Abstract
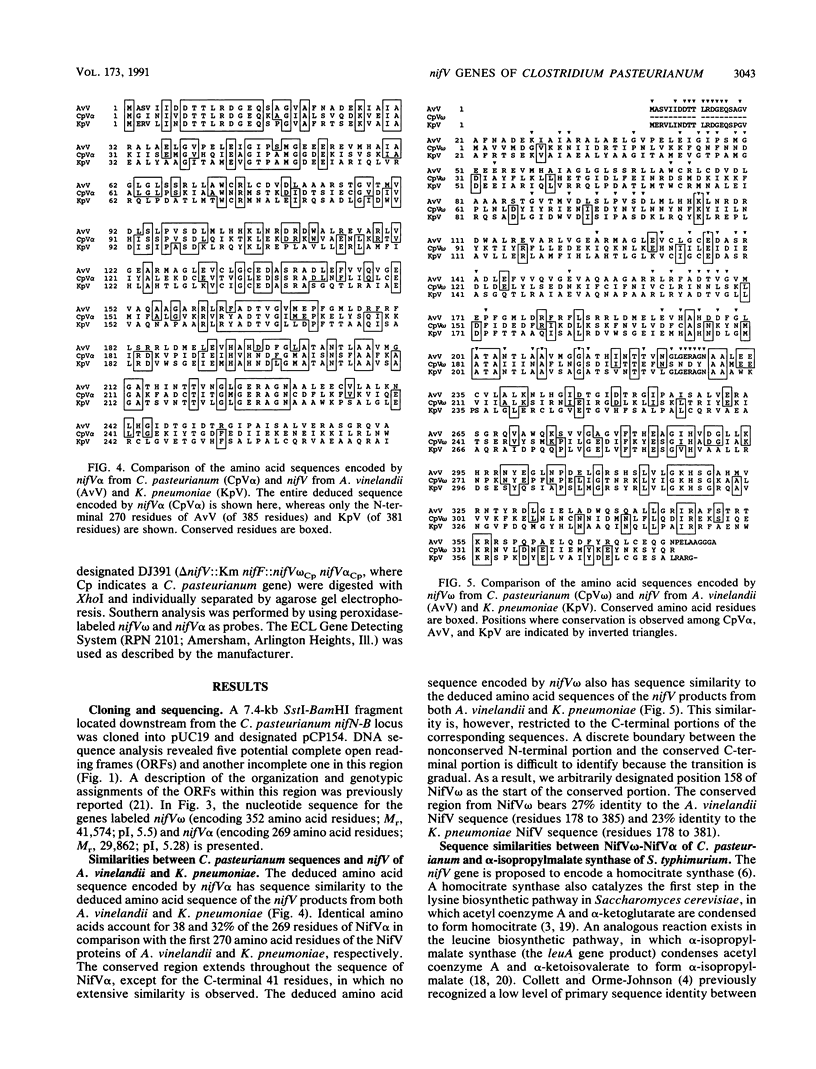
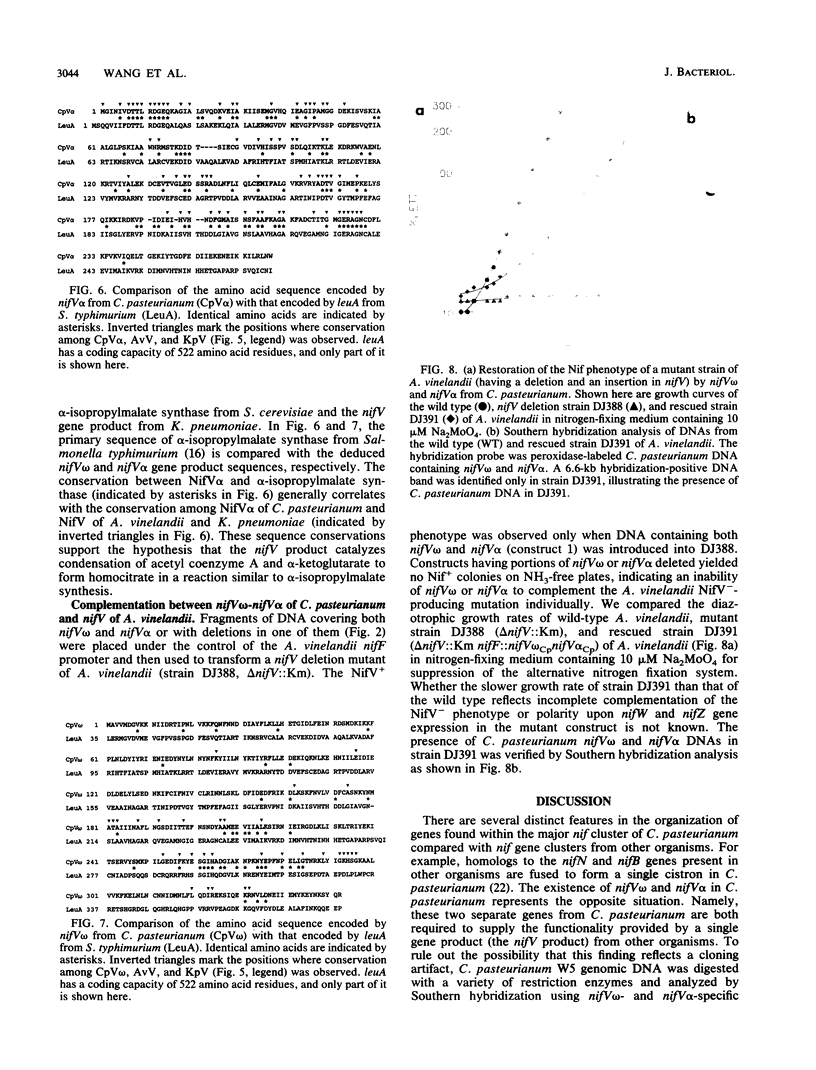
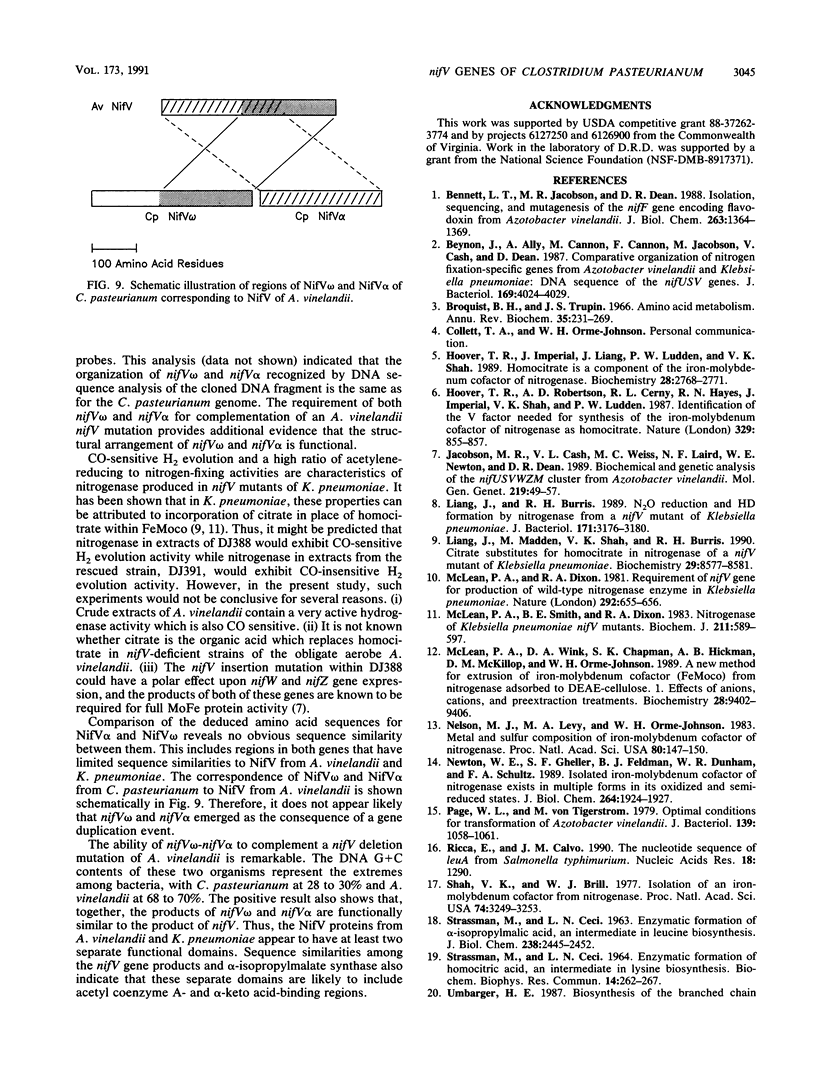
The nifV gene products from Azotobacter vinelandii and Klebsiella pneumoniae share a high level of primary sequence identity and are proposed to catalyze the synthesis of homocitrate. While searching for potential nif (nitrogen fixation) genes within the genomic region located downstream from the nifN-B gene of Clostridium pasteurianum, we observed two open reading frames (ORFs) whose deduced amino acid sequences exhibit nonoverlapping sequence identity to different portions of the nifV gene products from A. vinelandii and K. pneumoniae. Conserved regions were located between the C-terminal 195 amino acid residues of the first ORF and the C-terminal portion of the nifV gene product and between the entire sequence of the second ORF (269 amino acid residues) and the N-terminal portion of the nifV gene product. We therefore designated the first ORF nifV omega and the second ORF nifV alpha. The deduced amino acid sequences of nifV omega and nifV alpha were also found to have sequence similarity when compared with the primary sequence of the leuA gene product from Salmonella typhimurium, which encodes alpha-isopropylmalate synthase. Marker rescue experiments were performed by recombining nifV omega and nifV alpha from C. pasteurianum, singly and in combination, into the genome of an A. vinelandii mutant strain which has an insertion and a deletion mutation located within its nifV gene. A NifV+ phenotype was obtained only when both the C. pasteurianum nifV omega and nifV alpha genes were introduced into the chromosome of this mutant strain. These results suggest that the nifV omega and nifV alpha genes encode separate domains, both of which are required for homocitrate synthesis in C. pasteurianum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett L. T., Jacobson M. R., Dean D. R. Isolation, sequencing, and mutagenesis of the nifF gene encoding flavodoxin from Azotobacter vinelandii. J Biol Chem. 1988 Jan 25;263(3):1364–1369. [PubMed] [Google Scholar]
- Beynon J., Ally A., Cannon M., Cannon F., Jacobson M., Cash V., Dean D. Comparative organization of nitrogen fixation-specific genes from Azotobacter vinelandii and Klebsiella pneumoniae: DNA sequence of the nifUSV genes. J Bacteriol. 1987 Sep;169(9):4024–4029. doi: 10.1128/jb.169.9.4024-4029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. R., Imperial J., Ludden P. W., Shah V. K. Homocitrate is a component of the iron-molybdenum cofactor of nitrogenase. Biochemistry. 1989 Apr 4;28(7):2768–2771. doi: 10.1021/bi00433a004. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Robertson A. D., Cerny R. L., Hayes R. N., Imperial J., Shah V. K., Ludden P. W. Identification of the V factor needed for synthesis of the iron-molybdenum cofactor of nitrogenase as homocitrate. 1987 Oct 29-Nov 4Nature. 329(6142):855–857. doi: 10.1038/329855a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. R., Cash V. L., Weiss M. C., Laird N. F., Newton W. E., Dean D. R. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol Gen Genet. 1989 Oct;219(1-2):49–57. doi: 10.1007/BF00261156. [DOI] [PubMed] [Google Scholar]
- Liang J., Burris R. H. N2O reduction and HD formation by nitrogenase from a nifV mutant of Klebsiella pneumoniae. J Bacteriol. 1989 Jun;171(6):3176–3180. doi: 10.1128/jb.171.6.3176-3180.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Madden M., Shah V. K., Burris R. H. Citrate substitutes for homocitrate in nitrogenase of a nifV mutant of Klebsiella pneumoniae. Biochemistry. 1990 Sep 18;29(37):8577–8581. doi: 10.1021/bi00489a011. [DOI] [PubMed] [Google Scholar]
- McLean P. A., Dixon R. A. Requirement of nifV gene for production of wild-type nitrogenase enzyme in Klebsiella pneumoniae. Nature. 1981 Aug 13;292(5824):655–656. doi: 10.1038/292655a0. [DOI] [PubMed] [Google Scholar]
- McLean P. A., Smith B. E., Dixon R. A. Nitrogenase of Klebsiella pneumoniae nifV mutants. Biochem J. 1983 Jun 1;211(3):589–597. doi: 10.1042/bj2110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. A., Wink D. A., Chapman S. K., Hickman A. B., McKillop D. M., Orme-Johnson W. H. A new method for extraction of iron-molybdenum cofactor (FeMoco) from nitrogenase adsorbed to DEAE-cellulose. 1. Effects of anions, cations, and preextraction treatments. Biochemistry. 1989 Nov 28;28(24):9402–9406. doi: 10.1021/bi00450a023. [DOI] [PubMed] [Google Scholar]
- Nelson M. J., Levy M. A., Orme-Johnson W. H. Metal and sulfur composition of iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1983 Jan;80(1):147–150. doi: 10.1073/pnas.80.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton W. E., Gheller S. F., Feldman B. J., Dunham W. R., Schultz F. A. Isolated iron-molybdenum cofactor of nitrogenase exists in multiple forms in its oxidized and semi-reduced states. J Biol Chem. 1989 Feb 5;264(4):1924–1927. [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Calvo J. M. The nucleotide sequence of leuA from Salmonella typhimurium. Nucleic Acids Res. 1990 Mar 11;18(5):1290–1290. doi: 10.1093/nar/18.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASSMAN M., CECI L. N. Enzymatic formation of alpha-isopropylmalic acid, an intermediate in leucine biosynthesis. J Biol Chem. 1963 Jul;238:2445–2452. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman M., Ceci L. N. Enzymatic formation of homocitric acid, an intermediate in lysine biosynthesis. Biochem Biophys Res Commun. 1964;14:262–267. doi: 10.1016/0006-291x(64)90446-2. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Chen J. S., Johnson J. L. A nitrogen-fixation gene (nifC) in Clostridium pasteurianum with sequence similarity to chlJ of Escherichia coli. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1122–1128. doi: 10.1016/0006-291x(90)92012-o. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]