Abstract
Guanylate cyclase-activating proteins (GCAP1 and GCAP2) are thought to mediate the intracellular stimulation of guanylate cyclase (GC) by Ca2+, a key event in recovery of the dark state of rod photoreceptors after exposure to light. GCAP1 has been localized to rod and cone outer segments, the sites of phototransduction, and to photoreceptor synaptic terminals and some cone somata. We used in situ hybridization and immunocytochemistry to localize GCAP2 in human, monkey, and bovine retinas. In human and monkey retinas, the most intense immunolabeling with anti-GCAP2 antibodies was in the cone inner segments, somata, and synaptic terminals and, to a lesser degree, in rod inner segments and inner retinal neurons. In bovine retina, the most intense immunolabeling was in the rod inner segments, with weaker labeling of cone myoids, somata, and synapses. By using a GCAP2-specific antibody in enzymatic assays, we confirmed that GCAP1 but not GCAP2 is the major component that stimulates GC in bovine rod outer segment homogenates. These results suggest that although GCAP1 is involved in the Ca2+-sensitive regulation of GC in rod and cone outer segments, GCAP2 may have non-phototransduction functions in photoreceptors and inner retinal neurons.
Keywords: phototransduction, guanylate cyclase, Ca2+-binding proteins, cone photoreceptors
In photoreceptor cells, photoactivation of rhodopsin or cone visual pigment results in a transient decrease in the concentrations of Ca2+ and cGMP. These receptors and second messengers are linked through a cascade of specific activation/inactivation reactions in phototransduction (1, 2). The levels of Ca2+ and cGMP are strictly controlled and interconnected. cGMP is a gating ligand of the plasma membrane cation channels that are permeable to Ca2+ ions. After cGMP is hydrolyzed, the efflux of Ca2+ exceeds the influx, resulting in decreased [Ca2+] within the cell. The lowering of [Ca2+] triggers production of cGMP through activation of a photoreceptor-specific particulate guanylate cyclase (GC) (3). The Ca2+ sensitivity of GC (e.g., the higher activity at low levels of [Ca2+]) is mediated by one or more Ca2+-binding proteins, termed guanylate cyclase-activating proteins (GCAPs) (4, 5).
Two photoreceptor-specific GCs, GC1 and GC2, have been cloned (6–9). Although the localization of GC1 to rod and cone outer segments and synaptic terminals was established by both biochemical and immunocytochemical methods (5, 10–14), the localization of GC2 within photoreceptors is not known. Two GCAPs that stimulate GC1 and GC2 have also been cloned (15–17). There is abundant evidence that GCAP1 activates photoreceptor GC: (i) GCAP1 was isolated from rod outer segments (ROS) (4, 18); (ii) GCAP1 mRNA was found in the myoid region of rod and cone photoreceptor cells (15, 19); (iii) GCAP1 was localized to rod and cone outer segments and to some cone somata and synaptic terminals by immunocytochemistry (17, 18); (iv) cross-linking techniques revealed that GCAP1 was complexed with GC1 (20). In addition, the finding that GC1 is not expressed in the retina of the rd (retinal degeneration) chicken, which contains low levels of GCAP1, suggested that the two proteins are coupled at the transcriptional or posttranslational level (21, 22). The localization of GCAP2 is less well established. GCAP2 was isolated from bovine retinal homogenates (5) and localized by immunocytochemistry to rod outer segments, inner segments, and synapses but was not detected in cones (16). However, GCAP2 was not detected in bovine ROS preparations in other studies (17, 18).
In the present study, we have found that GCAP2 is localized mainly to the inner segments, somata, and synapses of primate cones, but in bovine retina, it is localized mainly to the inner segments of rods and, to a lesser degree, to the inner segments of cones. The differential compartmentalization of GCAP1 and GCAP2 suggests that these proteins have different physiologic functions in photoreceptors.
MATERIALS AND METHODS
Expression of GCAP2 in Escherichia coli.
Two fragments (PvuII–BglII of 288 bp and BglII–HindIII of 412 bp) encompassing the coding sequence for GCAP2 except for the first 7 amino acids were cloned together in the vector pNIV103 that had been cleaved with MscI and HindIII. This clone, named GCAP2–7, isolated as one HindII–HindIII 706-bp fragment, was then inserted into the SmaI–HindIII site of the pQE30 vector (Qiagen, Chatsworth, CA). The GCAP2–7 plasmid was grown in E. coli JM109 and then transformed into E. coli M15. Recombinant E. coli was produced in LB medium containing ampicillin (100 μg/ml) and kanamycin (25 μg/ml) overnight at 37°C. Cultures were diluted 1:50 and grown until the absorption at 600 nm reached 0.7 unit. GCAP2–7 expression was induced by incubation for 5 hr in the presence of 1 mM isopropyl β-d-galactopyranoside. Cells were pelleted by centrifugation at 4°C. The proteins were solubilized with urea, and GCAP2 was purified on a Chelex-Ni column according to protocol 2 of the manual provided by Qiagen. Urea was removed by dialysis against 10 mM sodium phosphate (pH 7.5). The yield of recombinant GCAP2–7 was 7 mg/liter of culture (the purity higher than 90%), which was partially active in GC stimulation.
Cloning of GCAP2 cDNA and Expression in Insect Cells.
A bovine cDNA library was screened with a degenerate oligonucleotide W285 (17) as described from our laboratories (15). The resulting single clone (GCAP2, 2.3 kb) was blunt-ended, cloned into the BamHI site in pVL941, and expressed in High Five insect cells. The expressed recombinant GCAP2 showed a transient product of Mr 30,000 that was unstable and rapidly degraded to yield a product of Mr 23,000. Analysis of the 5′ untranslated region of GCAP2 showed an in-frame extension for GCAP2 of 44 amino acids that was due to a 5′ artifact. The mutant protein was expressed in insect cells and purified (the purity higher than 90%) on a G2 monoclonal antibody column as described (17). GCAP2, a 23-kDa protein, was used to produce UW31 polyclonal antibodies.
Preparation of Anti-GCAP2 Polyclonal Antibodies.
Rabbit anti-GCAP2 polyclonal antibodies were raised in New Zealand White rabbits by subcutaneous immunization with ≈50 μg of GCAP2 (in ≈50 μl) mixed with an equal volume of complete Freund’s adjuvant (Cocalico Biologicals, Reamstown, PA). Animals were given booster injections at 1- to 2-week intervals with 25 μg of GCAP2 mixed with incomplete Freund’s adjuvant. UW50 was raised against bacterially expressed GCAP2 and UW31 against GCAP2 expressed in insect cells.
Affinity Chromatography.
For UW50 and UW31 purification, 6 ml of rabbit serum UW50 and 10 ml of rabbit serum UW31 were diluted 1:2 in 10 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane (BTP) (pH 7.5) containing 50 mM NaCl and loaded on GCAP2–7-Sepharose (1 × 4 cm; 0.25 mg of protein per 1 ml of the CNBr-activated Sepharose 4B). Columns were previously equilibrated in the same buffer. The material was eluted with 0.1 M glycine (pH 2.5). Approximately 4 mg of purified polyclonal antibodies was obtained.
Endogenous GCAP2 Purification.
A retinal extract containing GCAP1 and GCAP2 was prepared by homogenizing 50 bovine retinas in 50 ml of water, containing 2 mM benzamidine and leupeptin (20 μg/ml). The extract was separated from retinal particulates by centrifugation (48,000 × g for 50 min) at 4°C. The extract was then loaded onto a UW50 polyclonal antibody-coupled Sepharose column (affinity purified, 2 mg of antibody per 1 ml of the CNBr-activated Sepharose in a column 1 × 2.5 cm) equilibrated with buffer A (10 mM BTP, pH 7.5/2 mM benzamidine) at a flow rate of 15 ml/hr. The column was then washed successively with (i) buffer A, (ii) buffer A containing 200 mM NaCl, (iii) buffer A, (iv) buffer A containing 4 M urea, and (v) buffer A. Material was eluted with 0.1 M glycine (pH 2.5) containing 2 mM benzamidine. Fractions (1 ml) were collected, immediately neutralized with 1 M Tris⋅HCl (pH 8.4; 0.2 ml per fraction), and examined by SDS/PAGE and immunoblot analysis with the G2 monoclonal antibody (GCAP1) and UW31 polyclonal antibodies (GCAP2) (diluted 1:30,000).
SDS/PAGE and Immunoblotting.
SDS/PAGE was performed according to Laemmli (23) with 12% polyacrylamide gels. The electrotransfer of protein onto Immobilon-P (Millipore) was carried out with a Hoeffer mini-gel system. For immunoblotting, membranes were blocked with 3% (wt/vol) gelatin in 20 mM Tris⋅HCl (pH 8.0) containing 150 mM NaCl and 0.05% Tween 20 and incubated for 1–2 hr with primary antibody at dilutions of 1:30,000. A secondary antibody conjugated with alkaline phosphatase (Promega) was used at a dilution of 1:5,000. antibody binding was detected using 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium.
GC Assays.
The GC assays were performed with [α-32P]GTP and washed ROS in the presence of 1 mM 3-isobutyl-1-methylxanthine (IBMX) or using guanosine 5′-[α-[35S]thio]triphosphate as described (17).
In Situ Hybridization.
A full-length cDNA of the coding region of GCAP2 cloned into pBluescript was used to prepare hybridization probes. The digoxigenin-labeled probes were generated from linearized DNA using T3 RNA polymerase for the antisense and T7 polymerase for the sense probes (Ambion, Austin, TX). Both probes were hydrolyzed with 60 mM Na2CO3/40 mM NaHCO3/80 mM dithiothreitol at 60°C for 40 min to reduce the probe length to 150–300 bases. In situ hybridization was performed on human and bovine retinas as described by Harland (24). The retinas were embedded in JB-4 plastic (Polysciences), sectioned at 5 μm, and counterstained with 0.1% basic fuchsin in 20 mM Tris/maleate in 10% ethanol at pH 8.0. Previous Northern blot analysis showed that human GCAP2 is expressed in the retina and is not detectable in other tissues analyzed (25). The size of the mRNA was 2.2 kb, slightly larger than that for GCAP1, and there was no detectable cross-hybridization between GCAP1 and GCAP2 using Northern blot analysis.
Immunocytochemistry.
Immediately after animal sacrifice, the anterior segments of monkey (Macaca fasicularis) and bovine eyes were removed, and the eye cups were immersed in 4% paraformaldehyde/0.13 M sodium phosphate (pH 7.4), at 4°C. Human eyes were fixed in the same way within 4 hr post mortem. Retinal tissue used for cryosections was transferred from paraformaldehyde fixative to 30% sucrose/0.13 M sodium phosphate (pH 7.4) and stored overnight at 4°C. This tissue was then transferred to O.C.T. cryoembedding compound (Miles), frozen, and sectioned at 40 μm. Nonspecific labeling was blocked by incubating sections in the appropriate normal serum diluted 1:100 in PBS. Sections were incubated overnight at 4°C in affinity-purified anti-GCAP2 polyclonal antibodies (UW50; A280 = 0.4) diluted 1:100 to 1:1,500 in PBS. Triton X-100 (0.3%) was included in all PBS solutions to facilitate antibody penetration. Controls were processed by omitting primary antibodies from the incubation buffer. Additional controls were prepared by adsorbing the anti-GCAP2 polyclonal antibodies with excess (25 μg/ml) GCAP2–7 or GCAP1 prior to incubation. Some sections of monkey retina were double-labeled with antibodies to red/green cone opsin (generated against an N-terminal peptide in human red/green cone opsin; a gift from J. Saari, University of Washington) or blue cone opsin (JH455 serum, specific for the C terminus of human blue opsin; a gift from Jeremy Nathans, Johns Hopkins University School of Medicine). After incubation in primary antibodies, sections were rinsed with PBS and incubated overnight at 4°C in fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (diluted 1:100 in PBS) and/or indocarbocyanine (Cy3)-conjugated goat anti-mouse IgG (diluted 1:200 in PBS; Jackson ImmunoResearch). Sections were rinsed in PBS, mounted in 5% n-propylgallate in glycerol and cover-slipped. The immunolabeling was analyzed with a Bio-Rad MRC 600 laser scanning confocal microscope using a dual channel scan mode. Single plane and z series images were collected and stored as unprocessed files. To evaluate signal overlap, double-labeled samples were examined sequentially under excitation at 488 nm and 568 nm. Image files selected for publication were imported into Adobe photoshop 3.0 and dye sublimation prints were generated.
RESULTS
GCAP2 Is Expressed Predominantly in Photoreceptors.
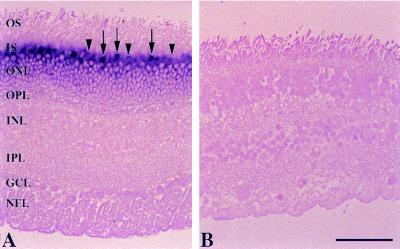
In situ hybridization using human and bovine retinas and digoxigenin-labeled antisense and sense GCAP2 RNA probes was employed to identify the cells of expression. In human retina, cells in the outer nuclear layer were strongly labeled with the antisense probe (Fig. 1A), whereas no hybridization signal was produced by the sense probe (Fig. 1B). The most intense staining was found in the cone myoids, with weaker labeling in rod inner segments. Similar results were obtained using bovine retinas, but the rod inner segments were more heavily labeled than those of the cones (data not shown).
Figure 1.
In situ hybridization of GCAP2 transcripts in the human retina using antisense (A) and sense (B) probes. The following layers are indicated: OS, photoreceptor outer segments; IS, photoreceptor inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; and NFL, nerve-fiber layer. The strongest labeling with the antisense probe is in the cone inner segments (arrows), with weaker labeling of the rod inner segments (arrowheads). (Bar = 50 μm.)
Anti-GCAP2 Polyclonal Antibodies Recognize GCAP2 but not GCAP1.
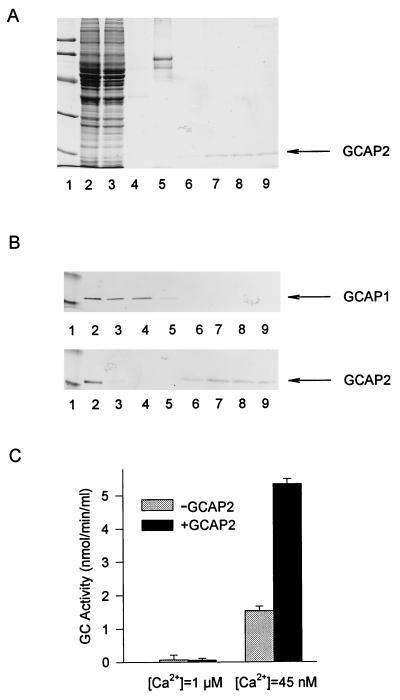
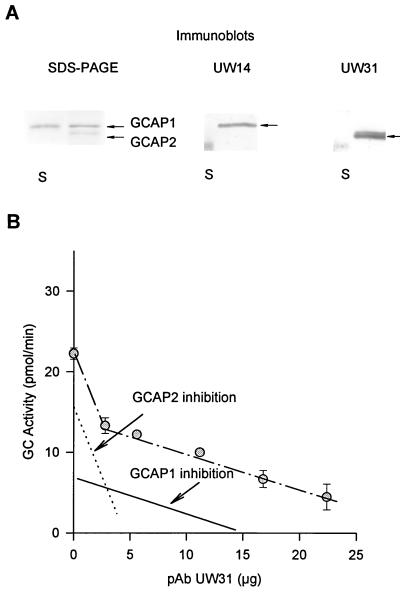
Immunoaffinity chromatography and immunoblots were employed to test the specificity of anti-GCAP2 polyclonal antibodies (UW50). When a retinal extract containing GCAP1 and GCAP2 was loaded on a UW50-Sepharose column, we found that the flow-through contained GCAP1 but that GCAP2 was retained on the column. Immunoblotting of the retinal extract with the anti-GCAP2 polyclonal antibodies (UW31 in Fig. 2B or with UW50, data not shown) produced a single band (see also ¶). The anti-GCAP2 did not cross-react with GCAP1 (Fig. 2 A and B), which had a slightly slower mobility in SDS/PAGE (17). The low pH elution yielded a single protein with Ca2+-dependent GC-stimulating activity (Fig. 2C). SDS/PAGE analysis (Fig. 2A) of these fractions revealed a single band of GCAP2 (≈19 kDa), free of GCAP1 and other contaminants. These experiments suggest that UW50 antibodies were specific for GCAP2 in solution and on immunoblots.
Figure 2.
Immunoaffinity chromatography of retinal extracts. The GCAP1 and GCAP2 were extracted from 50 retinas as described by Gorczyca et al. (17). The extract was loaded onto an immunoaffinity column containing affinity-purified anti-GCAP2 polyclonal antibodies (UW50) coupled to CNBr-activated Sepharose. The fractions that passed through the column during loading were collected, and the column was washed. Bound proteins were eluted with 0.1 M glycine (pH 2.5). Eluted fractions were immediately neutralized with 1 M Tris⋅HCl (pH 8.4). (A) SDS/PAGE analysis. The indicated fractions (in 15 μl) were analyzed on 12.3% polyacrylamide gels and stained with Coomassie brilliant blue R-250. Lanes: 1, standard proteins of known molecular masses (from the top in kDa: 92, 67, 43, 30, 21, 14; from Pharmacia); 2, extracts before chromatography; 3, fractions that passed through the column; 4, fractions that passed through the column in the BTP buffer; 5, fractions that passed through the column in 4 M urea; 6–9, fractions eluted with glycine (pH 2.5). The arrow indicates the position of GCAP2. (B) Reactivity of retinal extracts with anti-GCAP1 and anti-GCAP2 antibodies. One-microliter aliquots from fractions as described in A were probed with anti-GCAP1 monoclonal antibody (G-2) or with anti-GCAP2 polyclonal antibodies (UW31). The arrows indicate the positions of GCAP1 and GCAP2, respectively. Note that G-2 antibody does not react with GCAP2 on immunoblots, although at the high concentrations of G-2 coupled to the CNBr-activated Sepharose, GCAP2 was retained on the column [discussed by Gorczyca et al. (17)]. (C) GC-stimulating activity of GCAP2. Ten-microliter aliquots of a fraction with the highest concentration of GCAP2 was tested to determine its effect on GC activity with washed ROS membranes in the presence of either 1 μM Ca2+ or 45 nM Ca2+.
GCAP2 Is Localized to Monkey Cones.
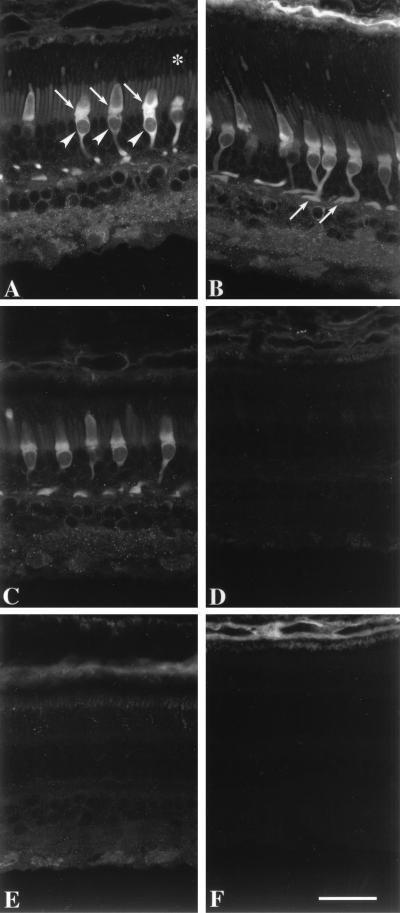
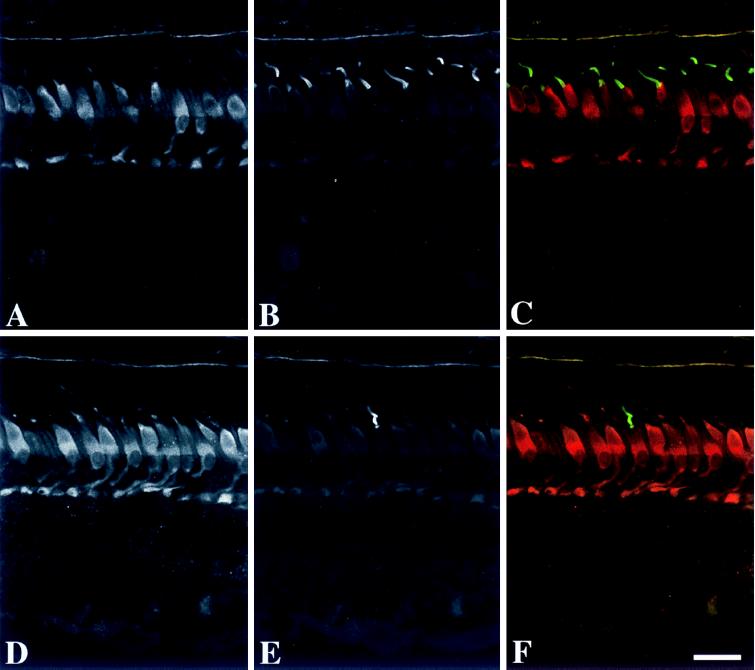
Immunofluorescence microscopy of monkey retina revealed that GCAP2 was present mainly in the inner segments, somata and synaptic terminals of the cones (Fig. 3A). Weak labeling was found in the cone outer segments, the inner and outer segments of rods, the inner plexiform layer, and somata of some inner retinal neurons (Fig. 3A). Using a z projection, immunolabeling was found throughout the cone cell cytoplasm (Fig. 3B). The immunolabeling was not altered by preabsorption of the anti-GCAP2 polyclonal antibodies (UW50) with GCAP1 (Fig. 3C) but was abolished by preincubation with GCAP2–7 (Fig. 3D). The immunolabeling of cone inner segments and synapses persisted at dilutions of the antibody that no longer produced immunolabeling of the other cell types. In double-labeled sections of monkey retina, the GCAP2 was localized to cones (Fig. 4 A and D), including those whose outer segments were reactive with anti-red/green (Fig. 4 B and C) and -blue (Fig. 4 E and F) cone opsins. These results demonstrate that GCAP2 is present in all classes of cone cells. Similar localization of GCAP2 was present in sections of human retina (data not shown).
Figure 3.
Immunofluorescence localization of GCAP2 in monkey retina. (A) GCAP2 immunolabeling with UW50 is strongest in the cone inner segments (arrows), somata (arrowheads), and synaptic terminals. Cone nuclei are negative images. Weak immunolabeling is present in the rod and cone outer segments (denoted by asterisk), rod inner segments, and neurons in the inner retina. (B) Although individual cones are rarely visible in their entirety within a single image plane, their cellular processes can be visualized with a projection of a z series. Arrows denote cone synaptic terminals. (C) Addition of purified GCAP1 (25 μg/ml) to anti-GCAP2 polyclonal antibodies produces minimal decrease in GCAP2 immunoreactivity, verifying that this antibody does not cross-react with GCAP1. (D) Addition of GCAP2–7 (25 μg/ml) to anti-GCAP2 polyclonal antibodies abolishes GCAP2 immunoreactivity. Sections incubated in preimmune serum (E) or buffer without anti-GCAP2 (F) show no immunolabeling of cones or other retinal cell types. The choroid (at the top) is weakly reactive with the secondary antibody. (Bar = 20 μm.)
Figure 4.
Immunofluorescence localization of GCAP2 and cone opsins in monkey retina. (A–C) Localization of GCAP2 and red/green cone opsin. (A) The cones are immunolabeled with anti-GCAP2 (UW50), with the strongest labeling in the inner segments, somata, and synaptic terminals. Some inner retinal neurons are weakly labeled. (B) Anti-red/green cone opsin labels the majority of cone outer segments. (C) Double labeling with anti-red/green cone opsin (green) and anti-GCAP2 (red) demonstrates that the red/green cones are immunopositive for GCAP2. (D–F) Localization of GCAP2 and blue cone opsin. (D) The cone photoreceptors are immunolabeled with anti-GCAP2. (E) Anti-blue cone opsin labels a single cone outer segment. (F) Double labeling with anti-blue cone opsin (green) and anti-GCAP2 (red) demonstrates that the blue cone is immunopositive for GCAP2. (Bar = 20 μm.)
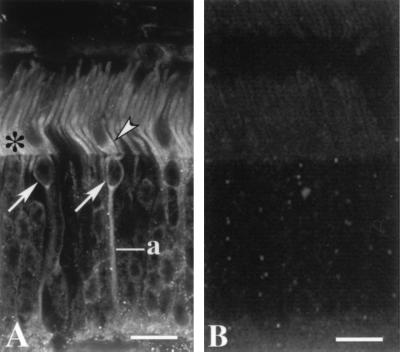
In bovine retina, the most intense immunolabeling was observed in the inner segments of rods, with weaker labeling of the rod and cone outer segments and the myoids of some cone inner segments (Fig. 5A). Some cone somata, axons, and synapses were labeled throughout (Fig. 5A). No labeling was present when the GCAP2 antibodies were preabsorbed with GCAP2–7 (Fig. 5B) or endogenous purified GCAP2 (data not shown). In addition, unrelated bacterially expressed rhodopsin kinase, purified in the same manner as GCAP2–7, did not block immunolabeling (data not shown).
Figure 5.
Immunofluorescence localization of GCAP2 in bovine retina. (A) Both rod and cone photoreceptors are labeled. In rods, labeling is strongest in the inner segments (asterisk). The cone inner segments are substantially wider than rod inner segments and the cell bodies of cones are restricted to the outermost tier of the outer nuclear layer. Labeling is present in the myoid region of a cone inner segment (arrowhead), cone somata (arrows) and a cone axon (a). Labeled photoreceptor synapses are at the bottom. (Bar = 15 μm.) (B) Addition of GCAP2–7 (25 μg/ml) to anti-GCAP2 polyclonal antibodies abolishes GCAP2 immunoreactivity.
GCAP2 Is a Minor Component of ROS Homogenates.
In immunocytochemistry, lack of specific labeling could be due to epitope masking. In addition, GCAP1 and GCAP2 could be coupled to different effector molecules found in separate cellular compartments. GCAP1 and GCAP2 contributions in stimulation of GC in ROS were estimated by using GC assays in the presence of anti-GCAP2 antibody (UW31). UW31 recognized specifically GCAP2 but not GCAP1 (Fig. 6A). Furthermore, in a reconstituted systems UW31 potently inhibited GC stimulation by GCAP2 (shown as dotted line in Fig. 6B), but had only a minor effect on GC stimulation by GCAP1 (Fig. 6B, solid line). The minor inhibition was also observed with several other unrelated IgGs in ROS homogenates and in the reconstituted systems (data not shown); thus, we believe that this effect is due to a general inhibition of GCAP1 by IgG. When freshly purified bovine ROS were used to assay for GC activity in the presence of UW31, a two-phase effect was observed: there was a sharp decrease in the activity followed by a slow gradual inhibition (Fig. 6B). Similar results were obtained in four experiments. One possible interpretation of these results is that GCAP2 was inhibited first, followed by a gradual nonspecific inhibition of GCAP1-dependent stimulation of GC. Extrapolation of these data by subtracting nonspecific inhibition of GCAP1 suggests that ≈28% of GC stimulation in ROS resulted from GCAP2. As expected, addition of GCAP1 to ROS homogenates decreased the contribution by GCAP2 in GC stimulation proportionally to the amount of added protein, whereas addition of GCAP2 to ROS homogenates increased the contribution by GCAP2 in GC stimulation proportionally to the amount of added activating protein. GCAP2 has a higher specific activity (2- to 3-fold) than GCAP1 (figure 8 in ref. 17); thus, the GCAP2 protein would be only between 5% and 15% of GCAP1 in ROS preparations, consistent with the results obtained by immunocytochemistry. When solutions collected during ROS preparations were loaded on immunoaffinity columns, only trace amounts of GCAP1 and GCAP2 were found, consistent with high affinity for biological membranes and minimal losses of the activators during biochemical manipulations.
Figure 6.
Inhibition of GC activity in ROS. (A) SDS/PAGE and immunoblots of GCAP1 and GCAP2 isolated from a retinal extract. UW14 was specific for GCAP1, and UW31 was specific for GCAP2. S represents molecular markers at 20 kDa. Note that GCAP2 appears in two molecular forms of the unknown origin and that UW31 does not recognize GCAP1. (B) The inhibition of GC activity in ROS homogenates. The GC assay was performed as described by Gorczyca et al. (4). Dotted line was determined experimentally from the inhibition of GCAP2-dependent stimulation of GC in reconstituted systems composed of purified GCAP2 (2 μg) and washed ROS membranes. Solid line was determined experimentally from the inhibition of GCAP1-dependent stimulation of GC in reconstituted systems composed of purified GCAP1 (6 μg) and washed ROS membranes.The amount of GCAP1 or GCAP2 (studied in the range of GC stimulation between 5 and 80 pmol/min) did not affect the slope (GC activity versus antibody concentration) of these inhibitions.
DISCUSSION
Recent biochemical studies on Ca2+-binding proteins disclosed that a subfamily of these proteins is expressed mainly in neuronal tissues (neuronal Ca2+-binding proteins, NCBPs) (2). The NCBPs contain four characteristic EF-hand Ca2+-binding loops, of which two or three sites have a high affinity for Ca2+ and the remaining sites are disabled by amino acid substitutions. In addition, NCBPs are N-acylated by a fatty acid at the penultimate Gly residue, after Met-1 is removed. In the retina, the NCBPs are heterogeneously acylated by at least four types of lipids [for review, see Polans et al. (2)]. A subfamily of the NCBPs, the GCAPs, are implicated in the regulation of GC (4, 5, 17).
An unresolved question is why photoreceptors contain two functionally similar GCAP molecules. In bovine retinal extracts, the ratio of GCAP1 to GCAP2 was between 3:1 and 4:1 (ref. 17 and Fig. 5). Our study demonstrates that GCAP2 has a different cellular localization from that of GCAP1 (17), which is localized to rod and cone outer segments, synaptic terminals, and some cone somata (17, 18). GCAP2 was found mainly in the inner segments, somata, and synaptic terminals of all classes of cones in primate retinas. Interestingly, in bovine retina, the inner segments of rod cells and the myoids of some cone cells were labeled, but the immunoreactivity for GCAP2 was weaker in cones than in rods. In the bovine retina, both rod and cone outer segments were very weakly labeled, consistent with the finding of minor amounts of GCAP2 in ROS homogenates. These data are also consistent with the biochemical results of Gorczyca et al. (17) and Frins et al. (18), who found that freshly prepared ROS do not contain significant amounts of GCAP2, but differ from the findings of Dizhoor et al. (16), who reported that GCAP2 is present in the inner and outer segments of rods but not in cones. The origin of these discrepancies is not known, although Dizhoor et al. (16) used a different polyclonal antibody produced against a truncated form of GCAP2 (≈150 amino acids instead of 204).
The genes encoding GCAP2 and GCAP1 are arranged in a tail-to-tail array on the short arm of human chromosome 6 and mouse chromosome 17 (25). Preliminary PCR results indicate that the corresponding bovine genes are also organized in a tail-to-tail array. In this type of arrangement, the regulatory elements governing tissue specificity are located on opposite ends of these two genes. Most tail-to-tail gene arrays reported in the literature [see reference in Surguchov et al. (25)] exhibit differential expression, either cell-specific or expression at different times during development. Our findings are consistent with a model in which GCAP1 is expressed in both rods and cones and transported to the outer segments to regulate cGMP synthesis during phototransduction and to the synapse to participate in unknown pathways. GCAP2 may interact with a yet unidentified target protein in the inner segments, somata, and synapses. Additional studies are needed to evaluate the functions of GCAP1 and GCAP2 in the physiology of the retina.
Acknowledgments
We thank Xinyu Zhao for the preparation of probes used in the in situ hybridization experiments; J. Preston Van Hooser for help during the course of these studies; D. Possin and J. Chang for assistance with tissue processing; and P. Brunner (University of Washington W. M. Keck Center for Advanced Studies of Natural Signaling) for help with the confocal microscopy. This research was supported by National Institutes of Health Grants EY08061, EY01311, EY08123, EY01730, and RR00166 (to the Regional Primate Center), and awards from Research to Prevent Blindness, Inc. (R.P.B.), to the Departments of Ophthalmology at the University of Washington and the University of Utah. W.B. and A.H.M. are recipients of grants from the Foundation Fighting Blindness, Inc. K.P. is a recipient of a Jules and Doris Stein Professorship from RPB, and A.H.M. is a Senior Scientific Investigator of RPB. Legal requirements for use of human donor postmortem retinas were met (University of Washington Human Subjects Approval 26-099E, dated January 24, 1996).
ABBREVIATIONS
- GCAP
guanylate cyclase-activating protein
- GC. guanylate cyclase
ROS, rod outer segments
Footnotes
Two bands (the lower molecular weight band being dominant) were observed also with several anti-GCAP2 monoclonal antibodies at higher magnification of the immunoblots as this shown in Fig. 5. The molecular basis of this heterogeneity is not known.
References
- 1.Koutalos Y, Yau K-W. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- 2.Polans A, Baehr W, Palczewski K. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- 3.Koch K-W, Stryer L. Nature. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- 4.Gorczyca W A, Gray-Keller M P, Detwiler P B, Palczewski K. Proc Natl Acad Sci USA. 1994;91:4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dizhoor A M, Lowe D G, Olshevskaya E V, Laura R P, Hurley J B. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 6.Shyjan A W, de-Sauvage F J, Gillett N A, Goeddel D V, Lowe D G. Neuron. 1992;9:727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- 7.Lowe D G, Dizhoor A M, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley J B. Proc Natl Acad Sci USA. 1995;92:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R B, Foster D C, Garbers D L, Fulle H J. Proc Natl Acad Sci USA. 1995;92:602–606. doi: 10.1073/pnas.92.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goraczniak R M, Duda T, Sitaramayya A, Sharma R K. Biochem J. 1994;302:455–461. doi: 10.1042/bj3020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch K-W. J Biol Chem. 1991;266:8634–8637. [PubMed] [Google Scholar]
- 11.Hayashi F, Yamazaki A. Proc Natl Acad Sci USA. 1991;88:4746–4750. doi: 10.1073/pnas.88.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Seno K, Nishizawa Y, Hayashi F, Yamazaki A, Matsumoto H, Wakabayashi T, Usukura J. Exp Eye Res. 1994;59:761–768. doi: 10.1006/exer.1994.1162. [DOI] [PubMed] [Google Scholar]
- 13.Aparicio J G, Applebury M L. Protein Expression Purif. 1995;6:501–511. doi: 10.1006/prep.1995.1067. [DOI] [PubMed] [Google Scholar]
- 14.Hallett M A, Delaat J L, Arikawa K, Schlamp C L, Kong F, Williams D S. J Cell Sci. 1996;109:1803–1812. doi: 10.1242/jcs.109.7.1803. [DOI] [PubMed] [Google Scholar]
- 15.Palczewski K, Subbaraya I, Gorczyca W A, Helekar B S, Ruiz C C, Ohguro H, Huang J, Zhao X, Crabb J W, Johnson R S, Walsh K A, Gray-Keller M P, Detwiler P B, Baehr W. Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 16.Dizhoor A M, Olshevskaya E V, Henzel W J, Wong S C, Stults J T, Ankoudinova I, Hurley J B. J Biol Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- 17.Gorczyca W A, Polans A S, Surgucheva I G, Subbaraya I, Baehr W, Palczewski K. J Biol Chem. 1995;270:22029–22036. doi: 10.1074/jbc.270.37.22029. [DOI] [PubMed] [Google Scholar]
- 18.Frins S, Bönigk W, Müller F, Kellner R, Koch K-W. J Biol Chem. 1996;271:8022–8027. doi: 10.1074/jbc.271.14.8022. [DOI] [PubMed] [Google Scholar]
- 19.Subbaraya I, Ruiz C C, Helekar B S, Zhao X, Gorczyca W A, Pettenati M J, Rao P N, Palczewski K, Baehr W. J Biol Chem. 1994;269:31080–31089. [PubMed] [Google Scholar]
- 20.Duda T, Goraczniak R, Surgucheva I, Rudnicka-Nawrot M, Gorczyca W, Palczewski K, Sitaramayya A, Baehr W, Sharma R K. Biochemistry. 1996;35:8478–8482. doi: 10.1021/bi960752z. [DOI] [PubMed] [Google Scholar]
- 21.Semple-Rowland S L, Gorczyca W A, Buczyłko J, Helekar B S, Ruiz C C, Subbaraya I, Palczewski K, Baehr W. FEBS Lett. 1996;385:47–52. doi: 10.1016/0014-5793(96)00345-6. [DOI] [PubMed] [Google Scholar]
- 22.Semple-Rowland S L, Buczyłko J, Palczewski K, Baehr W. Invest Ophthalmol Visual Sci. 1996;37:S810. [Google Scholar]
- 23.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Harland R M. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 25.Surguchov A, Bronson J D, Banerjee P, Knowles J A, Ruiz C C, Subbaraya I, Palczewski K, Baehr W. Genomics. 1997;39:312–322. doi: 10.1006/geno.1996.4513. [DOI] [PubMed] [Google Scholar]