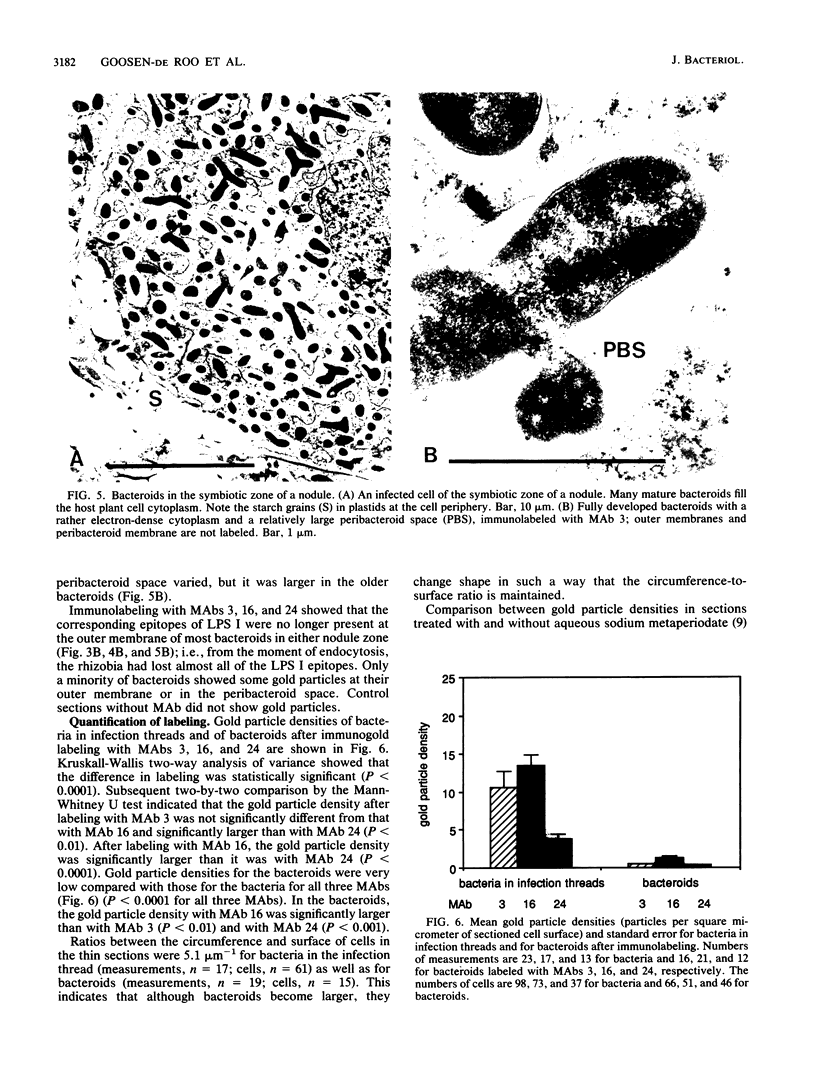
Abstract
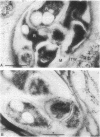
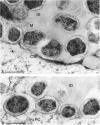
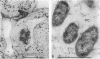
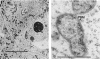
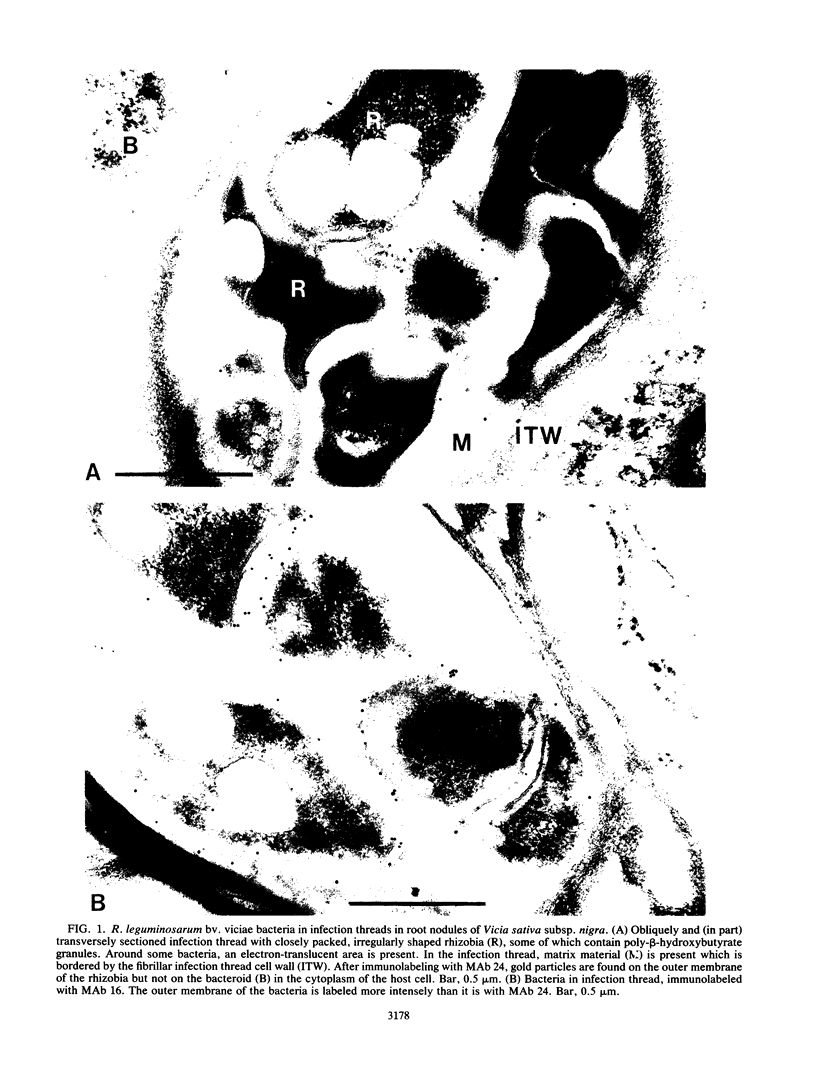
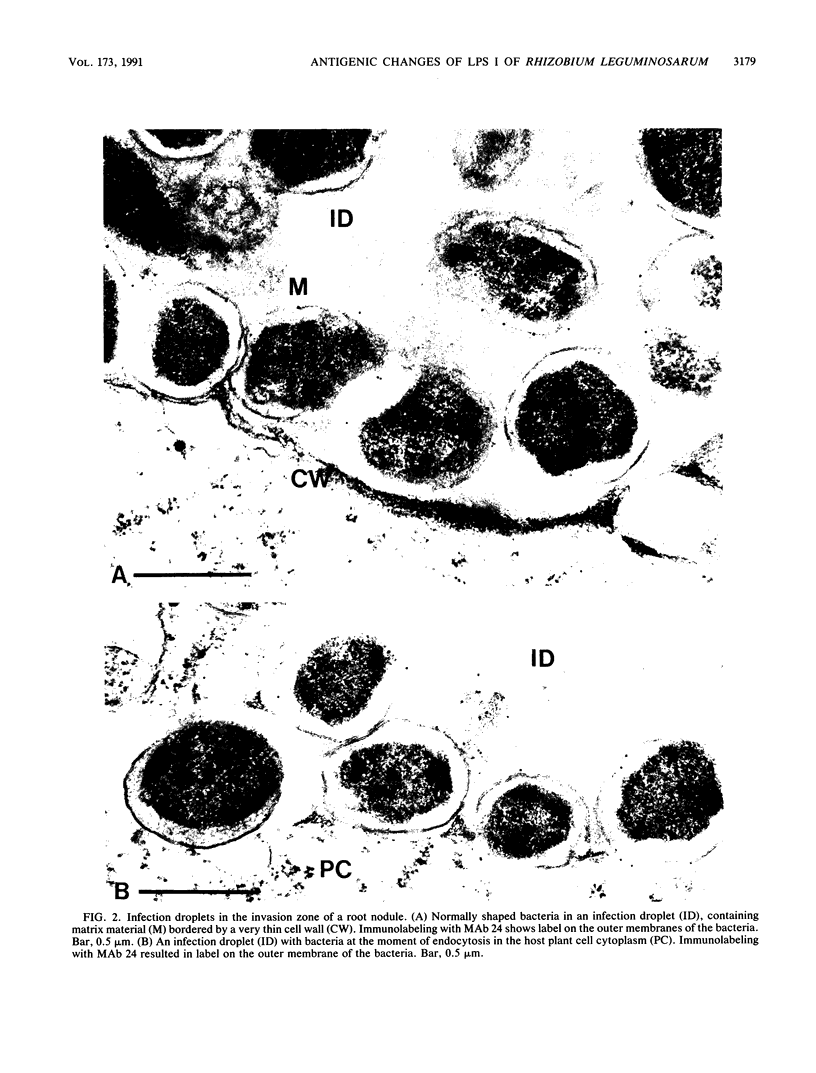
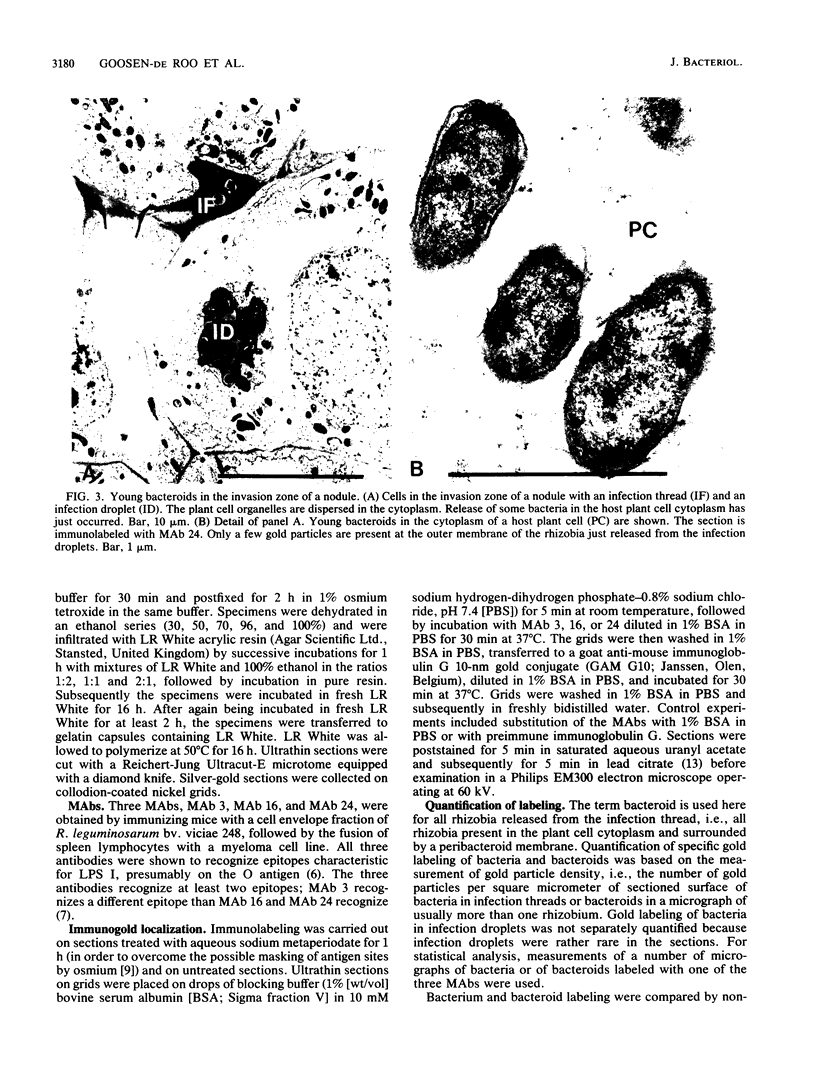
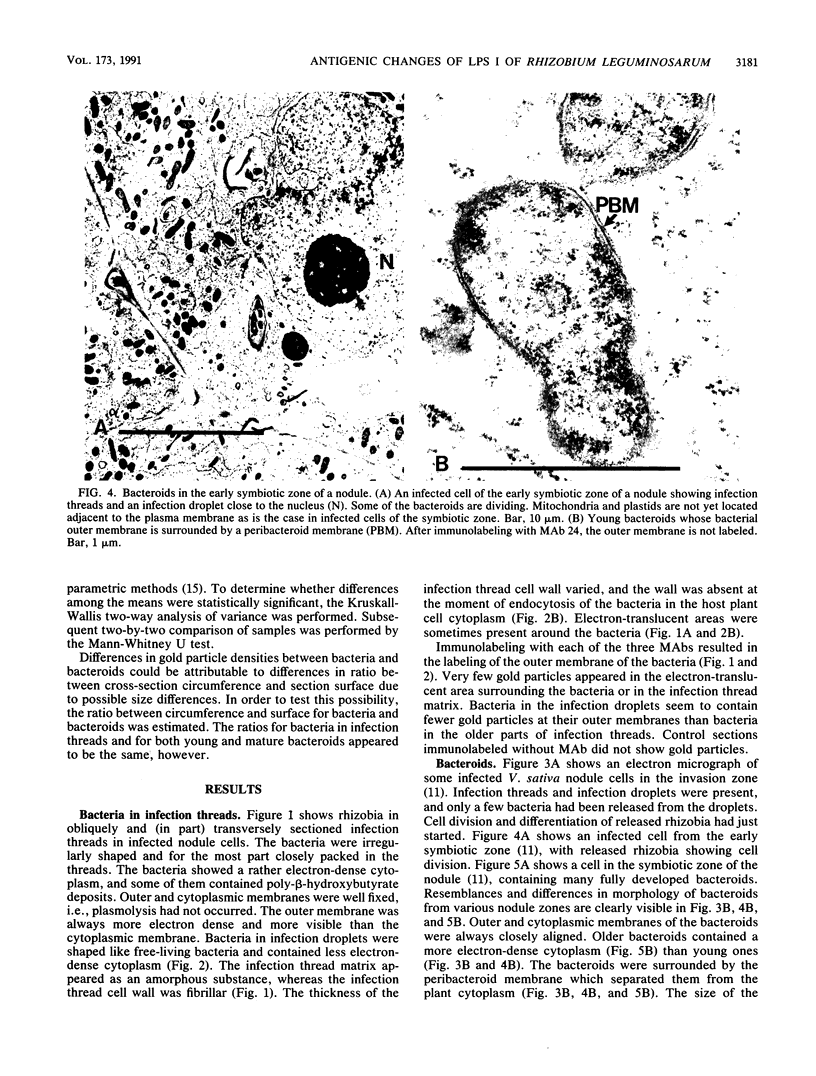
Three different monoclonal antibodies raised against the O antigen-containing lipopolysaccharide (LPS I) of free-living cells were used in an immunocytochemical study to follow the fate of LPS I on the outer membrane of Rhizobium leguminosarum bv. viciae 248 during the nodulation of Vicia sativa subsp. nigra. After immunogold labeling, the LPS I epitopes were detected on the outer membrane of bacteria present in infection threads throughout the nodule. Epitopes were not detectable on bacteria released from the infection thread. The data show that the LPS I epitopes present on rhizobia in infection droplets disappear shortly before or during endocytosis of the bacteria into the host plant cell cytoplasm. The abruptness of the change suggests an active degradation or modification of LPS I epitopes rather than only a repression of their synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal A. K., Shantharam S., Verma D. P. Changes in the outer cell wall of Rhizobium during development of root nodule symbiosis in soybean. Can J Microbiol. 1980 Sep;26(9):1096–1103. doi: 10.1139/m80-182. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Butcher G. W., Galfre G., Wood E. A., Brewin N. J. Physical association between the peribacteroid membrane and lipopolysaccharide from the bacteroid outer membrane in Rhizobium-infected pea root nodule cells. J Cell Sci. 1986 Sep;85:47–61. doi: 10.1242/jcs.85.1.47. [DOI] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Priefer U. B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol. 1989 Nov;171(11):6161–6168. doi: 10.1128/jb.171.11.6161-6168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. G., Wells B., Brewin N. J., Wood E., Knight C. D., Downie J. A. The legume-Rhizobium symbiosis: a cell surface interaction. J Cell Sci Suppl. 1985;2:317–331. doi: 10.1242/jcs.1985.supplement_2.17. [DOI] [PubMed] [Google Scholar]
- VandenBosch K. A., Brewin N. J., Kannenberg E. L. Developmental regulation of a Rhizobium cell surface antigen during growth of pea root nodules. J Bacteriol. 1989 Sep;171(9):4537–4542. doi: 10.1128/jb.171.9.4537-4542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R., de Rijk R., Mulders I. H., Lugtenberg B. J. Immunological characterization of Rhizobium leguminosarum outer membrane antigens by use of polyclonal and monoclonal antibodies. J Bacteriol. 1989 Feb;171(2):1136–1142. doi: 10.1128/jb.171.2.1136-1142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R., van Rossum C., Lugtenberg B. J. Recognition of individual strains of fast-growing rhizobia by using profiles of membrane proteins and lipopolysaccharides. J Bacteriol. 1988 Aug;170(8):3782–3785. doi: 10.1128/jb.170.8.3782-3785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]