarizes the smoking status and intensity (cigarettes/d) throughout the study. Among those smoking, the average number of cigarettes smoked per day was 31.7 at baseline and 23.8 at Year 5 for men and 27.6 at baseline and 20.4 at Year 5 for women. At baseline, men had smoked 42.8 pack-years (SD = 20.1) and women had smoked 36.2 (SD = 16.5) pack-years. Overall, 50.7% continued to smoke throughout the follow-up visits, 19.8% were sustained quitters, and 29.5% were intermittent smokers, according to LHS criteria.
Figure 2.
Smoking category at baseline and each follow-up year. The figure shows the proportion of subjects in each smoking intensity category at baseline (B) and each follow-up year. The lower segment of each bar (no fill) represents the % of nonsmokers (solid black segment). The segments above sequentially represent smoking category by cigarettes/day: 1–15 (horizontal bars); 16–25 (dots); 26–35 (vertical bars); 36–45 (dense horizontal bars); 46 or more (solid black).
Table 2 summarizes the exposure status and employment status for participants at baseline and at Year 5. Most remained actively employed; 91% of participating men were employed at baseline, and 78% were employed at the Year 5 follow-up. At baseline and at each follow-up visit, men reported significantly more dust and fume exposure than did women. For example, at baseline, 19.8% of men and 8.9% of women reported occupational fume exposure (P < 0.0001 by Chi-square test). Part of this difference was due to the higher proportion of men who were actively employed; for example, at baseline, 91% of men and 84.3% of women were currently employed. However, even limiting analysis to those currently employed, differences in exposure between men and women were seen (at baseline, 21.8% of employed men reported fume exposure, but only 8.9% of employed women did so).
TABLE 2.
EMPLOYMENT AND EXPOSURE STATUS
| Baseline | Year 5 | |
|---|---|---|
| Characteristics | n (%) | n (%) |
| Men | ||
| Employment | ||
| Yes | 3,269 (91.0) | 2,602 (77.8) |
| No | 323 (9.0) | 740 (22.1) |
| Unknown | 0 (0.0) | 1 (0.0) |
| Exposure | ||
| Dust only | 287 (8.0) | 268 (8.0) |
| Fume only | 213 (5.9) | 128 (3.8) |
| Both | 499 (13.9) | 690 (20.6) |
| Neither | 2,593 (72.2) | 2,257 (67.5) |
| Women | ||
| Employment | ||
| Yes | 1,797 (84.3) | 1,322 (66.4) |
| No | 335 (15.7) | 669 (33.6) |
| Unknown | 0 (0.0) | 1 (0.1) |
| Exposure | ||
| Dust only | 121 (5.7) | 167 (8.4) |
| Fume only | 85 (3.8) | 50 (2.5) |
| Both | 109 (5.1) | 186 (9.3) |
| Neither | 1,822 (85.5) | 1,589 (79.8) |
The analyses of the impact of self-reported dust and fume exposure among men is summarized in Table 3. Four outcome variables were assessed (all are % predicted, which, by definition, are adjusted for age, height, gender, and race): FEV1 prebronchodilator; FEV1 post-bronchodilator; FVC prebronchodilator; and FVC post-bronchodilator (the latter two are not shown in the table). All models include baseline lung function as a covariate; therefore, the regression coefficients express the influence of predictor variables upon the annual rate of decline of lung function.
TABLE 3.
EFFECT OF EXPOSURE AND OTHER CHARACTERISTICS ON POST-BRONCHODILATOR FEV1 (MEN)
| Exposure Effect
|
Smoking
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline
|
Follow-Up
|
Baseline (Cigs/d) | Follow-Up
|
Baseline Characteristics
|
|||||
| Model | Dust | Fume | Dust | Fume | Status | Intensity | Age | AR | |
| 1 | — | — | −0.143 (NS) | — | −0.006 (NS) | −1.986 (<0.0001) | — | −0.103 (<0.0001) | 0.032 (<0.0001) |
| 2 | — | — | −0.132 (NS) | — | 0.005 (NS) | −1.248 (<0.0001) | −0.353 (<0.0001) | −0.105 (<0.0001) | 0.032 (<0.0001) |
| 3 | 0.185 (NS) | — | −0.126 (NS) | — | −0.006 (NS) | −1.985 (<0.0001) | — | −0.103 (<0.0001) | 0.032 (<0.0001) |
| 4 | 0.154 (NS) | — | −0.118 (NS) | — | 0.005 (NS) | −1.248 (<0.0001) | −0.352 (<0.0001) | −0.106 (<0.0001) | 0.032 (<0.0001) |
| 5 | — | — | — | −0.263 (0.011) | −0.006 (NS) | −1.986 (<0.0001) | — | −0.103 (<0.0001) | 0.032 (<0.0001) |
| 6 | — | — | — | −0.25 (0.015) | 0.005 (NS) | −1.249 (<0.0001) | −0.352 (< 0.0001) | −0.106 (<0.0001) | 0.032 (<0.0001) |
| 7 | — | 0.376 (NS) | — | −0.228 (0.03) | −0.006 (NS) | −1.983 (<0.0001) | — | −0.104 (<0.0001) | 0.031 (<0.0001) |
| 8 | — | 0.354 (NS) | — | −0.218 (0.038) | 0.005 (NS) | −1.249 (<0.0001) | −0.351 (<0.0001) | −0.107 (<0.0001) | 0.032 (<0.0001) |
Definition of abbreviations: AR = airway responsiveness; Cigs = cigarettes; NS = not significant.
The table shows results of analyses for men. Each row represents an individual analysis (model); em-dashes indicate variables not included in the model; the regression coefficients in FEV1 % predicted per year and P values (in parentheses) are shown. Effects of exposure to dust and to fume are shown for baseline and for follow-up years. Baseline smoking is described as cigarettes per day; smoking at follow-up years by smoking status (yes/no) and/or smoking intensity category (see Methods).
Fume exposure in men was associated with a reduction of the post-bronchodilator FEV1% predicted. This was observed consistently in several models using alternative methods of adjustment for smoking. The magnitude of effect of fume exposure was consistent across the models with post-bronchodilator FEV1, and represents a rate of decline in FEV1 of approximately 0.25% predicted per year of fume exposure. This was a smaller effect than the effect of continued smoking, which was approximately 1.2–1.9% predicted.
Baseline airway responsiveness was strongly associated with FEV1 in every model. Additional models (not shown) that did not include a measure of airway responsiveness (SMCT) showed similar results for the effects of fume exposure to those that included airway responsiveness as a covariate.
Fume exposure was not associated with the rate of decline in prebronchodilator FEV1. Likewise, continuing fume exposure was not associated with the rate of decline in the FVC, either pre- or post-bronchodilator (data not shown). Results of FVC models not including airway responsiveness were not different from those using the SMCT.
Analyses of data for women did not demonstrate statistically significant relationships between fume exposure and the rate of decline in FEV1, nor were there consistent trends. Analyses of dust exposure did not show statistically significant relationships between dust exposure and the rate of decline of either FEV1 or FVC during follow-up in either men or women. In women, there was a nonstatistically significant trend for dust exposure to be associated with lower prebronchodilator FEV1.
DISCUSSION
This study examined the influence of occupational exposure to dust and fume on respiratory function in individuals with early chronic obstructive pulmonary disease COPD. A significant effect of fume exposure on the rate of lung function decline over 5 years of follow-up was demonstrated in men. Fume exposure was associated with significant decrements of post-bronchodilator FEV1, suggesting that ongoing occupational fume exposure may be particularly adverse for individuals with early COPD. A comparable effect was not shown for the prebronchodilator FEV1.
Fume exposure affected the rate of decline of lung function even when adjusted for baseline lung function. The mixed-effects model allows the differentiation of the effect of ongoing (time-variant) exposure from that of cumulative exposures that occurred before the onset of the longitudinal study follow-up. Thus, these analyses largely reflect the influence of ongoing exposures during the course of the longitudinal follow-up, because the models are adjusted for the effect of previous and baseline exposure on the baseline level of lung function. In addition to the effect of long-term cumulative exposures, ongoing exposure has an additional adverse impact.
Several cross-sectional and longitudinal studies have shown that occupational dust exposure affects the level and the rate of decline of lung function. Many of these studies were conducted within a single industry or job category (such as mining [24–26]), or were related to a single exposure (e.g., coal dust, silica, carbon black, silicon carbide) (24, 26–30). Other studies were conducted in community-based cohorts with a variety of occupational exposures (2, 27, 31–35). Nearly all of the foregoing studies focused on determining the effect of dust exposures on persons without known lung disease or on the role of dust in initiation of COPD. The current study did not demonstrate an effect of ongoing dust exposure upon rate of decline of lung function. One study of persons with established COPD showed that prior exposure to “vapors, gas, dust, or fumes” was associated with increased symptoms over a 1-year follow-up (36).
The current study addresses different questions: (1) does ongoing exposure affect the course of existing COPD? (2) How do exposures affect lung function in persons who are developing COPD, recognizing that persons with early obstructive disease may differ from the general population with regard to susceptibility to adverse effects of exposure (1, 37).
“Fume” describes a variety of exposures. In a technical sense, fume is defined as an “airborne particulate formed by the condensation of solid particles from the gaseous state. Usually… from … combustion … or … melting process” (38). However, our study participants may have used this term to describe a great variety of agents, such as smoke and perhaps strong odors or vapors of volatile liquids. The overall distribution of jobs in the study population suggests that “fume” was interpreted by participants in a broad sense, not strictly limited to solid aerosols related to combustion (39).
The effect of fume exposure demonstrated in this longitudinal analysis is consistent with the impact of fume exposure noted in a cross-sectional analysis of this population (12, 40). In the latter analysis, fume exposure was described both by participants' self-reports and by estimates based upon an expert panel, job-derived exposure matrix. The experts used the term fume in its technically correct sense (“fine condensation-related solid aerosol”); results based upon self-report and by expert ratings were the same. Therefore, the results in this article are unlikely to represent an artifact due to misinterpretation by participants of the term fume. Work categories in which a high proportion of participants reported fume exposure include agriculture, metal products, construction, and personal appearance workers (41).
Baseline FEV1, which was included as a covariate, reflects the influence of prior exposures; any residual effect of prior exposures not adjusted by baseline FEV1 is likely to be statistically overshadowed by that of ongoing exposure. Therefore, the absence of demonstrated statistically significant coefficients for baseline exposures in the analyses reported here does not imply that such prior exposure is unimportant. Indeed, in our cross-sectional analysis of the baseline LHS data with regard to prior cumulative exposure, we found that fume exposure was closely associated with reductions in FEV1 (12). The effect of including baseline FEV1 as a covariate is illustrated in Table 3 by the baseline smoking intensity coefficients. Despite the well known influence of smoking on the rate of lung function decline (21), no coefficients for smoking intensity at baseline are statistically related to longitudinal change, because the effect of baseline smoking intensity is similarly overadjusted by including it in the baseline FEV1 covariate. Furthermore, age was a baseline covariate in the regression analyses, as the rate of decline may be influenced by age; the correlation of age and cumulative exposure might overadjust and reduce the likelihood of detecting an effect of prior exposures.
Despite comparable analytic methods, adverse effects in women were not demonstrated in this study. Although this may reflect a fundamental difference in responsiveness of women to occupational fume exposure, it is more likely that the smaller number of women in the study, and the smaller proportion with exposure, significantly reduced the statistical power. In addition, exposures for women may differ qualitatively and quantitatively from reported exposures for men, such that reported women's exposures might be less intense than those reported by men. Also, airway hyperresponsiveness was more common in women than in men (15), and those hyperresponsive women who are particularly prone to irritative effects of fume may have changed occupations well before the onset of the study.
Implications
These results have several important implications. First, they indicate that continuing occupational exposure to fume after onset of COPD adversely affects lung function. Although the average magnitude of this effect on the rate of decline (0.25% of predicted FEV1/yr) is smaller than the average effect of continued cigarette smoking (1.2–1.9% predicted/yr), this can lead to a significant functional loss over a prolonged period of time, particularly when added to the loss due to smoking per se. Furthermore, some sensitive individuals may experience a significantly greater effect than the average effect estimated by the regression coefficients. Finally, the metric of self-reported exposure to fume was relatively crude, so that the regression coefficient may underestimate the actual effect (i.e., any misclassification error is likely to bias toward the null).
Second, the data suggest the possibility that materials that participants subjectively identify as fume (whether because of odor or due to direct mucosal irritation) may have a particular propensity to induce inflammatory responses. Thus, materials referred to as fume may be more likely to produce an impact on lung function than those reflected in the term “dust” (39).
Third, although there is a specific effect of fume, the precise meaning of this term is ambiguous in common parlance. In the past, many epidemiologic studies have focused on dust, and included fume only in conjunction with other exposure terms in the same question (e.g., “exposure to fumes, vapors, and gases”). Future studies should inquire explicitly about fume per se to define which aspect of fume is associated with reduction of lung function.
Fourth, there is a clear implication for secondary screening and secondary prevention. Persons with early COPD should be very cautious about ongoing, uncontrolled occupational fume exposure. Particularly because they already have existing lung damage and typically have an accelerated lung function decline over time in comparison with normal persons, any additional lung function loss due to fume exposure is likely to be more significant. Clinicians should ask patients with COPD about occupational factors and recommend methods to limit exposures, particularly those capable of irritating airways or inducing airway inflammation. Self-reporting of fume exposure may be a surrogate indicator of exposure to materials with particular risk.
Fifth, patients with COPD may be more sensitive to fume exposure than are members of the general population. In broad, community-based populations, dust has a more prominent effect on lung function than does fume (2, 5, 26, 27, 35, 42, 43). In light of these previous findings, the findings from the current study may indicate that persons with COPD are peculiarly sensitive to fume.
Because all participants had early COPD, this study cannot directly determine if a similar effect would be seen among persons without COPD. It is possible that similar personal genetic factors predispose some individuals to the adverse pulmonary effects of both tobacco smoke and occupational exposures. Most LHS participants were middle aged, and it is also possible that the effect observed might be different at other ages. COPD is a disorder that progresses over a prolonged period, and it is therefore likely that the adverse effect of occupational fume exposure is not limited to the 5 years of follow-up in the LHS.
Because this study depends on self-report, it is possible that there may be a recall bias. However the results, showing a particular effect of fume exposure, are consistent with those of the cross-sectional analysis of baseline data that we performed (12). The latter study included “expert” exposure assessments that are not subject to recall bias.
The analyses did not include explicit adjustment of significance tests for multiple comparisons. It is therefore possible that the observed effect of fume exposure may represent type I error. However, the results were observed in a consistent fashion across several of the analytic approaches. The analyses were conducted based upon explicit a priori hypotheses developed before conducting the actual analysis.
Conclusions
This study shows an adverse effect of ongoing fume exposure on the rate of decline in lung function in men with early COPD. These findings suggest that fume exposure may have a particularly adverse effect on persons with COPD. These results support the need and opportunity for secondary prevention of COPD by controlling occupational exposures to fume for persons with early COPD.
Acknowledgments
Boehringer Ingelheim Pharmaceuticals, Inc. (Ridgefield, CT) supplied Atrovent and placebo inhalers.
Supported by the American Association of Medical Colleges/National Institute for Occupational Safety and Health/Centers for Disease Control and Prevention grant MM0060 02/02 and contract N01-HR-46002 from the Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health (Lung Health Study).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Originally Published in Press as DOI: 10.1164/rccm.200605-730OC on July 12, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
The principal investigators and senior staff of the clinical and coordinating centers, the National Heart, Lung, and Blood Institute, and members of the Safety and Data Monitoring Board of the Lung Health Study are as follows:
Case Western Reserve University, Cleveland, OH: M.D. Altose, M.D. (Principal Investigator), C.D. Deitz, Ph.D. (Project Coordinator); Henry Ford Hospital, Detroit, MI: M.S. Eichenhorn, M.D. (Principal Investigator), K.J. Braden, A.A.S. (Project Coordinator), P.A. Fantuz, R.N., B.S.N., R.L. Jentons, M.A.L.L.P. (Project Coordinator); Johns Hopkins University School of Medicine, Baltimore, MD: R.A. Wise, M.D. (Principal Investigator), C.S.R. and, Ph.D. (Co–Principal Investigator), K.A. Schiller (Project Coordinator); Mayo Clinic, Rochester, MN: P.D. Scanlon, M.D. (Principal Investigator), G.M. Caron (Project Coordinator), K.S. Mieras, L.C. Walters; Oregon Health Sciences University, Portland, OR: A.S. Buist, M.D. (Principal Investigator), L.R. Johnson, Ph.D. (LHS Pulmonary Function Coordinator), V.J. Bortz (Project Coordinator); University of Alabama at Birmingham, AL: W.C. Bailey, M.D. (Principal Investigator), L.B. Gerald, Ph.D., M.S.P.H. (Project Coordinator); University of California, Los Angeles, CA: D.P. Tashkin, M.D. (Principal Investigator), I.P. Zuniga (Project Coordinator); University of Manitoba, Winnipeg, MB, Canada: N.R. Anthonisen, M.D. (Principal Investigator, Steering Committee Chair), J. Manfreda, M.D. (Co–Principal Investigator), R.P. Murray, Ph.D. (Co–Principal Investigator), S.C. Rempel-Rossum (Project Coordinator); University of Minnesota Coordinating Center, Minneapolis, MN: J.E. Connett, Ph.D. (Principal Investigator), P.G. Lindgren, M.S., M.A. Skeans, M.S., H.T. Voelker; University of Pittsburgh, Pittsburgh, PA: R.M. Rogers, M.D. (Principal Investigator), M.E. Pusateri (Project Coordinator); University of Utah, Salt Lake City, UT: R.E. Kanner, M.D. (Principal Investigator), G.M. Villegas (Project Coordinator); Data Monitoring Board: C. Furberg, M.D., Ph.D., J.R. Landis, Ph.D., E. Mauger, Ph.D., J.R. Maurer, M.D., Y. Phillips, M.D., J.K. Stoller, M.D., I. Tager, M.D., A. Thomas, Jr., M.D.; Morbidity and Mortality Review Board: T.E. Cuddy, M.D., R.S. Fontana, M.D., R.E. Hyatt, M.D., C.T. Lambrew, M.D., B.A. Mason, M.D., D.M. Mintzer, M.D., R.B. Wray, M.D.; National Heart, Lung, and Blood Institute Staff, Bethesda, MD: J.P. Kiley, Ph.D. (Director, Division of Lung Diseases), G. Weinmann, M.D. (Director, Airway Biology and Disease Program, DLD), M.C. Wu, Ph.D. (Division of Epidemiology and Clinical Applications).
References
- 1.Mayer AS, Newman LS. Genetic and environmental modulation of chronic obstructive pulmonary disease. Respir Physiol 2001;128:3–11. [DOI] [PubMed] [Google Scholar]
- 2.Anto JM, Vermeire P, Vestbo J, Sunyer J. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J 2001;17:982–994. [DOI] [PubMed] [Google Scholar]
- 3.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Airflow obstruction attributable to work in industry and occupation among US race/ethnic groups: a study of NHANES III data. Am J Ind Med 2004;46:126–135. [DOI] [PubMed] [Google Scholar]
- 4.Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, Milton D, Schwartz D, Toren K, Viegi G. American Thoracic Society statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med 2003;167:787–797. [DOI] [PubMed] [Google Scholar]
- 5.Trupin L, Earnest G, San Pedro M, Balmes JR, Eisner MD, Yelin E, Katz PP, Blanc PD. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J 2003;22:462–469. [DOI] [PubMed] [Google Scholar]
- 6.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA Jr, Enright PL, Kanner RE, O'Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: The Lung Health Study. JAMA 1994;272:1497–1505. [PubMed] [Google Scholar]
- 7.Krzyzanowski M, Jedrychowski W, Wysocki M. Factors associated with the change in ventilatory function and the development of chronic obstructive pulmonary disease in a 13-year follow-up of the Cracow Study: Risk of chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;134:1011–1019. [DOI] [PubMed] [Google Scholar]
- 8.Humerfelt S, Gulsvik A, Skjaerven R, Nilssen S, Kvale G, Sulheim O, Ramm E, Eilertsen E, Humerfelt SB. Decline in FEV1 and airflow limitation related to occupational exposures in men of an urban community. Eur Respir J 1993;6:1095–1103. [PubMed] [Google Scholar]
- 9.Kauffmann F, Drouet D, Lellouch J, Brille D. Occupational exposure and 12-year spirometric changes among Paris area workers. Br J Ind Med 1982;39:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulvestad B, Bakke B, Eduard W, Kongerud J, Lund MB. Cumulative exposure to dust causes accelerated decline in lung function in tunnel workers. Occup Environ Med 2001;58:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergdahl IA, Toren K, Eriksson K, Hedlund U, Nilsson T, Flodin R, Jarvholm B. Increased mortality in COPD among construction workers exposed to inorganic dust. Eur Respir J 2004;23:402–406. [DOI] [PubMed] [Google Scholar]
- 12.Harber P, Simmons M, Tashkin D, Hnizdo E, Crawford L, Connett J. Occupational dust and fume exposure effect on lung function in early COPD. Am J Respir Crit Care Med 2004;169:A46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buist AS, Connett JE, Miller RD, Kanner RE, Owens GR, Voelker HT. Chronic obstructive pulmonary disease early intervention trial (Lung Health Study): baseline characteristics of randomized participants. Chest 1993;103:1863–1872. [DOI] [PubMed] [Google Scholar]
- 14.Enright PL, Johnson LR, Connett JE, Voelker H, Buist AS. Spirometry in the Lung Health Study: 1. Methods and quality control. Am Rev Respir Dis 1991;143:1215–1223. [DOI] [PubMed] [Google Scholar]
- 15.Tashkin DP, Altose MD, Bleecker ER, Connett JE, Kanner RE, Lee WW, Wise R. The Lung Health Study: airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation: the Lung Health Study Research Group. Am Rev Respir Dis 1992;145:301–310. [DOI] [PubMed] [Google Scholar]
- 16.Connett JE, Bjornson-Benson WM, Daniels K. Recruitment of participants in the Lung Health Study, II: Assessment of recruiting strategies. Control Clin Trials 1993;14:38S–51S. [DOI] [PubMed] [Google Scholar]
- 17.Tashkin DP, Altose MD, Connett JE, Kanner RE, Lee WW, Wise RA. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease: the Lung Health Study Research Group. Am J Respir Crit Care Med 1996;153:1802–1811. [DOI] [PubMed] [Google Scholar]
- 18.Connett JE, Kusek JW, Bailey WC, O'Hara P, Wu M. Design of the Lung Health Study: a randomized clinical trial of early intervention for chronic obstructive pulmonary disease. Control Clin Trials 1993;14:3S–19S. [DOI] [PubMed] [Google Scholar]
- 19.O'Hara P, Grill J, Rigdon MA, Connett JE, Lauger GA, Johnston JJ. Design and results of the initial intervention program for the Lung Health Study: the Lung Health Study Research Group. Prev Med 1993;22:304–315. [DOI] [PubMed] [Google Scholar]
- 20.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981;123:659–664. [DOI] [PubMed] [Google Scholar]
- 21.Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease: the Lung Health Study. Am J Respir Crit Care Med 2000;161:381–390. [DOI] [PubMed] [Google Scholar]
- 22.Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol 1975;56:323–327. [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute. SAS/STAT, Version 8. Cary, NC: SAS Institute; 1999.
- 24.Hnizdo E. Health risks among white South African goldminers: dust, smoking and chronic obstructive pulmonary disease. S Afr Med J 1992;81:512–517. [PubMed] [Google Scholar]
- 25.Meijers JM, Swaen GM, Slangen JJ, van Vliet K, Sturmans F. Long-term mortality in miners with coal workers' pneumoconiosis in The Netherlands: a pilot study. Am J Ind Med 1991;19:43–50. [DOI] [PubMed] [Google Scholar]
- 26.Soutar CA, Hurley JF. Relation between dust exposure and lung function in miners and ex-miners. Br J Ind Med 1986;43:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hnizdo E, Vallyathan V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence. Occup Environ Med 2003;60:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijers JM, Swaen GM, Slangen JJ. Mortality of Dutch coal miners in relation to pneumoconiosis, chronic obstructive pulmonary disease, and lung function. Occup Environ Med 1997;54:708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harber P, Muranko H, Solis S, Torossian A, Merz B. Effect of carbon black exposure on respiratory function and symptoms. J Occup Environ Med 2003;45:144–155. [DOI] [PubMed] [Google Scholar]
- 30.Romundstad P, Andersen A, Haldorsen T. Non-malignant mortality among Norwegian silicon carbide smelter workers. Occup Environ Med 2002;59:345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulsvik A. The global burden and impact of chronic obstructive pulmonary disease worldwide. Monaldi Arch Chest Dis 2001;56:261–264. [PubMed] [Google Scholar]
- 32.Goren AI, Bruderman I. Effects of occupational exposure and smoking on respiratory symptomatology and PFT in healthy panelists and COPD patients. Eur J Epidemiol 1989;5:58–64. [DOI] [PubMed] [Google Scholar]
- 33.Hendrick DJ. Occupational and chronic obstructive pulmonary disease (COPD). Thorax 1996;51:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastrangelo G, Tartari M, Fedeli U, Fadda E, Saia B. Ascertaining the risk of chronic obstructive pulmonary disease in relation to occupation using a case–control design. Occup Med (Lond) 2003;53:165–172. [DOI] [PubMed] [Google Scholar]
- 35.Oxman AD, Muir DC, Shannon HS, Stock SR, Hnizdo E, Lange HJ. Occupational dust exposure and chronic obstructive pulmonary disease: a systematic overview of the evidence. Am Rev Respir Dis 1993;148:38–48. [DOI] [PubMed] [Google Scholar]
- 36.Blanc PD, Eisner MD, Trupin L, Yelin EH, Katz PP, Balmes JR. The association between occupational factors and adverse health outcomes in chronic obstructive pulmonary disease. Occup Environ Med 2004;61:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandford AJ, Chagani T, Weir TD, Connett JE, Anthonisen NR, Pare PD. Susceptibility genes for rapid decline of lung function in the Lung Health Study. Am J Respir Crit Care Med 2001;163:469–473. [DOI] [PubMed] [Google Scholar]
- 38.Plog BA, Niland J, Quinlan P. Fundamentals of industrial hygiene, 4th ed. Itasca, IL: National Safety Council; 1996.
- 39.Harber P, Simmons M, Tashkin DP, Hnizdo E, Schachter L. What do “dust,” “fume,” “mask use” really mean? [abstract] Am J Respir Crit Care Med 2003;167:A506. [Google Scholar]
- 40.Harber P, Simmons M, Tashkin D, Hnizdo E, Crawford L, Connett J. Comparison of 3 methods for assigning exposure metrics for evaluating occupation effect upon COPD [abstract]. Am J Respir Crit Care Med 2005;171:A818. [Google Scholar]
- 41.Harber P, Crawford L, Cheema A, Schachter L. Computer algorithm for automated work group classification from free text: the DREAM technique. J Occup Environ Med 2007;49:41–49. [DOI] [PubMed] [Google Scholar]
- 42.Lundback B, Gulsvik A, Albers M, Bakke P, Ronmark E, van den Boom G, et al. Epidemiological aspects and early detection of chronic obstructive airway diseases in the elderly. Eur Respir J Suppl 2003;40:3s–9s. [DOI] [PubMed] [Google Scholar]
- 43.Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989;140:S85–S91. [DOI] [PubMed] [Google Scholar]