Abstract
Rationale: Asthma is characterized by increases in airway resistance, pulmonary remodeling, and lung inflammation. The cytokine transforming growth factor (TGF)-β has been shown to have a central role in asthma pathogenesis and in mouse models of allergic airway disease.
Objectives: To determine the contribution of TGF-β to airway hyperresponsiveness (AHR), we examined the time course, source, and isoform specificity of TGF-β production in an in vivo mouse asthma model. To then elucidate the function of TGF-β in AHR, inflammation, and pulmonary fibrosis, we examined the effects of blocking TGF-β signaling with neutralizing antibody.
Methods: Mice were sensitized and challenged with ovalbumin (OVA) to establish allergic airway disease. TGF-β activity was neutralized by intranasal administration of monoclonal antibody.
Measurements and Main Results: TGF-β1 protein levels were increased in OVA-challenged lungs versus naive controls, and airway epithelial cells were shown to be a likely source of TGF-β1. In addition, TGF-β1 levels were elevated in OVA-exposed IL-5–null mice, which fail to recruit eosinophils into the airways. Neutralization of TGF-β1 with specific antibody had no significant effect on airway inflammation and eosinophilia, although anti–TGF-β1 antibody enhanced OVA-induced AHR and suppressed pulmonary fibrosis.
Conclusions: These data show that TGF-β1 is the main TGF-β isoform produced after OVA challenge, with a likely cellular source being the airway epithelium. The effects of blocking TGF-β1 signaling had differential effects on AHR, fibrosis, and inflammation. While TGF-β neutralization may be beneficial to abrogating airway remodeling, it may be detrimental to lung function by increasing AHR.
Keywords: lung, mice, hypersensitivity, cytokines
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Transforming growth factor (TGF)-β1 is known to play a critical role in promoting pulmonary remodeling; however, its contribution to airway hyperresponsiveness (AHR) is less well defined.
What This Study Adds to the Field
TGF-β1 is the predominant TGF-β isoform produced in a mouse asthma model and neutralization of TGF-β1 results in less fibrosis and increased AHR.
Transforming growth factor (TGF)-β has emerged as a key mediator of pulmonary fibrosis, and accordingly a good candidate responsible for the subepithelial fibrosis observed in asthma (1, 2). The cellular source of TGF-β in asthma is unclear; TGF-β1 and TGF-β3 have been shown to be expressed in the bronchial epithelium (3), whereas TGF-β1 production has been reported in eosinophils and fibroblasts (4, 5). Moreover, patients with asthma express increased TGF-β1 in the bronchoalveolar lavage (BAL) fluid in response to segmental allergen challenge and TGF-β1 levels in the airway epithelium and submucosa correlate to airway basement membrane thickness, suggesting a direct role for TGF-β1 in airway remodeling (6, 7). In addition, TGF-β1 promoter polymorphisms have been linked to asthma susceptibility (8). TGF-β1 instillation into mouse lungs and TGF-β1 adenoviral expression or transgenic overexpression in the airway epithelium induce airway collagen mRNA and protein deposition, indicating that TGF-β1 is sufficient to induce fibrosis (9–11). In addition, a causal role for TGF-β1 has been elucidated in IL-13–mediated fibrosis (12).
Recent studies have clarified some of the mechanisms involved in TGF-β1 stimulation of airway remodeling. TGF-β1 activates gene transcription via binding to a heterodimeric receptor, part of the activin receptor-like kinase (ALK) family. TGF-β receptor I (ALK5) phosphorylates members of the Smad (Similar to mothers against decapentaplegic) protein family to initiate nuclear translocation and transcription (13). Blockade of ALK5 kinase activity by an oral drug inhibits adenoviral TGF-β1–induced lung fibrosis (14). Furthermore, anti–TGF-β antibody has been shown to inhibit airway remodeling in an ovalbumin (OVA) model by blocking activation of the Smad signaling cascade (15). In the latter study, an antibody specific for all three TGF-β isoforms did not affect pulmonary inflammation but significantly reduced collagen deposition, smooth muscle cell proliferation, and goblet cell mucus production. This raises the interesting question as to whether blocking the actions of TGF-β might ameliorate the airway hyperresponsiveness (AHR) associated with allergic inflammation. Reduction of smooth muscle or airway mucus would be expected to potentially reduce AHR (16); however, fibrotic remodeling of the airway wall may actually reduce AHR by stiffening the airway and making it more resistant to constriction (17).
The goal of the present study was to examine the effects of TGF-β1 neutralizing antibody on lung pathophysiology in terms of fibrosis, inflammation, and lung function. We also sought to elucidate the specific isoform and cellular source of TGF-β in a common allergic inflammation mouse model. We report that anti-TGF-β1 antibody suppressed airway fibrosis induced by OVA challenge, while on the other hand, anti–TGF-β1 antibody increased AHR to inhaled methacholine (Mch). These results suggest that TGF-β1, although implicated in promoting structural remodeling of the airway wall, plays a suppressive role in the pathogenesis of airway responsiveness induced by antigen.
METHODS
Animals and Reagents
Female BALB/c mice, C57BL/6 mice, or IL-5−/− mice ages 2–4 months were purchased from Jackson Laboratories (Bar Harbor, ME). All chemicals used were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Vermont. Antibody to human TGF-β1, TGF-β2, and TGF-β3, and nonspecific IgG1 were purchased from R&D Systems (Minneapolis, MN) and total TGF-β protein levels were measured by ELISA (R&D Systems). Mouse monoclonal anti–TGF-β1 antibody was obtained from R&D Systems (catalog no. MAB240, clone 9016). Phospho-Smad 2 antibody was purchased from Cell Signaling Technology (Danvers, MA).
Model of Allergic Airway Disease
Allergic airway inflammation was induced as previously reported (18). Briefly, mice were injected intraperitoneally with 40 μg of OVA in the adjuvant aluminum hydroxide (Alum) (Pierce Chemical, Rockford, IL) on Days 1 and 14 to induce sensitization. Sham-sensitized mice received Alum alone. Mice were then exposed to an aerosolized 1% OVA solution in sterile phosphate-buffered saline (PBS) for 30 minutes on Day 21 (1×), Days 21 through 23 (3×), or Days 21 through 26 (6×). Lung tissues were harvested 2 days after the final OVA challenge, except in TGF-β1 time-course studies where tissues were collected as indicated (Figure E1 of the online supplement).
TGF-β Blocking Antibody Studies
TGF-β1 neutralizing antibody (75 μg) in sterile PBS was delivered 1 hour before OVA aerosol challenges via intranasal administration. Mice received four total doses of TGF-β1 antibody, three doses preceding the three OVA challenges, and one dose on the day before lung function was assessed.
Respiratory Mechanics
Airway resistance and tissue elastance were measured as previously described (19). Multiple linear regression was used to fit measured pressure and volume in each individual mouse to the model of linear motion of the lung (20, 21). See online supplement for details of AHR measurements.
Lung Histology and Immunohistochemistry
Lungs were inflated and fixed with 4% paraformaldehyde, followed by paraffin embedding. Blocks were cut into 5-μm sections. Airway inflammation was assessed by hematoxylin-and-eosin staining or TGF-β1 immunohistochemistry was performed (see online supplement). Phospho-Smad 2 staining was performed according to the antibody manufacturer's instructions.
Assessment of Pulmonary Fibrosis
Lung sections of 5 μm were then stained with picosirius red reagent that selectively stains collagen when visualized by polarized light microscopy (22). Slides were then scored using a scale of 0 to 3 (0 being the least stain intensity, 3 the highest intensity) for airway-associated collagen deposition by two independent, blinded observers. The cumulative score from each mouse was then averaged according to treatment group.
Digital Image Analysis
Digital images of immunohistochemistry and picosirius red–stained slides were captured using a Zeiss Axioskop2 plus microscope and the Zeiss Axiocam digital camera (Carl Zeiss Microimaging, Thornwood, NY). Color photos were then converted to 8-bit gray-scale images and mean pixel density was measured using NIH Image J software (National Institutes of Health, Bethesda, MD). Three sections of the airway wall were sampled per image. Images were obtained from a minimum of three different mice per group.
Cytokine Analyses in BAL
BAL samples from OVA-exposed mice were analyzed using the Bioplex System and a 23-plex cytokine array (BioRad Laboratories, Hercules, CA).
Statistical Analyses
Data presented in the figures were subjected to one-way analysis of variance followed by Tukey test for multiple comparisons. Comparisons of two means were conducted by unpaired Student's t test assuming unequal variance. Analyses with resultant P values less than 0.05 were determined significant, except where noted. Statistics were performed (Microsoft Excel software package; Microsoft Corp., Redmond, WA), and data are presented as mean values ± SEM.
RESULTS
TGF-β1 Is the Primary Isoform of TGF-β Produced after OVA Challenge
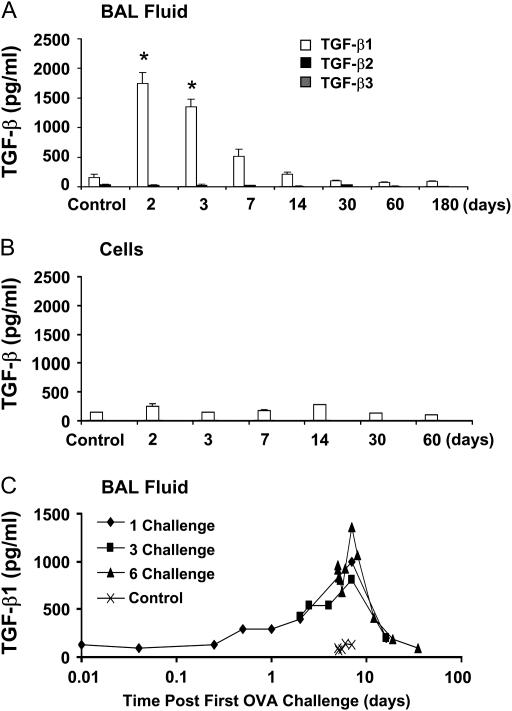
To determine the effects of OVA challenge on TGF-β production, mice were challenged with OVA for 6 consecutive days followed by 2 to 180 days of recovery. Specific TGF-β isoform levels were measured in the BAL fluid by ELISA. TGF-β1 was the only isoform of TGF-β significantly increased by OVA challenge in the cell-free BAL fluid; total TGF-β1 protein reached a peak level of 1,745.3 ± 188.2 pg/ml 2 days after the final OVA challenge (7 d after the first OVA challenge) and remained elevated for 1 week after OVA exposure (Figure 1A). TGF-β1 levels were not significantly elevated above controls at later time points. Levels of active TGF-β1 protein in BAL fluid were undetectable at the time points analyzed. No significant changes in the levels of TGF-β2 or TGF-β3 were found, nor were changes identified in the levels of TGF-β in BAL cell lysates (Figure 1B).
Figure 1.
Detection of transforming growth factor (TGF)-β isoforms in the bronchoalveolar lavage (BAL) fluid after ovalbumin (OVA) sensitization and challenge. BALB/c mice were sensitized to OVA and challenged with 1% aerosolized OVA on 6 consecutive days, and BAL samples were analyzed at the indicated time points after the last challenge (n = 4 mice each). Control mice were not sensitized or challenged with OVA. TGF-β levels were measured by isoform-specific ELISA on the BAL supernatant (A) or cell lysate (B). TGF-β1 levels in the BAL fluid were measured at the indicated times after one, three, or six OVA challenges (C). *P < 0.05.
We next examined the kinetics of TGF-β1 release into the BAL fluid. Peak TGF-β1 production was observed 7 days from the first OVA exposure regardless of the number (one, three, or six) of OVA challenges (Figure 1C). Additional OVA challenges increased the maximal release of TGF-β1 from 803.9 pg/ml after one challenge, to 1,000.0 and 1,359.8 pg/ml for three and six challenges, respectively. Collectively, these data indicate that TGF-β1 is perhaps the most prominent isoform of TGF-β in the OVA mouse model of allergic airway disease and that the timing of peak TGF-β1 release appears to be independent of the OVA challenge protocol.
The Airway Epithelium Is an Important Source of TGF-β1 Production in Allergic Airway Inflammation
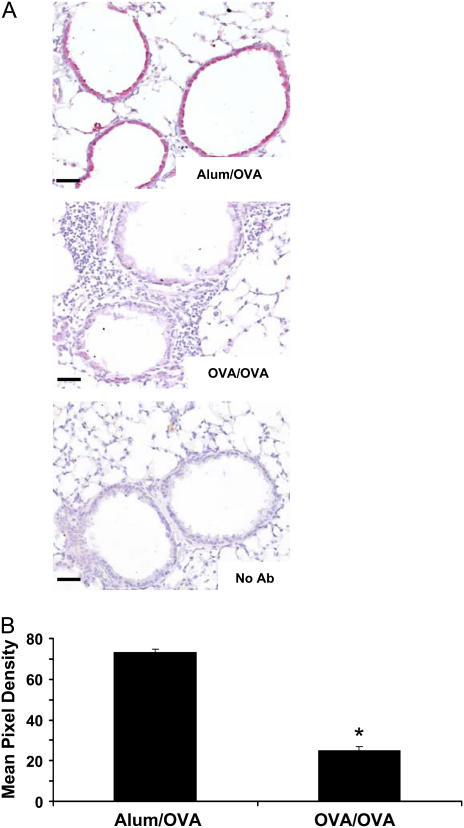
To identify the source of TGF-β1 in mouse lungs, in situ, immunohistochemical analyses for TGF-β1 protein were performed on lung sections. In naive mice, marked TGF-β1 immunoreactivity occurred in the bronchial airway epithelium of control animals (Figure 2A). Significantly, after antigen challenge in sensitized mice, the TGF-β1 immunoreactivity of the epithelium was lost, potentially indicating release of TGF-β1 into the airspaces, which is supported by the timing of the loss of staining in the epithelium coinciding with the increase in BAL fluid TGF-β1 protein presented in Figure 1. TGF-β1 immunoreactivity was quantified by digital analysis and revealed a significant reduction in TGF-β1 protein levels after OVA sensitization and challenge (Figure 2B).
Figure 2.
Transforming growth factor (TGF)-β1 protein colocalizes with the bronchial epithelium and is decreased upon ovalbumin (OVA) sensitization and challenge. BALB/c mice were challenged six times with 1% aerosolized OVA, and paraffin-embedded sections were prepared. Immunohistochemistry specific for TGF-β1 was then performed as described in Methods (A) (n = 5 mice each). Alum/OVA indicates control mice that were sham sensitized then challenged with OVA, OVA/OVA indicates OVA-sensitized and -challenged mice, No Ab indicates a no primary antibody control. TGF-β1 is indicated by red staining at ×200 original magnification; scale bar equals 25 μm. TGF-β1 staining was then quantified by mean pixel density (B) (n = 12), OVA/OVA-treated mice had decreased TGF-β1 immunoreactivity compared with Alum/OVA. *P < 0.05.
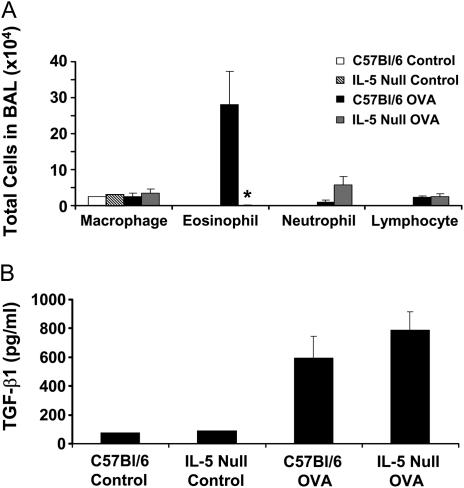
The time course of TGF-β1 protein increases also mirrored the OVA-induced eosinophilic inflammation. To examine the potential role of eosinophils in OVA-induced TGF-β1 production, we measured the BAL fluid levels of TGF-β1 in wild-type C57Bl/6 or IL-5–deficient mice after OVA challenge. It has been previously shown that IL-5–deficient mice fail to recruit eosinophils after OVA challenge (23). We therefore exposed IL-5–null mice and their background controls, C57Bl/6 mice, to six OVA challenges and assessed airway inflammation and TGF-β1 BAL levels. IL-5–null mice indeed failed to exhibit airway eosinophilia (Figure 3A). Despite the absence of airway eosinophils, IL-5–null mice produced similar or higher levels of BAL TGF-β1 (787.7 ± 129.4 pg/ml) compared with C57Bl/6 control mice (596.0 ± 148.2 pg/ml) (Figure 3B). These data suggest that TGF-β1 levels in BAL fluid increase independently of IL-5 or eosinophilic inflammation in response to OVA antigen.
Figure 3.
IL-5–null mice produce similar transforming growth factor (TGF)-β1 levels to control animals after ovalbumin (OVA) challenge despite decreased eosinophilia. IL-5–null mice or C57BL/6 controls were sensitized to OVA and challenged six times with 1% aerosolized OVA. Bronchoalveolar lavage (BAL) samples were then taken and analyzed for cell number, identity, and TGF-β1 levels (n = 3 mice each). IL-5–null mice failed to recruit eosinophils into the airspaces compared with controls (A). Both control and IL-5–null mice produce similar levels of TGF-β1 protein in the BAL fluid (B). *P = 0.058.
Anti–TGF-β Antibody Does Not Inhibit OVA–induced Airway Inflammation
To understand the functional significance of increased airway TGF-β1 in mice with allergic airway disease, we next interfered with the effects of TGF-β1 by neutralizing TGF-β1 activity using intratracheal administration of a TGF-β1–specific antibody. To confirm that our instilled anti–TGF-β1 antibody blocked TGF-β1 activity and signaling in the airways, we challenged mice three times with OVA 30 minutes after instillation of anti–TGF-β1 neutralizing antibody. The transcriptional regulator Smad 2 is activated by phosphorylation after TGF-β1 receptor binding, leading to nuclear accumulation (13). We stained OVA-treated lung sections for phospho-Smad 2 levels (Figure 4A). TGF-β1 neutralizing antibody blocked phospho-Smad 2 nuclear accumulation in the airways of OVA-treated mice; control nonspecific IgG1 had no effect. Phospho-Smad 2 levels were quantified by digital pixel density, which confirmed that anti–TGF-β1 treatment did indeed significantly decrease phospho-Smad 2 levels (Figure 4B). These data confirm that anti–TGF-β1 antibody effectively abrogates TGF-β signaling in vivo.
Figure 4.
Ovalbumin (OVA)-induced phosphorylation of Smad 2 is attenuated by anti–transforming growth factor (TGF)-β1 antibody treatment. BALB/c mice were sensitized to OVA and challenged on 3 consecutive days with 1% aerosolized OVA. TGF-β1 neutralizing antibodies (75 μg) or control nonspecific IgG1 were administered once daily for 4 days starting the day of the first OVA challenge. Paraffin-embedded lung sections were then stained for phospho-Smad 2 (red) and counterstained with the nuclear dye Sytox Green (green) (A). Nuclear phospho-Smad 2 appears yellow due to the overlapping stains. Images were taken at ×200 original magnification by confocal microscopy; scale bar equals 25 μm. Phospho-Smad 2 staining was then quantified by mean pixel density (B) (n = 21, 24, 24, respectively). Anti–TGF-β1 significantly decreased phospho-Smad 2 immunoreactivity. *P < 0.05.
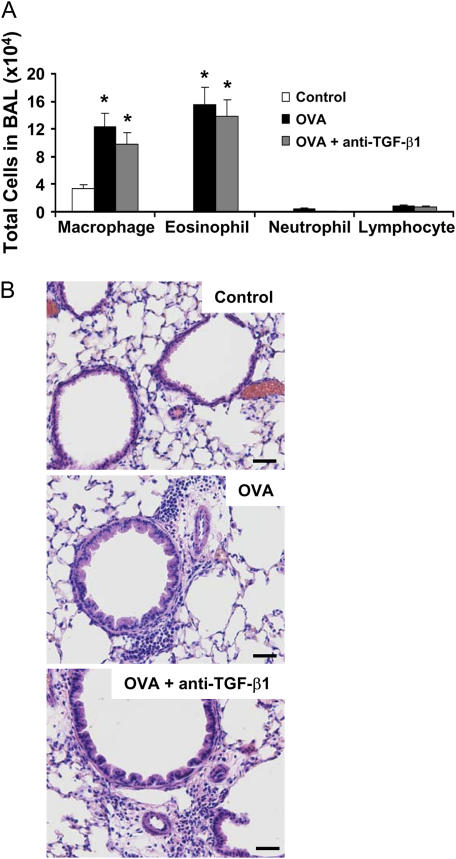
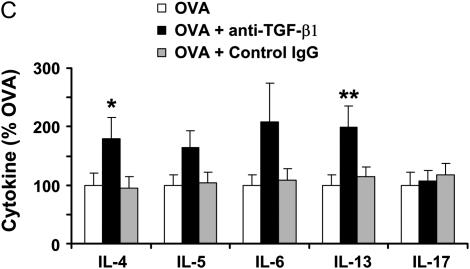
To next address the functional consequence of antibody instillation on airway inflammation induced by OVA, we analyzed BAL cell numbers, differential cell counts, and BAL cytokine levels. Administration of anti–TGF-β1 antibody did not significantly alter OVA-induced airway eosinophilia (Figure 5A). In addition, lung histology revealed peribronchial and perivascular tissue inflammation in both antibody-treated and control mice (Figure 5B). Finally, anti–TGF-β1 antibody treatment significantly enhanced Th2 cytokine levels of IL-4 and IL-13, as well as nonsignificantly increased IL-5 and IL-6 (Figure 5C). However, these data indicate that TGF-β1 activity is not required for inflammatory cell recruitment to the airways in OVA allergic airway disease, because TGF-β1 neutralization did not alter inflammatory cell numbers and it appears that TGF-β1 may have a suppressive effect on Th2 cytokine production.
Figure 5.
Neutralization of transforming growth factor (TGF)-β1 does not alter pulmonary inflammation in mice subjected to ovalbumin (OVA) sensitization and challenge. BALB/c mice were sensitized to OVA and challenged on 3 consecutive days with 1% aerosolized OVA. TGF-β1 neutralizing antibodies (75 μg) were administered once daily for 4 days starting the day of the first OVA challenge. Cell number and identity were determined in the bronchoalveolar lavage (BAL) (n = 4, 8, 9 mice, respectively) (A). Whole lung sections were prepared by paraffin embedding and stained with hematoxylin and eosin to examine the tissue inflammation in the treatment groups (B), ×200 original magnification. BAL cytokine profiles were assessed by Bioplex analysis (n = 7, 8, 8, respectively) (C). *P < 0.06 versus OVA mice; **P < 0.05 versus OVA mice.
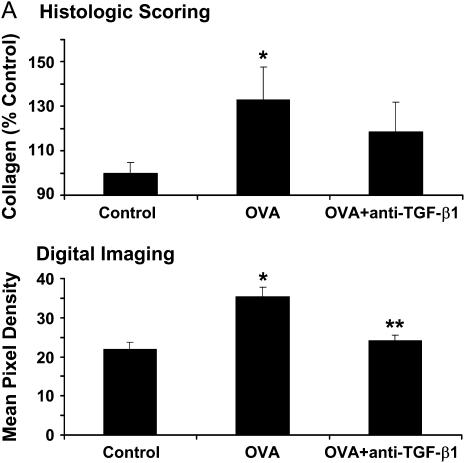
TGF-β Antibody Neutralization Inhibits Airway Collagen Deposition
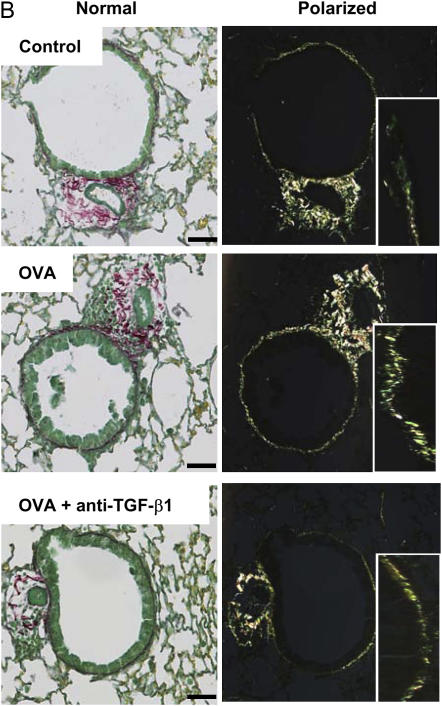
To confirm that TGF-β neutralization in our study reduced antigen-induced airway fibrosis, as previously reported (15), we quantified the amount of subepithelial collagen deposition after OVA challenge. Histologic scoring of bronchial collagen deposition was performed after OVA challenge with or without anti–TGF-β1 antibody administration. OVA-induced collagen deposition in control animals significantly increased by 132.8 ± 15.0%, and this increase in airway collagen deposition was partially inhibited by anti–TGF-β1 antibody, 118.5 ± 13.4% (Figures 6A, top, and 6B). Digital pixel density analysis of subepithelial collagen deposition showed that OVA treatment significantly increased peribronchial collagen levels compared with controls (optical density [o.d.], 35.3 ± 2.4 vs. 22.0 ± 1.7, respectively), and anti–TGF-β1 significantly decreased this response (o.d., 24.0 ± 1.5) (Figure 6A, bottom). Subepithelial collagen deposition was not statistically different between control mice and mice treated with anti–TGF-β1. These data confirm that TGF-β1 neutralization blocks OVA-induced peribronchial collagen deposition.
Figure 6.
Neutralization of transforming growth factor (TGF)-β1 decreases subepithelial collagen deposition after ovalbumin (OVA) challenge. BALB/c mice were sensitized to OVA and challenged on 3 consecutive days with 1% aerosolized OVA. TGF-β1 neutralizing antibodies (75 μg) were administered once daily for 4 days starting the day of the first OVA challenge. Picosirius red–stained paraffin sections were assessed by blinded scorers for collagen deposition (A, top) (n = 6, 12, 15 mice, respectively). Picosirius red images were then analyzed by mean pixel density for collagen deposition (A, bottom) (n = 27, 27, 30 airways analyzed, respectively). Differential interference contrast images of Picosirius red–stained lung sections (B, ×200 original magnification, scale bar = 25 μm). Insets depict a portion of the airway wall at increased magnification. (A, top) *P = 0.059 versus control mice (histologic scoring); (A, bottom) *P < 0.05 versus control; **P < 0.05 versus OVA (digital imaging); OVA + anti–TGF-β1 were not statistically different from control.
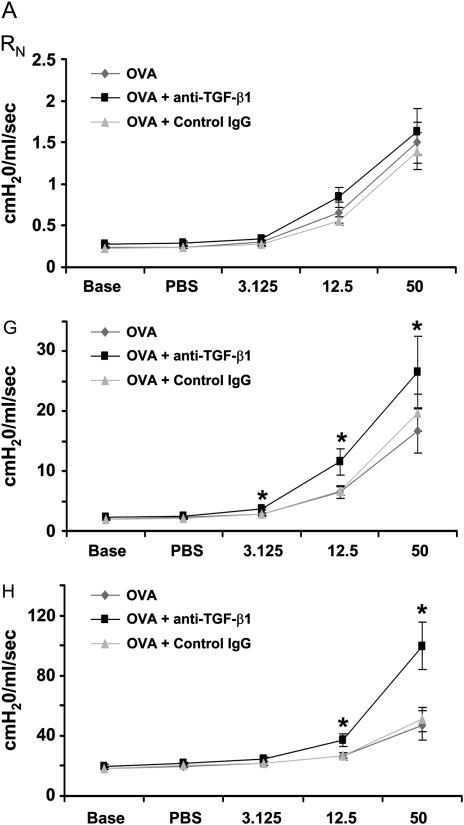
Anti–TGF-β1 Antibody Enhances AHR in Response to Inhaled Mch in the OVA Model
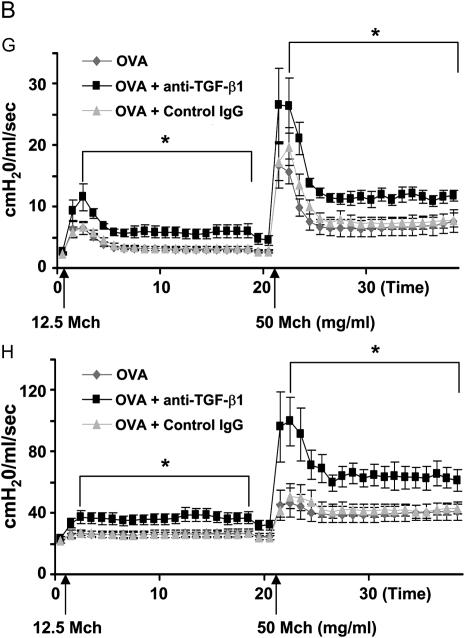
Because AHR is a significant functional outcome of allergic airway disease, we assessed the effects of TGF-β neutralization on OVA-induced AHR. Anti–TGF-β1 antibody was administered to mice during three OVA challenges. OVA plus control IgG or OVA plus anti–TGF-β1 antibody instillation did not alter baseline lung mechanical values from those of OVA controls (Table 1). TGF-β1 blockade significantly enhanced AHR induced by OVA in terms of tissue resistance (G) and tissue elastance (H) throughout the Mch dose–response curve. Peak G responses after Mch challenge were significantly elevated by anti–TGF-β1 treatment compared with OVA controls (11.57 ± 2.18 vs. 6.46 ± 1.06 at 12.5 mg/ml Mch and 26.57 ± 6.00 vs. 16.64 ± 3.71 at 50 mg/ml Mch). Peak H responses were also significantly increased by TGF-β1 neutralization (37.2 ± 4.4 vs. 26.4 ± 2.3 at 12.5 mg/ml Mch and 99.6 ± 15.7 vs. 46.8 ± 9.7 at 50 mg/ml Mch) (Figure 7A). AHR as assessed by G and H parameters remained significantly elevated between Mch challenges in mice treated with anti–TGF-β1 compared with OVA controls (Figure 7B). TGF-β1 neutralization had no significant effect on airway resistance (RN). Control IgG antibody treatment had no significant effects on OVA-induced AHR. Anti–TGF-β1 antibody in the absence of OVA challenge did not alter AHR from control levels (data not shown). These data show that neutralization of TGF-β1 results in significantly increased AHR in the mouse OVA model of asthma.
TABLE 1.
BASELINE LUNG FUNCTION VALUES FOR MICE BEFORE METHACHOLINE TREATMENT
| OVA | OVA + Anti–TGF-β1 | OVA + Control IgG | |
|---|---|---|---|
| RN cm H2O/(ml/s) | 0.239 ± 0.014 | 0.275 ± 0.021 | 0.233 ± 0.011 |
| G cm H2O/(ml/s) | 1.94 ± 0.13 | 2.22 ± 0.13 | 2.02 ± 0.11 |
| H cm H2O/(ml/s) | 18.2 ± 1.0 | 19.8 ± 0.9 | 18.4 ± 1.2 |
Definition of abbreviations: G = tissue resistance; H = tissue elastance; OVA = ovalbumin; RN = airway resistance; TGF = transforming growth factor.
Values represent mean ± SEM.
Figure 7.
Neutralization of transforming growth factor (TGF)-β1 enhances ovalbumin (OVA)-induced airway hyperresponsiveness (AHR). BALB/c mice were sensitized to OVA and challenged on 3 consecutive days with 1% aerosolized OVA. TGF-β1 neutralizing antibodies (75 μg) were administered once daily for 4 days starting the day of the first OVA challenge. After challenge, airway function was assessed by Flexivent (SCIREQ, Montreal, PQ, Canada). TGF-β1 neutralization enhanced OVA sensitization and challenge induced AHR (A) (peak response; n = 7, 8, 8 mice, respectively). In addition to increasing peak methacholine (Mch) responses, TGF-β1 antibody enhanced prolonged AHR between Mch doses (B). There were no effects of control IgG treatment on OVA-induced AHR. AHR as measured by the G (tissue resistance) and H (tissue elastance) parameters were significantly increased. *P < 0.05 versus OVA mice.
DISCUSSION
TGF-β has been suggested to be an important cytokine in the genesis of airway remodeling that underlies diverse pulmonary diseases, and which has been shown to be sufficient in mouse models to drive airway fibrosis. Numerous studies in vitro and in vivo have shown that TGF-β1 stimulates production of fibrotic genes, such as collagen, connective tissue growth factor (CTGF), or plasminogen activator inhibitor (PAI)-1, in diverse cell types (1). In addition, the mouse has been reported to have TGF-β1, TGF-β2, and TGF-β3 protein expression in the lung (24). Therefore, we investigated the production of specific TGF-β isoforms in an OVA model of allergic airway disease and showed that TGF-β1 is the predominant isoform released into the airspaces. Time-course experiments indicated that TGF-β1 release is initiated early in the disease process after OVA challenge and peaks 7 days after the first OVA exposure. We also demonstrated that the bronchial epithelium of the conducting airways is a key localization of TGF-β1 protein in control mice, its expression is decreased in the OVA model, and eosinophil recruitment is not required for TGF-β1 production.
The functional role of TGF-β signaling in the lung was then assessed using anti–TGF-β1 antibody. TGF-β1 antibody neutralization indeed blocked downstream phospho-Smad 2 accumulation in the airways, confirming that the approach used attenuates canonical TGF-β1 signaling. Anti–TGF-β1 antibody treatment failed to effect the establishment of eosinophilic or monocytic inflammation in the lung; however, it did increase Th2 cytokine profiles and it enhanced OVA-induced AHR, whereas it inhibited subepithelial collagen deposition. These data show that, although inhibition of pulmonary fibrosis with TGF-β1 neutralizing antibody may be a possible outcome, anti–TGF-β1 antibody may, in fact, exacerbate asthma pathology in terms of enhancing AHR. Furthermore, these data suggest a complex and apparently paradoxical role for TGF-β1 in disease pathophysiology: on the one hand, it may contribute to the fibrosis, whereas on the other hand, it has an ameliorating role in regard to the genesis of AHR.
The role of TGF-β signaling in lung fibrosis has been elucidated in recent years. Indeed, inhibition of TGF-β1 signaling via downstream ALK5 receptor inhibition blocks lung expression of several fibrotic genes, including type I collagen and PAI-1 (14). Conversely, gain of function mutations in TGF-β receptors correlate to increased CTGF gene expression (25). Treatment of OVA-induced mice with pan–TGF-β antibody reduced airway collagen deposition, epithelial mucus metaplasia, and smooth muscle cell proliferation (15). It is therefore not surprising that, in the current study, anti–TGF-β1 antibody was effective in inhibiting pulmonary fibrosis; however, the above studies did not assess the impact of the inhibition of fibrosis on either airway function or AHR.
The observation that immunoreactive TGF-β1 protein is primarily present in airway epithelial cells is supported by recent studies using laser capture microdissection to identify the source of OVA-induced fibrotic cytokines (26). In those studies, TGF-β1, CTGF, and PAI-1 mRNA levels were all specifically enriched in the epithelial layer and not in the underlying smooth muscle. In addition, recent studies have shown activation of signaling pathways downstream of TGF-β receptor engagement in the bronchial epithelium in both animal models and biopsies from humans with asthma (15, 26–29). Specifically, phospho-Smad 2, the activated receptor Smad, colocalizes with the epithelium in both antigen-challenged mice (including in this study) and humans with asthma. Taken together, these data implicate TGF-β1 as potentially playing an autocrine role in the airway epithelium to induce pulmonary remodeling and other pulmonary pathologies. The relative contribution of inflammatory cells to TGF-β1 production may be an important difference between human and mouse allergic airway disease, and we cannot exclude the possibility that eosinophils produced and released TGF-β1 at time points different to those studied in our model.
In the current study, anti–TGF-β1 antibody had no effect on eosinophilic inflammation but did enhance Th2 cytokine profiles induced by OVA challenge. The finding that inflammatory cell recruitment is unaltered by TGF-β1 neutralization is consistent with two recent studies using anti–pan-TGF-β antibody or ALK5 inhibition (14, 15). Further support for these results comes from an additional study using anti–TGF-β antibody followed by a detailed assessment of systemic immune cell function. These authors found that anti–pan-TGF-β antibody had no effect on lymphocyte proliferation, phagocytic activity, cytokine production, or immunoglobulin production (30). However, studies conducted using the OVA model in TGF-β1 heterozygous mice, systemically lacking an allele of the TGF-β1 gene, showed increased eosinophil accumulation and increased Th2 cytokine production (31). In these mice, TGF-β1 protein levels in the lung were reduced to 30% of normal wild-type levels after OVA challenge and it is unclear why this study produced significantly different results from our current work, although we did observe increased Th2 cytokine production similar to the results found in TGF-β1 heterozygous mice. It is important to note that disruption of the TGF-β1 gene has been shown to produce partial lethality and severe inflammatory abnormalities in mice (32–34). Elevated Th2 cytokine levels have been linked with AHR and allergic airway disease severity in numerous studies, suggesting that TGF-β1 neutralization may impact AHR through modulation of Th2 cell responses (35, 36).
Interference with TGF-β1 function using neutralizing antibody had a significant effect on the pathophysiology induced by antigen challenge. Although anti–TGF-β1 antibody increased all measures of baseline (pre-Mch) lung mechanics, this effect was not significant (Table 1). However, anti–TGF-β1 antibody did significantly enhance AHR as assessed with G (tissue resistance) and H (elastance) but not RN (airway resistance), which is consistent with a peripheral (small) airway effect. These findings are both surprising and at first glance inconsistent with the commonly held view that airway remodeling is a major cause of AHR.
Some potential explanations for this outcome can be considered. One possible explanation for the results is the well-known effect that TGF-β1 has on T-cell regulation. TGF-β1 overexpression in helper T cells has been shown to inhibit OVA-induced AHR in mice (37). Moreover, in two recent studies, one in which TGF-β1 was overexpressed in airway epithelial cells (38) and another in which TGF-β1 was administered intratracheally (39), both treatments were associated with increased TGF-β1 and a decrease in AHR. On the other hand, there are other reports where reduced levels of TGF-β1 (31) or ectopically administered TGF-β1 (9) did not alter airway resistance or AHR. Unfortunately, the determination of the mechanical response and AHR in all of the above studies was obtained noninvasively with a whole body plethysmographic technique, a measurement that has been shown to have severe shortcomings and uncertain interpretation (40).
Another potential immunologic mechanism by which TGF-β1 may decrease antigen-induced AHR is through effects on regulatory T lymphocyte (Treg) activity. Treg cells were initially identified as a specific class of TGF-β1–producing T cells that make up a small percentage of the T-cell pool (41). The Treg cells are important in the regulation of Th2 immunity and are believed to play an important role in the control of allergic responses because Treg cells have been shown to suppress OVA-induced AHR in mice, via a TGF-β1–dependent mechanism (42). In that study, anti–TGF-β antibody blocked the inhibitory effects of Tregs on AHR, analogous to our findings. In addition, Treg cells have been shown to induce Th2 cell apoptosis (43) and increased tolerance to inhaled antigen (44), promoting resolution of AHR. Recent work has shown that TGF-β1 induces maturation of naive T cells to a Treg phenotype via expression of the Treg-specific transcription factor Foxp3 (45). These data suggest that TGF-β1 activity is required for normal Treg function in the lung. By inhibiting TGF-β1 activity, it is possible that lung T-cell function is skewed toward an exacerbated allergic phenotype, which would include increased AHR. The role of Treg activity in the structure/function relationships in the lung and the link to TGF-β1 remain to be determined.
Last, it is likely that, during the inflammatory events caused by antigen challenge, elaboration of TGF-β1 inhibits AHR by a structural mechanism. Thickening of the airway walls of patients with asthma is related to asthma severity (46, 47) and has shown to be greatest in those who die of asthma (48, 49). Mathematical modeling (50, 51) suggests that thickening of the airway wall contributes to AHR in vivo by a geometric mechanism, in which a thickened airway wall internal to the airway smooth muscle (ASM) will narrow the airway lumen more for any given stimuli to the ASM. MacParland and colleagues (17) have recently reviewed the evidence that structural remodeling of the airway could hypothetically either contribute to AHR or ameliorate AHR; however, the evidence directly linking AHR to airway wall alterations is both limited and inconsistent (52). To explain the present findings, we suggest that antigen-induced inflammation leads to the well-documented elaboration of mediators (e.g., leukotrienes and interleukins) and, in this case, TGF-β1 (Figure 1), which, in turn, causes subepithelial fibrosis (Figure 6). This airway wall fibrosis would then result in any one or combination of events, including the following: (1) stiffening of the wall preventing the ASM from narrowing the lumen in response to Mch; (2) as the fibrosis occurs within and between the cells of the ASM, this would increase the parallel elastic load to ASM which prevents shortening; or (3) increasing the series load against which the ASM must contract (17, 53). It has been noted that chronic antigen challenge leads to more substantial airway wall remodeling but a decline in AHR (54), consistent with this proposed mechanism of an ameliorating effect of airway fibrosis. Consistent with this explanation is the spatial location of the antigen-induced deposition of collagen with and outside of the ASM (Figure 6B) and its reduction by an anti–TGF-β1 antibody. Moreover, the pattern of the temporal response to a single dose of inhaled Mch of both elastance (H) and tissue resistance (G) (Figure 7B) shows a markedly enhanced peak response consistent with enhanced ASM narrowing. Anti–TGF-β1 antibody, by decreasing the amount of submucosal collagen, would allow ASM to contract more, leading to a marked increase in the peak response of H, a temporal pattern that we have not observed previously even with antigen challenge (55, 56). In this way, airway fibrosis would serve to lessen airway narrowing, but prevention of its formation by blocking the effects of TGF-β1 results in enhanced airway narrowing.
In conclusion, the data presented in this study show that, although anti–TGF-β antibody strategies may indeed provide desired antifibrotic potential, the effects of anti–TGF-β1 antibody in fact exacerbates the AHR associated with the allergic airway response. The mechanism by which TGF-β1 regulates AHR remains unclear and further study is warranted to elucidate the pathways involved. In addition, we identify TGF-β1 as the primary TGF-β isoform produced in the antigen challenge model and the airway epithelium as a potential source of TGF-β. We also demonstrate that the mechanisms of antigen-induced inflammation, remodeling, and AHR appear to be independent with regard to the TGF-β signaling pathway. We conclude that the function of TGF-β1 in allergic airway disease is to shift the lung toward a fibrotic phenotype while dampening the AHR induced by antigen challenge, perhaps by either an immunologic or mechanical mechanism. Collectively, these findings suggest that the commonly held notion that airway remodeling is directly involved in the genesis of AHR may need to be reconsidered.
Supplementary Material
Acknowledgments
The authors thank Amy L. Brown and Jennifer L. Ather for technical assistance in these studies.
Supported by grants from the National Institute of Health HL P01-67004 and P20 RL 15557 National Center for Research Resources, Centers of Biomedical Research Excellence.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200702-334OC on August 29, 2007
Conflict of Interest Statement: J.F.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.M.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.F.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.v.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.H.T.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.M.W.J.-H. received $1,500 from Sepracor for a lecture given in January 2005. C.G.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816–827. [DOI] [PubMed] [Google Scholar]
- 2.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 2004;125:754–765. [DOI] [PubMed] [Google Scholar]
- 3.Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy 2004;34:567–575. [DOI] [PubMed] [Google Scholar]
- 4.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1997;17:326–333. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 2004;31:62–68. [DOI] [PubMed] [Google Scholar]
- 6.Batra V, Musani AI, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy 2004;34:437–444. [DOI] [PubMed] [Google Scholar]
- 7.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-β expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med 1997;156:591–599. [DOI] [PubMed] [Google Scholar]
- 8.Silverman ES, Palmer LJ, Subramaniam V, Hallock A, Mathew S, Vallone J, Faffe DS, Shikanai T, Raby BA, Weiss ST, et al. Transforming growth factor-β1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med 2004;169:214–219. [DOI] [PubMed] [Google Scholar]
- 9.Kenyon NJ, Ward RW, McGrew G, Last JA. TGF-beta1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax 2003;58:772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu YD, Hua J, Mui A, O'Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2003;285:L527–L539. [DOI] [PubMed] [Google Scholar]
- 11.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001;194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–584. [DOI] [PubMed] [Google Scholar]
- 14.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, et al. Progressive transforming growth factor β1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med 2005;171:889–898. [DOI] [PubMed] [Google Scholar]
- 15.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol 2005;174:5774–5780. [DOI] [PubMed] [Google Scholar]
- 16.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 1999;104:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol 2003;95:426–434. [DOI] [PubMed] [Google Scholar]
- 18.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 2002;160:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 2001;281:L394–L402. [DOI] [PubMed] [Google Scholar]
- 20.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178. [DOI] [PubMed] [Google Scholar]
- 21.Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 2003;94:1297–1306. [DOI] [PubMed] [Google Scholar]
- 22.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979;11:447–455. [DOI] [PubMed] [Google Scholar]
- 23.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest 2004;113:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol 1991;115:1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275–281. [DOI] [PubMed] [Google Scholar]
- 26.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, Martin G, Panju M, Inman MD, Gauldie J. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol 2005;32:99–107. [DOI] [PubMed] [Google Scholar]
- 27.Sagara H, Okada T, Okumura K, Ogawa H, Ra C, Fukuda T, Nakao A. Activation of TGF-beta/Smad2 signaling is associated with airway remodeling in asthma. J Allergy Clin Immunol 2002;110:249–254. [DOI] [PubMed] [Google Scholar]
- 28.Phipps S, Benyahia F, Ou TT, Barkans J, Robinson DS, Kay AB. Acute allergen-induced airway remodeling in atopic asthma. Am J Respir Cell Mol Biol 2004;31:626–632. [DOI] [PubMed] [Google Scholar]
- 29.Rosendahl A, Checchin D, Fehniger TE, ten Dijke P, Heldin CH, Sideras P. Activation of the TGF-beta/activin-Smad2 pathway during allergic airway inflammation. Am J Respir Cell Mol Biol 2001;25:60–68. [DOI] [PubMed] [Google Scholar]
- 30.Ruzek MC, Hawes M, Pratt B, McPherson J, Ledbetter S, Richards SM, Garman RD. Minimal effects on immune parameters following chronic anti-TGF-beta monoclonal antibody administration to normal mice. Immunopharmacol Immunotoxicol 2003;25:235–257. [DOI] [PubMed] [Google Scholar]
- 31.Scherf W, Burdach S, Hansen G. Reduced expression of transforming growth factor beta 1 exacerbates pathology in an experimental asthma model. Eur J Immunol 2005;35:198–206. [DOI] [PubMed] [Google Scholar]
- 32.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992;359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boivin GP, Molina JR, Ormsby I, Stemmermann G, Doetschman T. Gastric lesions in transforming growth factor beta-1 heterozygous mice. Lab Invest 1996;74:513–518. [PubMed] [Google Scholar]
- 34.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S. Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood 1996;87:1439–1445. [PubMed] [Google Scholar]
- 35.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 1999;17:255–281. [DOI] [PubMed] [Google Scholar]
- 36.Hamelmann E, Gelfand EW. IL-5-induced airway eosinophilia: the key to asthma? Immunol Rev 2001;179:182–191. [DOI] [PubMed] [Google Scholar]
- 37.Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, DeKruyff RH, Umetsu DT. CD4(+) T helper cells engineered to produce latent TGF-beta1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest 2000;105:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon SG, Lee CG, Oh MH, Chun EY, Gho YS, Cho SH, Kim JH, Min KU, Kim YY, Kim YK, et al. Recombinant basic fibroblast growth factor inhibits the airway hyperresponsiveness, mucus production, and lung inflammation induced by an allergen challenge. J Allergy Clin Immunol 2007;119:831–837. [DOI] [PubMed] [Google Scholar]
- 39.Fu CL, Ye YL, Lee YL, Chiang BL. Effects of overexpression of IL-10, IL-12, TGF-beta and IL-4 on allergen induced change in bronchial responsiveness. Respir Res 2006;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol 2002;93:1198–1207. [DOI] [PubMed] [Google Scholar]
- 41.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol 2005;5:271–283. [DOI] [PubMed] [Google Scholar]
- 42.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol 2007;178:1433–1442. [DOI] [PubMed] [Google Scholar]
- 43.Finotto S, Eigenbrod T, Karwot R, Boross I, Doganci A, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, et al. Local blockade of IL-6R signaling induces lung CD4+ T cell apoptosis in a murine model of asthma via regulatory T cells. Int Immunol 2007;19:685–693. [DOI] [PubMed] [Google Scholar]
- 44.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med 2006;203:2649–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chetta A, Foresi A, Del Donno M, Bertorelli G, Pesci A, Olivieri D. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest 1997;111:852–857. [DOI] [PubMed] [Google Scholar]
- 47.Boulet L, Belanger M, Carrier G. Airway responsiveness and bronchial-wall thickness in asthma with or without fixed airflow obstruction. Am J Respir Crit Care Med 1995;152:865–871. [DOI] [PubMed] [Google Scholar]
- 48.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993;147:405–410. [DOI] [PubMed] [Google Scholar]
- 49.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, Nishimura K, Itoh H, Izumi T. Airway wall thickness in asthma assessed by computed tomography: relation to clinical indices. Am J Respir Crit Care Med 2000;162:1518–1523. [DOI] [PubMed] [Google Scholar]
- 50.Moreno RH, Hogg JC, Pare PD. Mechanics of airway narrowing. Am Rev Respir Dis 1986;133:1171–1180. [DOI] [PubMed] [Google Scholar]
- 51.Wagers S, Lundblad L, Moriya HT, Bates JH, Irvin CG. Nonlinearity of respiratory mechanics during bronchoconstriction in mice with airway inflammation. J Appl Physiol 2002;92:1802–1807. [DOI] [PubMed] [Google Scholar]
- 52.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 2007;30:1420–1441. [DOI] [PubMed] [Google Scholar]
- 53.Lambert RK. Role of bronchial basement membrane in airway collapse. J Appl Physiol 1991;71:666–673. [DOI] [PubMed] [Google Scholar]
- 54.Vanacker NJ, Palmans E, Pauwels RA, Kips JC. Fluticasone inhibits the progression of allergen-induced structural airway changes. Clin Exp Allergy 2002;32:914–920. [DOI] [PubMed] [Google Scholar]
- 55.Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 2004;114:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol 2007;102:221–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.