Abstract
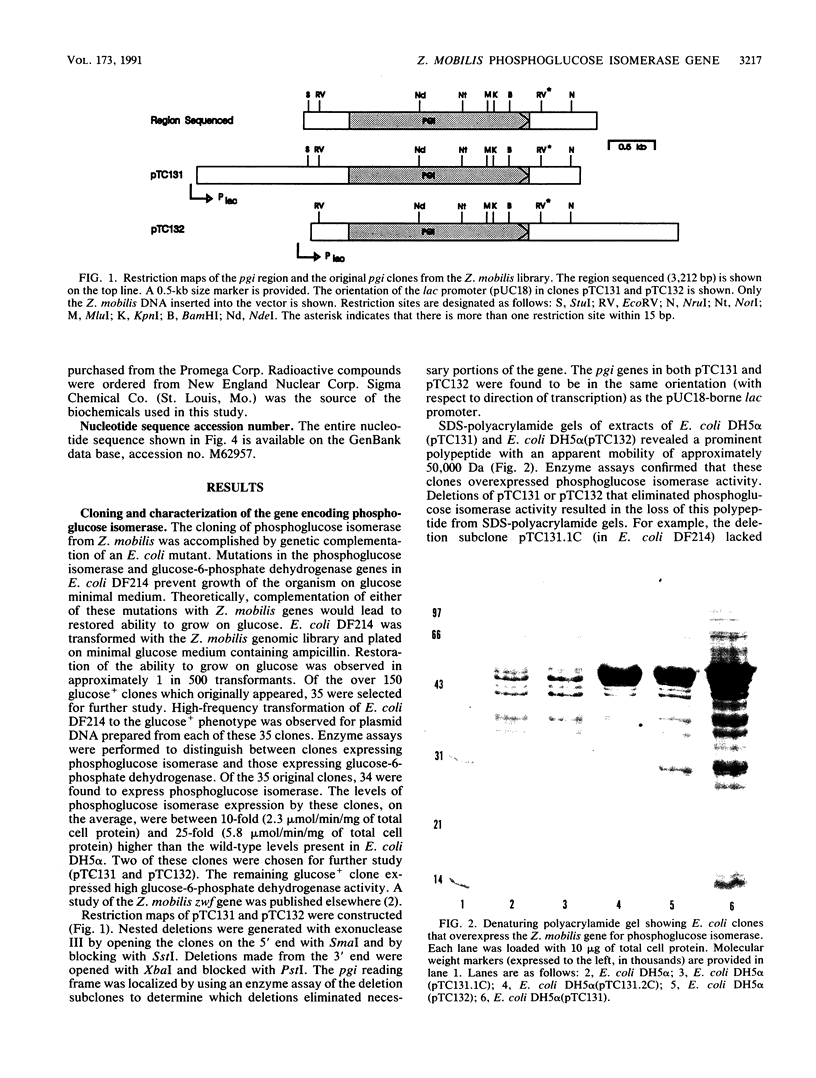
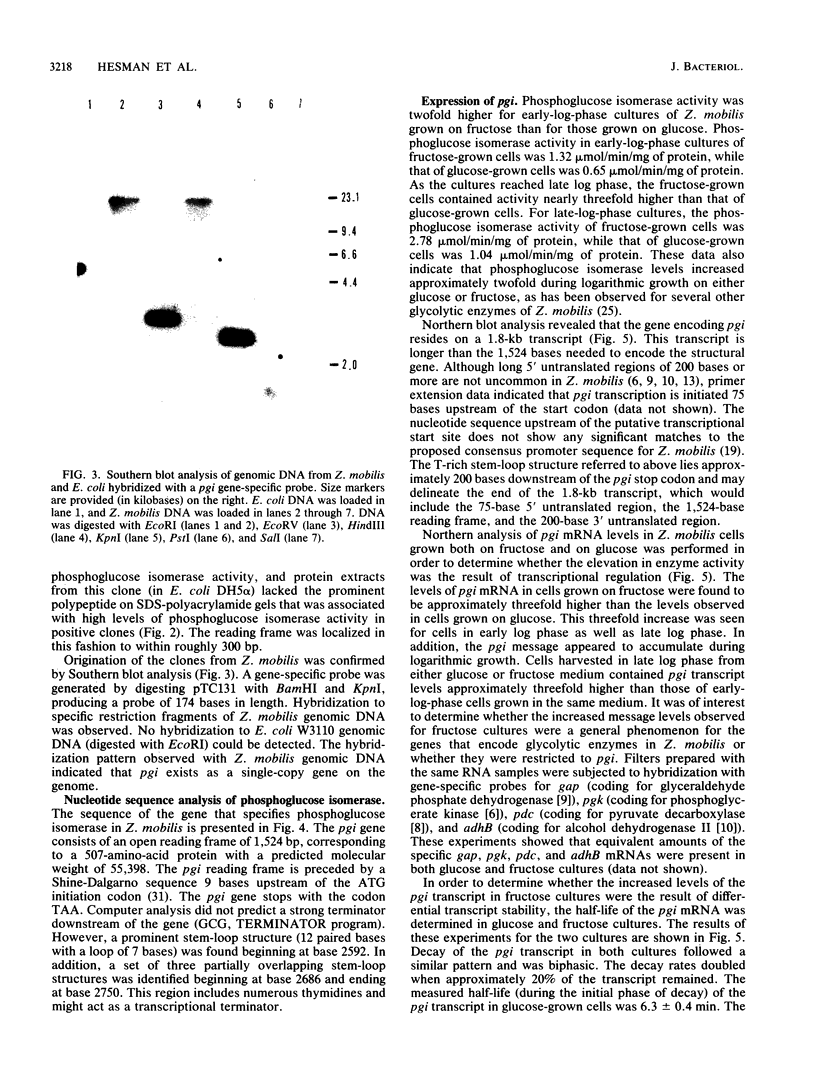
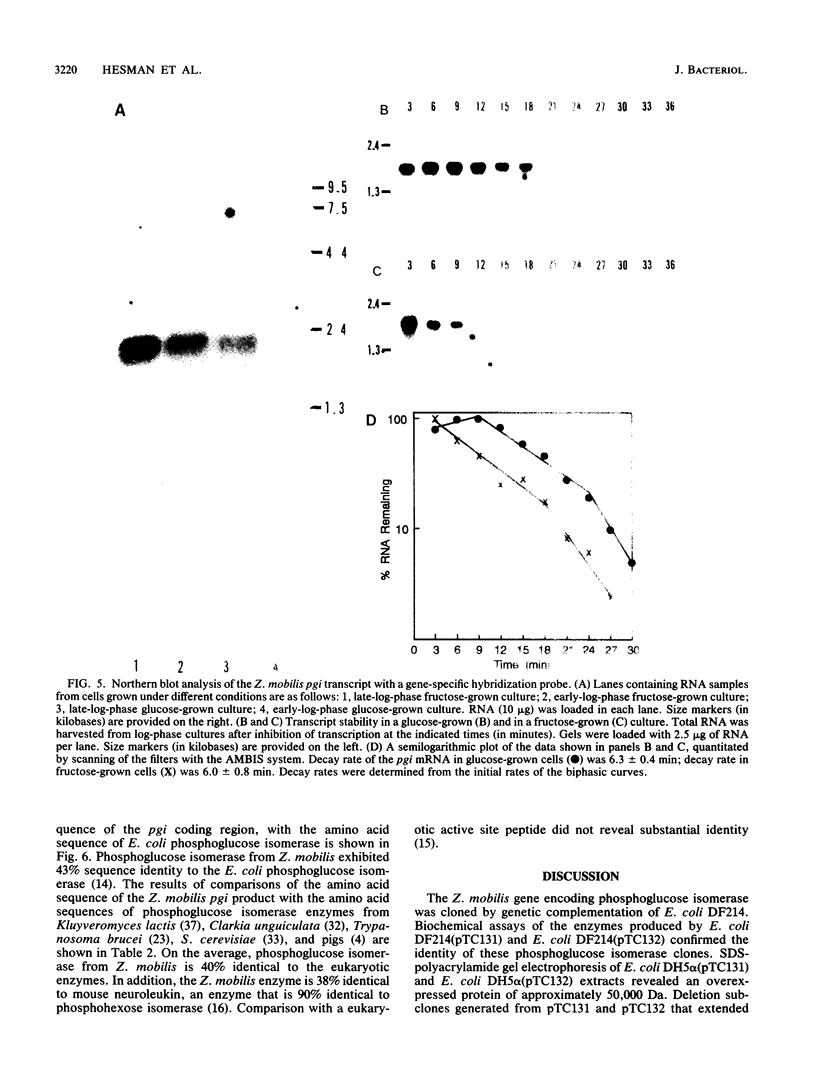
The Zymomonas mobilis gene encoding phosphoglucose isomerase (pgi) was cloned by genetic complementation of an Escherichia coli pgi mutant. An enzyme assay and sodium dodecyl sulfate-polyacrylamide gel electrophoresis confirmed the presence of excess amounts of phosphoglucose isomerase in E. coli clones carrying the Z. mobilis pgi gene. The pgi gene is present in only one copy on the Z. mobilis genome. Nucleotide sequence analysis of the pgi region revealed an open reading frame of 1,524 bp preceded by a strong Shine-Dalgarno sequence. The pgi gene encodes a 507-amino-acid protein with a predicted molecular weight of 55,398. Z. mobilis phosphoglucose isomerase is between 38 and 43% identical to the enzyme from other species. Northern (RNA) blot analysis showed that the pgi transcript is 1.8 kb in length. The level of the pgi transcript was found to be influenced by the phase of growth and by the carbon and energy sources. Transcript levels increased with respect to total RNA during logarithmic growth and were threefold higher when grown on fructose than on glucose. These changes in transcript levels paralleled phosphoglucose isomerase activities in the cultures. Differential mRNA stability was not a factor, since the half-life of the pgi transcript was 6.3 min in glucose-grown cells and 6.0 min in fructose-grown cells. Thus, an increase in the rate of transcription appears to be at least partially responsible for the increased levels of phosphoglucose isomerase observed for Z. mobilis grown on fructose.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnell W. O., Yi K. C., Conway T. Sequence and genetic organization of a Zymomonas mobilis gene cluster that encodes several enzymes of glucose metabolism. J Bacteriol. 1990 Dec;172(12):7227–7240. doi: 10.1128/jb.172.12.7227-7240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput M., Claes V., Portetelle D., Cludts I., Cravador A., Burny A., Gras H., Tartar A. The neurotrophic factor neuroleukin is 90% homologous with phosphohexose isomerase. Nature. 1988 Mar 31;332(6163):454–455. doi: 10.1038/332454a0. [DOI] [PubMed] [Google Scholar]
- Conway T., Byun M. O., Ingram L. O. Expression Vector for Zymomonas mobilis. Appl Environ Microbiol. 1987 Feb;53(2):235–241. doi: 10.1128/aem.53.2.235-241.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Ingram L. O. Phosphoglycerate kinase gene from Zymomonas mobilis: cloning, sequencing, and localization within the gap operon. J Bacteriol. 1988 Apr;170(4):1926–1933. doi: 10.1128/jb.170.4.1926-1933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Ingram L. O. Similarity of Escherichia coli propanediol oxidoreductase (fucO product) and an unusual alcohol dehydrogenase from Zymomonas mobilis and Saccharomyces cerevisiae. J Bacteriol. 1989 Jul;171(7):3754–3759. doi: 10.1128/jb.171.7.3754-3759.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Osman Y. A., Konnan J. I., Hoffmann E. M., Ingram L. O. Promoter and nucleotide sequences of the Zymomonas mobilis pyruvate decarboxylase. J Bacteriol. 1987 Mar;169(3):949–954. doi: 10.1128/jb.169.3.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Sewell G. W., Ingram L. O. Glyceraldehyde-3-phosphate dehydrogenase gene from Zymomonas mobilis: cloning, sequencing, and identification of promoter region. J Bacteriol. 1987 Dec;169(12):5653–5662. doi: 10.1128/jb.169.12.5653-5662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Sewell G. W., Osman Y. A., Ingram L. O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987 Jun;169(6):2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy C. K., Mejia J. P., Conway T., Ingram L. O. Differential expression of gap and pgk genes within the gap operon of Zymomonas mobilis. J Bacteriol. 1989 Dec;171(12):6549–6554. doi: 10.1128/jb.171.12.6549-6554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froman B. E., Tait R. C., Gottlieb L. D. Isolation and characterization of the phosphoglucose isomerase gene from Escherichia coli. Mol Gen Genet. 1989 May;217(1):126–131. doi: 10.1007/BF00330951. [DOI] [PubMed] [Google Scholar]
- Gibson D. R., Gracy R. W., Hartman F. C. Affinity labeling and characterization of the active site histidine of glucosephosphate isomerase. Sequence homology with triosephosphate isomerase. J Biol Chem. 1980 Oct 10;255(19):9369–9374. [PubMed] [Google Scholar]
- Gurney M. E., Heinrich S. P., Lee M. R., Yin H. S. Molecular cloning and expression of neuroleukin, a neurotrophic factor for spinal and sensory neurons. Science. 1986 Oct 31;234(4776):566–574. doi: 10.1126/science.3764429. [DOI] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Krieg N. R., Phibbs P. V., Jr Transport and catabolism of D-fructose by Spirillum itersomii. J Bacteriol. 1974 Jan;117(1):144–150. doi: 10.1128/jb.117.1.144-150.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A. Stabilization of the 3' one-third of Escherichia coli ribosomal protein S20 mRNA in mutants lacking polynucleotide phosphorylase. J Bacteriol. 1989 Aug;171(8):4112–4120. doi: 10.1128/jb.171.8.4112-4120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand M., Kooystra U., Wierenga R. K., Lambeir A. M., Van Beeumen J., Opperdoes F. R., Michels P. A. Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of the enzyme. Eur J Biochem. 1989 Sep 15;184(2):455–464. doi: 10.1111/j.1432-1033.1989.tb15038.x. [DOI] [PubMed] [Google Scholar]
- Osman Y. A., Conway T., Bonetti S. J., Ingram L. O. Glycolytic flux in Zymomonas mobilis: enzyme and metabolite levels during batch fermentation. J Bacteriol. 1987 Aug;169(8):3726–3736. doi: 10.1128/jb.169.8.3726-3736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K., Testolin V., Stoter A., Griffiths-Smith K., Algar E. M. Simultaneous purification and characterization of glucokinase, fructokinase and glucose-6-phosphate dehydrogenase from Zymomonas mobilis. Biochem J. 1985 Jun 15;228(3):627–634. doi: 10.1042/bj2280627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekamp-Olson P., Najarian R., Burke R. L. The isolation, characterization and nucleotide sequence of the phosphoglucoisomerase gene of Saccharomyces cerevisiae. Gene. 1988 Dec 15;73(1):153–161. doi: 10.1016/0378-1119(88)90321-6. [DOI] [PubMed] [Google Scholar]
- Thomson J., Gerstenberger P. D., Goldberg D. E., Gociar E., Orozco de Silva A., Fraenkel D. G. ColE1 hybrid plasmids for Escherichia coli genes of glycolysis and the hexose monophosphate shunt. J Bacteriol. 1979 Jan;137(1):502–506. doi: 10.1128/jb.137.1.502-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Hillman J. D., Schulman H., Reznikoff W. S., Fraenkel D. G. New phosphoglucose isomerase mutants of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1172–1174. doi: 10.1128/jb.122.3.1172-1174.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wésolowski-Louvel M., Goffrini P., Ferrero I. The RAG2 gene of the yeast Kluyveromyces lactis codes for a putative phosphoglucose isomerase. Nucleic Acids Res. 1988 Sep 12;16(17):8714–8714. doi: 10.1093/nar/16.17.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]