Abstract
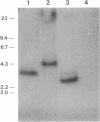
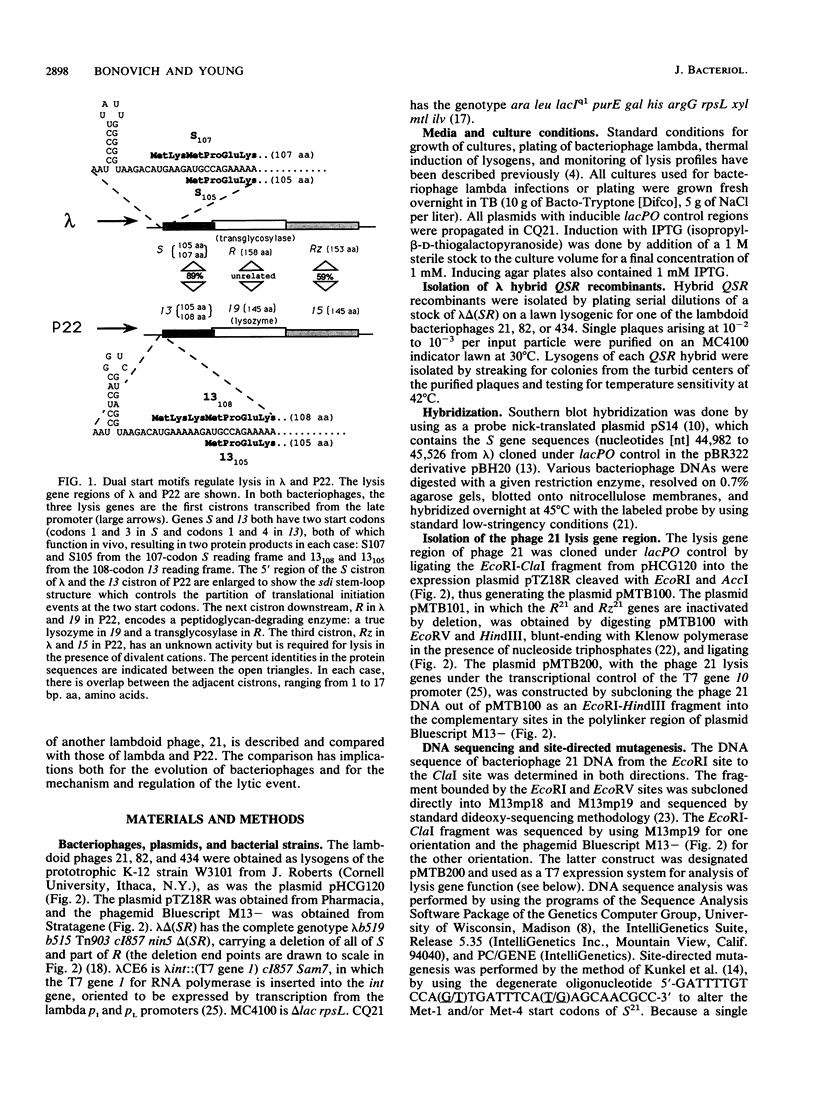
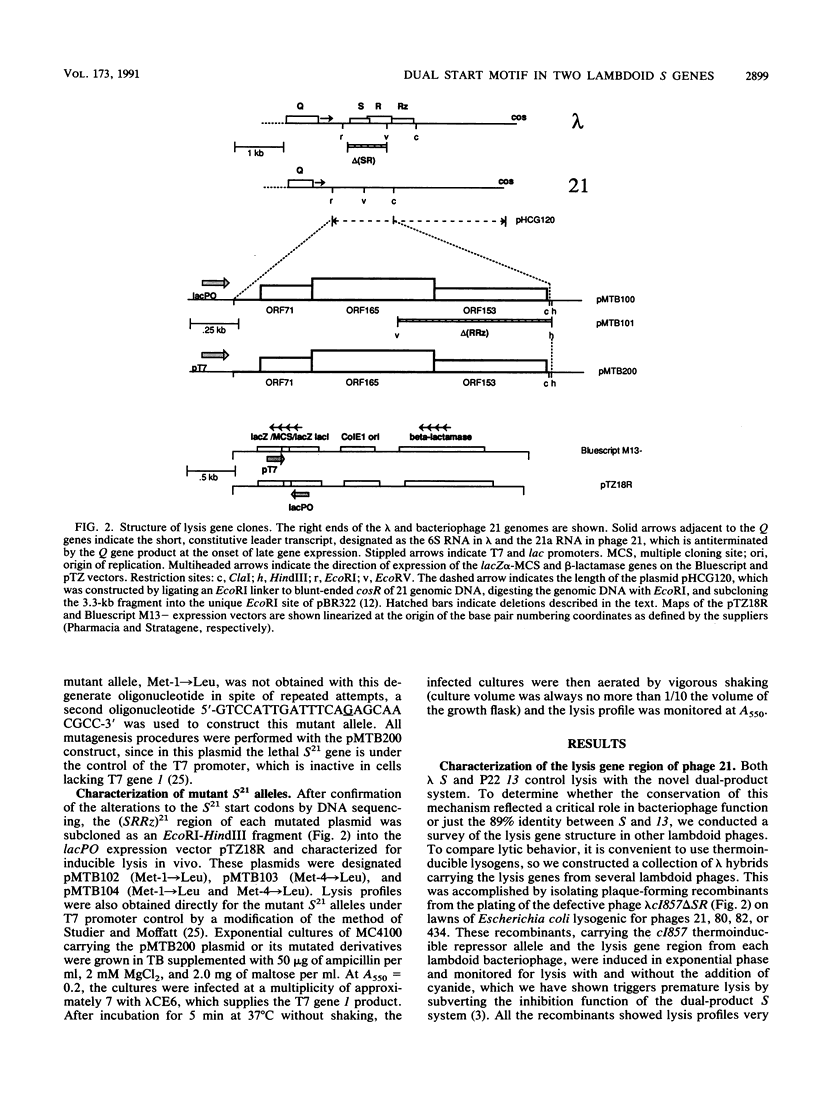
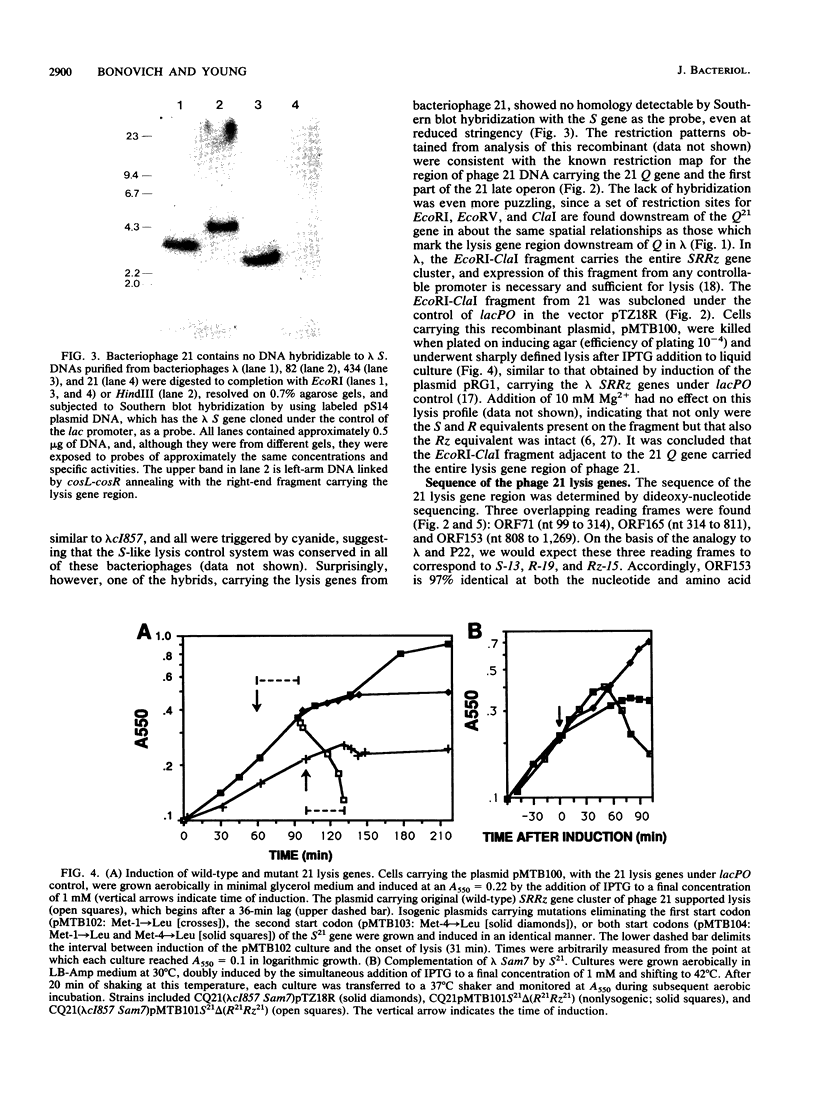
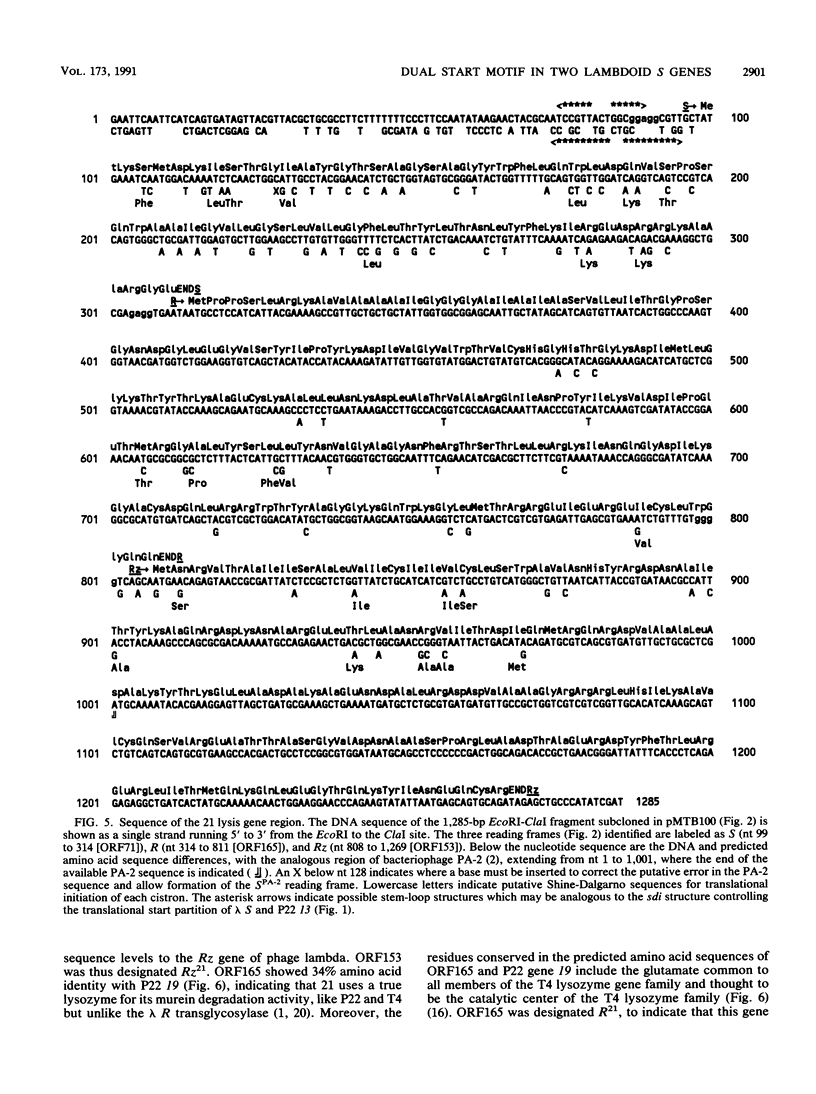
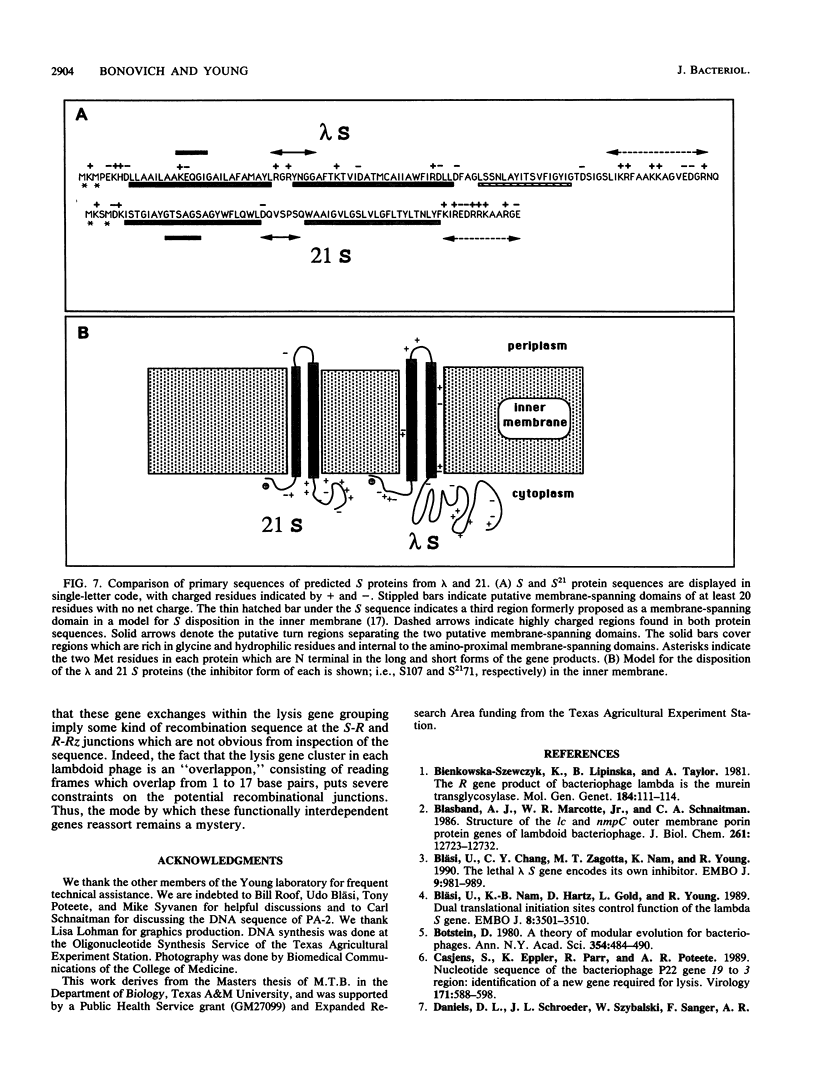
The lysis gene region of phage 21 contains three overlapping reading frames, designated S21, R21, and Rz21 on the basis of the analogy with the SRRz gene cluster of phage lambda. The 71-codon S21 gene complements lambda Sam7 for lysis function but shows no detectable homology with S lambda in the amino acid or nucleotide sequence. A highly related DNA sequence from the bacteriophage PA-2 was found by computer search of the GenBank data base. Correction of this sequence by insertion of a single base revealed another 71-codon reading frame, which is accordingly designated the SPA-2 gene and is 85% identical to S21. There are thus two unrelated classes of S genes; class I, consisting of the homologous 107-codon S lambda and 108-codon P22 gene 13, and class II, consisting of the 71-codon S21 and SPA-2 genes. The codon sequence Met-Lys-(X)-Met...begins all four genes. The two Met codons in S lambda and 13 have been shown to serve as translational starts for distinct polypeptide products which have opposing functions: the shorter polypeptide serves as the lethal lysis effector, whereas the longer polypeptide acts as a lysis inhibitor. To test whether this same system exists in the class II S genes, the Met-I and Met-4 codons of S21 were altered in inducible plasmid clones and the resultant lysis profiles were monitored. Elimination of the Met-1 start results in increased toxicity, and lysis, although not complete, begins earlier, which suggests that both starts are used in the scheduling of lysis by S21 and is consistent with the idea that the 71- and 68-residue products act as a lysis inhibitor and a lysis effector, respectively. In addition, the R gene of 21 was shown to be related to P22 gene 19, which encodes a true lysozyme activity, and was also found to be nearly identical to PA-2 ORF2. We infer that the 21 and PA-2 R genes both encode lysozymes in the T4 e gene family. These three genes form a second class lambdoid R genes, with the lambda R gene being the sole member of the first class. The existence of two interchangeable but unrelated classes of S genes and R genes is discussed in terms of a model of bacteriophage evolution in which the individual gene is the unit of evolution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowska-Szewczyk K., Lipinska B., Taylor A. The R gene product of bacteriophage lambda is the murein transglycosylase. Mol Gen Genet. 1981;184(1):111–114. doi: 10.1007/BF00271205. [DOI] [PubMed] [Google Scholar]
- Blasband A. J., Marcotte W. R., Jr, Schnaitman C. A. Structure of the lc and nmpC outer membrane porin protein genes of lambdoid bacteriophage. J Biol Chem. 1986 Sep 25;261(27):12723–12732. [PubMed] [Google Scholar]
- Bläsi U., Chang C. Y., Zagotta M. T., Nam K. B., Young R. The lethal lambda S gene encodes its own inhibitor. EMBO J. 1990 Apr;9(4):981–989. doi: 10.1002/j.1460-2075.1990.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsi U., Nam K., Hartz D., Gold L., Young R. Dual translational initiation sites control function of the lambda S gene. EMBO J. 1989 Nov;8(11):3501–3510. doi: 10.1002/j.1460-2075.1989.tb08515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D. A theory of modular evolution for bacteriophages. Ann N Y Acad Sci. 1980;354:484–490. doi: 10.1111/j.1749-6632.1980.tb27987.x. [DOI] [PubMed] [Google Scholar]
- Casjens S., Eppler K., Parr R., Poteete A. R. Nucleotide sequence of the bacteriophage P22 gene 19 to 3 region: identification of a new gene required for lysis. Virology. 1989 Aug;171(2):588–598. doi: 10.1016/0042-6822(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Garrett J. M., Young R. Lethal action of bacteriophage lambda S gene. J Virol. 1982 Dec;44(3):886–892. doi: 10.1128/jvi.44.3.886-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J., Bruno C., Young R. Lysis protein S of phage lambda functions in Saccharomyces cerevisiae. J Bacteriol. 1990 Dec;172(12):7275–7277. doi: 10.1128/jb.172.12.7275-7277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Nam K., Bläsi U., Zagotta M. T., Young R. Conservation of a dual-start motif in P22 lysis gene regulation. J Bacteriol. 1990 Jan;172(1):204–211. doi: 10.1128/jb.172.1.204-211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab R., Neal G., Garrett J., Grimaila R., Fusselman R., Young R. Mutational analysis of bacteriophage lambda lysis gene S. J Bacteriol. 1986 Sep;167(3):1035–1042. doi: 10.1128/jb.167.3.1035-1042.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab R., Neal G., Sohaskey C., Smith J., Young R. Dominance in lambda S mutations and evidence for translational control. J Mol Biol. 1988 Jan 5;199(1):95–105. doi: 10.1016/0022-2836(88)90381-6. [DOI] [PubMed] [Google Scholar]
- Rennell D., Poteete A. R. Phage P22 lysis genes: nucleotide sequences and functional relationships with T4 and lambda genes. Virology. 1985 May;143(1):280–289. doi: 10.1016/0042-6822(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Taylor A. Endopeptidase activity of phage lamba-endolysin. Nat New Biol. 1971 Dec 1;234(48):144–145. doi: 10.1038/newbio234144a0. [DOI] [PubMed] [Google Scholar]
- Young R., Way J., Way S., Yin J., Syvanen M. Transposition mutagenesis of bacteriophage lambda: a new gene affecting cell lysis. J Mol Biol. 1979 Aug 15;132(3):307–322. doi: 10.1016/0022-2836(79)90262-6. [DOI] [PubMed] [Google Scholar]