Abstract
Microinjection of plasmids encoding human tau (htau) protein into identified lamprey reticulospinal neurons (anterior bulbar cells, or ABCs) in situ induces chronic htau expression. htau protein is transported to both the axon and dendrites of expressing ABCs by mechanisms that require the C-terminal domain of htau protein but do not require directed htau mRNA transport. htau becomes phosphorylated at the PHF-1 (Ser-396/404) and TAU-1/AT8 (Ser-199/202) epitopes throughout ABCs with heavy htau accumulations; many such cells also exhibit degenerative changes, which include the development of extracellular htau deposits. Finally, expression of htau protein fused to green fluorescent protein induced the somatodendritic accumulation of filaments containing htau when examined by immunoelectron microscopy. These results suggest that chronic expression of htau in lamprey ABCs may be useful for studying cellular mechanisms underlying tau hyperphosphorylation and filament formation in vertebrate central neurons in situ.
The microtubule-associated protein tau is thought to be important for the development and maintenance of axonal identity and function in vertebrate neurons (1–3). In addition, human tau (htau) appears to play a role in the cytopathology of Alzheimer disease (AD), where it becomes hyperphosphorylated and accumulates abnormally in the somata and dendrites of affected cells (4–6) and forms pathological filamentous inclusions (paired helical filaments, or PHFs), which aggregate to form neurofibrillary tangles (NFTs). These events suggest that neurons with NFTs in AD may have lost their ability to degrade htau and control its phosphorylation state. Because the occurrence and distribution of NFTs and PHF–tau-positive neurites in AD brain are closely correlated with the development of dementia (6–9), the roles played by htau phosphorylation in the development of neurofibrillary pathology in mature neurons in situ are of particular interest.
In this study, we have used the unique accessibility of giant, identified neurons (anterior bulbar cells or ABCs) in the central nervous system of a lower vertebrate, the sea lamprey, to analyze the effects of expressing htau in mature vertebrate central neurons in situ. ABCs have been morphologically characterized and studied in detail on a single-cell level (10–13), and they can be microinjected in situ with drugs for chronic experiments (14, 15). We have microinjected ABCs with vectors expressing two full-length htau isoforms and deletion mutants expressing the N- or C-terminal halves of htau in intact lampreys (see Fig. 1). We show that exogenous htau is overexpressed in some of the injected cells. Heavy htau accumulation is accompanied by the somatodendritic accumulation of htau-immunopositive, 10- to 15-nm filaments and may be followed by the formation of condensed intracellular accumulations of phosphorylated htau, the development of extracellular htau deposits, and cellular degeneration. Such changes are not seen with the overexpression of tau deletion mutants. These results thus suggest that the overexpression of full-length htau isoforms in ABCs may provide an excellent in situ model of cellular mechanisms underlying the development of the cytoskeletal pathology seen in AD and related neurodegenerative conditions.
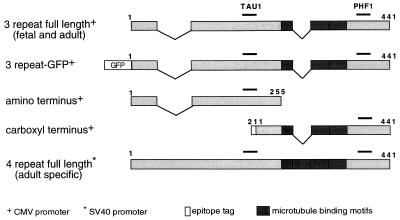
Figure 1.
Schematic of htau constructs expressed in ABCs. Three of the five plasmids used in this study—those containing the shortest full-length tau isoform that is present in both fetal and adult brain (pRC/CMVn123c, top), the N-terminal construct (pRC/CMVn591, third from top), and the C-terminal construct (pRC/CMVflag123c458, fourth from top)—were constructed in pRC/CMV, which uses the CMV promoter and enhancer sequences for expression. The fourth plasmid (pC2-GFPn123c, second from the top) encodes the GFP-tagged three-repeat tau construct and was constructed from the pGFP-C2 parent plasmid. The fifth plasmid (pEN1234c, bottom) is driven by the simian virus 40 promoter. The TAU-1 and PHF-1 epitopes are indicated by bars. All numbers shown above refer to the 441 residue isoform.
EXPERIMENTAL PROCEDURES
Microinjection.
Plasmid was spun down in EtOH (30 min, 13,000 rpm, 4°C), resuspended in microtubule stabilizing buffer (14), and injected at a final concentration of ≈1 mg/ml (with 0.5% Fast Green and 25 mg/ml Lucifer Yellow–dextran). Pressure injection was accomplished with a Picospritzer II unit (General Valve, Fairfield, NJ) as described (14). Lampreys were then permitted to recover at 4°C in lamprey saline (16) for 12–24 hr before being returned to well water at 15°C.
Immunocytochemistry.
Lamprey brains were fixed, sectioned, and immunostained as described (13, 14). Immunocytochemistry was performed on 10-μm transverse sections of paraffin-embedded lamprey heads that had been fixed by immersion in FAA (10% formalin, 10% glacial acetic acid, and 80% ethanol). This fixative does not permit the cross-reaction of PHF-1 with lamprey tau that is seen in axotomized ABCs fixed in Carnoy’s fixative (unpublished observations). TAU-1 has never been seen to cross react with lamprey brain under any fixation conditions. Alkaline phosphatase (AP) treatment consisted of the application of 100 units of calf intestinal AP (Sigma) to slides for 3 hr at 37°C before staining.
Plasmid Constructs.
Tau cDNA inserts were synthesized using PCR with Vent polymerase (New England Biolabs) and subcloned into the parent vectors [pRC/CMV (Invitrogen), pGFP-C2 (CLONTECH), or pECE (17)] using standard methods. The pRC/CMV123c construct expresses the 352 residue isoform of fetal and adult htau (18), which is missing two N-terminal exons (58 residues) and a microtubule (MT) binding repeat (31 residues; see ref. 1) present in the longest htau isoform (see Fig. 1). The pECE vector was used to express the longest htau isoform under the control of the early simian virus 40 promoter. htau N-terminal fragment was expressed by pRC/CMVn591, which encodes the N-terminal residues 1–255 of the longest htau isoform minus the two N-terminal exons. The protein expressed by pRC/CMVflag123c458 contains the C-terminal half of htau (residues 211–441 of the longest isoform, minus one MT-binding repeat), plus an epitope tag fused at the N terminus (19). Finally, pGFP-C2 was used to express a three-repeat htau construct (pC2-GFPn123c), which has the green fluorescent protein (GFP) coding sequence fused to the htau N terminus. Plasmid DNA was prepared using the Qiagen protocol (Qiagen, Chatsworth, CA).
In Situ Hybridization.
Fixation, tissue processing, and sectioning was done as described above for immunocytochemistry. Slides were pretreated for nonisotopic in situ hybridization as described by Swain et al. (20). Digoxigenin-labeled RNA probes were transcribed from CsCl-purified cDNA templates that had been digested with ClaI using SP6 polymerase in the presence of digoxigenin-labeled NTP. Sense probes were produced by digesting the plasmid with ApaI and then transcribed with T7 polymerase.
Electron Microscopy.
ABCs expressing GFP-tagged htau were targeted in living lamprey brains that had recently been isolated (10) and were maintained in cold lamprey Ringer’s solution (16) after being transected immediately rostral to the ABCs. These brains were examined on a Nikon Diaphot inverted microscope under conventional fluorescein epifluorescence. Fluorescent ABCs were photographed and then fixed in 4% paraformaldehyde/0.1% glutaraldehyde in cacodylate buffer (0.1 M, pH 7.3) for 2 hr before embedding in LR White (Electron Microscopy Sciences, Fort Washington, PA) per the manufacturer’s instructions. Transverse sections through ABCs (thick, 1 μm; and thin, 80 nm) transverse sections through ABCs were then taken and immunostained with an anti-GFP antiserum (1:50; CLONTECH) and an anti-rabbit secondary antibody decorated with 10 nm colloidal gold particles (Sigma). In some cases, GFP-expressing ABCs were fixed in 3.5% glutaraldehyde and processed for conventional electron microscopy with OsO4 and lead citrate.
Data Analysis.
Sections through ABCs expressing htau that had been stained with TAU-1 following AP pretreatment were scored blindly by two investigators as “lightly” or “heavily” stained according to the following criteria: lightly expressing cells had distinct labeling throughout cytoplasm, translucent appearance, and a clearly visible nucleus; and heavily expressing cells had opaque staining through most or all of soma, partly or completely obscuring the nucleus. Axonal staining was scored (as positive or negative) in sections taken 1–2 mm from the soma of the parent cell. Similarly, axonal staining of anti htau probe was scored in sections taken within 200 μm of the cell in question. The χ2 test was used to determine the significance of differences between samples from different treatments or timepoints.
RESULTS
htau Expression in ABCs.
A total of 1680 ABCs in 336 ammocoete sea lampreys (Petromyzon marinus) 11–15 cm long were microinjected in situ with plasmids expressing various htau constructs under the control of either the cytomegalovirus (CMV) or early simian virus 40 promoter (Fig. 1). Of these, 924 ABCs survived injection and were fixed and examined between 2 and 76 days postinjection (p.i.), with 119 cells (12.9%) expressing enough htau to permit further study. In situ hybridization and immunocytochemistry were performed on adjacent transverse sections through expressing ABCs. The monoclonal antibodies PHF-1 and TAU-1 were used to analyze htau expression in these experiments, because (i) phosphorylation of these epitopes is important in regulating tau binding to MTs; (21, 22), and (ii) immunostaining with both antibodies under the fixation conditions used was entirely specific to htau. PHF-1 recognizes htau phosphorylated at Ser-396/404, whereas TAU-1 recognizes htau dephosphorylated at Ser-199/202. TAU-1 staining of sections treated with AP was used to reveal total htau independent of phosphorylation state. In some experiments, the distribution of total htau was revealed by the use of a GFP epitope tag (Fig. 1). There was a significant increase in the percentage of injected cells expressing three-repeat full-length htau over the first month p.i.; this was accompanied by a significant reduction in the survival of ABCs over time (Table 1). These changes did not occur with the overexpression of N-terminal fragments lacking the MT-binding region.
Table 1.
Expression rate versus cell survival over time of ABCs expressing full-length versus N-terminal htau constructs
| Days p.i. | Three-repeat full-length htau
|
N-terminal htau fragment
|
||
|---|---|---|---|---|
| Expression | Cell survival | Expression | Cell survival | |
| 2–6 | 4/54 (7.4) | 54/82 (66) | 2/20 (10) | 20/35 (62.5) |
| 7–15 | 16/75 (21.3) | 75/150 (50) | 9/45 (20) | 45/80 (56.5) |
| 16–38 | 16/31 (51.6) | 31/65 (47) | 8/44 (18.2) | 44/75 (58.6) |
Expression and cell survival rates are compared between ABCs expressing full-length (three-MT binding repeat, no GFP tag) and N-terminal constructs of htau. Only sections stained with TAU-1 after AP treatment were scored for expression. ABCs expressing htau or surviving injection (numerators) are given as a fraction of the total number of surviving ABCs at the time of fixation or the total number of injected cells, respectively (denominators). Figures in parentheses give these data as percentages. The increase in the percentage of ABCs expressing full-length tau between each timepoint was significant (P < 0.005, χ2 test), whereas no significant change was seen with time in the proportion of ABCs expressing N-terminal fragments. The decrease in survival rate between the first and last timepoints was also significant for full-length but not N-terminal constructs (P < 0.05, χ2 test).
Distribution of htau mRNA and Domain-Specific Fragments of htau Protein.
In situ hybridization was used to demonstrate the presence and distribution of htau mRNA in expressing ABCs. Digoxigenin label was only seen in ABCs staining for htau protein. In all of 27 cells examined, label was perinuclear (Fig. 2 Left). Staining of the axonal initial segment was not observed in any of eight such cells examined. To determine whether the htau MT-binding domain mediates htau translocation within ABCs, two htau deletion mutants were tested. One encodes the N-terminal “projection” domain of htau, which lacks MT binding activity. The other deletion mutant encodes a C-terminal fragment capable of promoting MT assembly (ref. 23; see Fig. 1). Overexpression of the N-terminal tau fragment resulted in relatively strong somatic staining tapering off with distance from the soma (Fig. 2 Center). This pattern closely resembles that of htau mRNA. In contrast, the expression of the C-terminal htau fragment resulted in the preferential accumulation of immunolabel in the distal dendrites of all six ABCs examined (Fig. 2 Right), a pattern similar to that of full-length htau protein (described below).
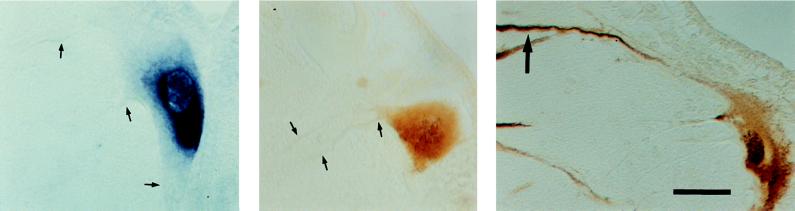
Figure 2.
Comparison of htau mRNA and protein distribution in ABCs. Transverse sections through ABCs showing the distribution of htau mRNA (Left), N-terminal htau fragment (Center), and C-terminal htau fragment (Right). Note that the htau mRNA and the N-terminal protein fragment are distributed perisomatically with htau-negative dendrites (small arrows), whereas immunostaining for the C-terminal htau fragment (which contains the MT-binding region) is strong in the distal dendrites (Right, large arrow) as well, suggesting that the C terminus is specifically involved in htau transport. The ventricular surface of the brain can be seen at the top right of each section. (Bar = 50 μm.)
Full-Length htau Protein Is Found Throughout the Cell and Is Most Heavily Phosphorylated in ABC Dendrites.
Immunostaining for full-length htau proteins extended to the dendritic tips and up to 1 cm or more along the axon in all htau-expressing cells examined. Total htau was detectable with the GFP epitope tag by fluorescence in freshly dissected living brain (Fig. 3A) or by pretreating sections with AP and then staining with TAU-1. PHF-1 and TAU-1 were used to examine the spatial distribution of htau phosphorylation. In lightly expressing ABCs (see Experimental Procedures), PHF-1 staining was restricted to the dendrites and, to a lesser extent, the soma (Fig. 3). TAU-1 staining, by contrast, was largely restricted to the soma, rapidly decreasing with distance distally in the dendrites (Fig. 3B). Tau-1 stain was variable in the axon (data not shown). htau thus was least phosphorylated in the soma and axon (where either PHF-1 or TAU-1 sites were often unphosphorylated) and most phosphorylated in the distal dendrites, where it was always heavily phosphorylated at both sites. This pattern was evident in all ABCs (of a total of 34 examined) expressing full-length htau constructs up to and including 8 days p.i. and in lightly staining ABCs examined at all times. However, in heavily expressing ABCs that had been injected with full-length htau constructs (without the GFP tag) at 9 days or more p.i., both PHF-1 and TAU-1 staining could be seen throughout the axons, somata, and dendrites of almost all (15/16) cells examined (Fig. 3C). The intensity of staining for TAU-1 was less than that of PHF-1 and was increased by AP pretreatment, suggesting that the TAU-1 site was not always phosphorylated. The change from the pattern seen earlier and in lightly staining cells (i.e., axons PHF-1-negative) was highly significant (P ≪ 0.005, χ2 test). Dense htau accumulations were phosphorylated at both PHF-1 and TAU-1 sites (Fig. 3D). Apparent degenerative changes present in most (10/16) heavily staining cells included nuclear staining and extracellular tau deposits near the somata and dendrites of expressing ABCs (Fig. 3D, asterisk), suggesting the loss of nuclear and plasma membrane integrity. This was in contrast to ABCs expressing N- and C-terminal htau fragments, where expression was never associated with the formation of dense htau deposits or degenerative changes. However, none of the 31 ABCs expressing GFP-htau (examined between 8 and 66 days p.i.) showed signs of degeneration, suggesting that filament formation per se is not necessarily correlated with cytodegenerative changes (see below).
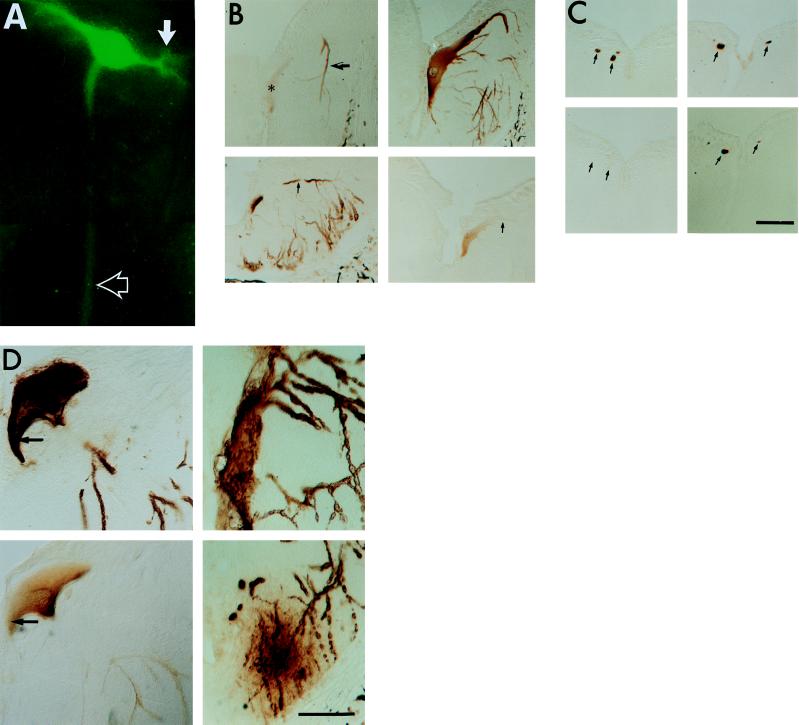
Figure 3.
Distribution and phosphorylation of full-length htau in lightly versus heavily expressing ABCs. (A) GFP fluorescence of a single ABC in the living lamprey hindbrain. GFP–htau is distributed throughout the dendrites (solid arrow), soma, and axon (open arrow). (B) Adjacent transverse sections through the somata and dendrites of lightly (Upper, 17 days p.i.) and heavily (Lower, 8 days p.i.) expressing ABCs in the hindbrain, showing phosphorylation of dendritic htau at the PHF-1 and TAU-1 sites. Both cells expressed three-repeat full-length htau without GFP. Sections were stained with PHF-1 (Left) and TAU-1 (Right). (Upper Right) Section was pretreated with AP to show total htau distribution. Note that normal morphology and an even somatodendritic distribution of total htau is combined with the selective accumulation of PHF-1-positive htau in the distal dendrites of a lightly expressing ABC (Upper, arrow) relative to the soma (Upper, asterisk). A heavily expressing ABC (Lower Left) shows PHF-1 staining throughout the soma and dendrites (arrow). Staining for TAU-1 is absent from dendrites (Lower Right, arrow). (C) Transverse sections through ABC axons ≈1 mm from their parent somata in the caudal hindbrain. (Left) Adjacent sections through the axons of three ABCs 8 days p.i. expressing three-repeat full-length htau without GFP; (Right) Adjacent sections from another lamprey examined 11 days p.i. expressing the same construct in which all of the axons (arrows) from ABCs were judged to be staining heavily. Note the absence of PHF-1 staining in the 8-day axons (Lower Left, arrows) and its strong presence in 11-day axons (Lower Right). (D) In heavily staining ABCs examined 9 days or later p.i., staining with both PHF-1 (Upper Left) and TAU-1 (Lower Left) was observed throughout the cells. Most htau accumulations were distributed in a clumped, uneven pattern in the soma and were hyperphosphorylated (i.e., PHF-1-positive and TAU-1-negative; Left, arrows). Some of these ABCs were undergoing degeneration (Upper Right); these often exhibited distal dendrites surrounded by extracellular htau accumulations in the adjacent neurophil (Lower Right, ∗). (Bars = 50 μm.)
htau Expression Induces Somatodendritic Accumulation of 10- to 15-nm Filaments Containing tau.
Eight ABCs expressing GFP-tagged htau constructs were targeted for examination in the electron microscope between 18 and 33 days p.i. Each of these exhibited a marked increase in the occurrence of 10- to 15-nm filaments throughout their somata and dendrites over that seen in the somata and dendrites of normal ABCs (12). These changes were not seen in nonexpressing cells in the same sections (Fig. 4A). Many of these filaments stained specifically with an anti-GFP polyclonal antibody in each of the three cells examined with this technique, indicating that htau has been incorporated into filaments (Fig. 4B). Because of the superficial resemblance of these filaments to neurofilaments (NFs), we immunostained sections through GFP–htau-expressing ABCs with antibodies that specifically recognize lamprey NF protein (20). When the somata and dendrites of 21 ABCs expressing GFP tagged htau were immunostained with NF specific mAbs in paraffin sections, 6 of 11 heavily expressing ABCs also showed a slight increase in somatic NF staining in adjacent sections at 18–22 days p.i. Immunostaining for phosphorylated as well as phosphorylation independent epitopes was seen in half of these cells (Fig. 4 D–F). However, NF staining was not observed in the dendrites of any ABC expressing htau (Fig. 4 E and F).
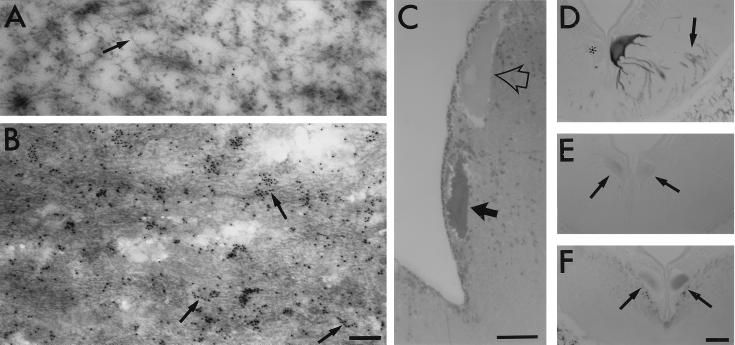
Figure 4.
Incorporation of htau into 10- to 15-nm filaments in ABCs. (A–C) ABCs expressing GFP-tagged htau were identified by the localization of fluorescent cells in freshly isolated brains before fixation. htau-expressing ABCs targeted in this manner stained more strongly with toluidine blue (C, solid arrow) than did adjacent nonexpressing cells (open arrow). Ultrastructural analysis of adjacent thin (80 nm) sections through these cells showed that htau-expressing ABCs contained densely packed bundles of 10- to 15-nm filaments (B), which are absent from the somata and dendrites of nonexpressing cells in the same section (A). Many of the filaments in the htau-expressing cells became decorated with 10-nm colloidal gold particles (B, arrows) when thin sections embedded in LR White were probed with an antiserum directed against GFP (CLONTECH) followed by a gold-labeled secondary antibody (Sigma). The patchy labeling in B is probably due to poor penetration of immunogold and the preferential decoration of filaments on the section surface. Note that NFs in nonexpressing cells were not labeled at all (A, arrow). [Bars = 200 nm (A and B) and 50 μm (C).] (D–F) Adjacent sections through the somata and dendrites of a pair of ABCs, one of which expresses htau (darkly stained cell at right) and one that does not (∗), were stained with the monoclonal antibodies PHF-1 (D), RMO44 (which selectively recognizes non phosphorylated NFs; E), and RMO62 (which recognizes a phosphorylated epitope on lamprey NF protein; F). Note that no increase in staining for somatodendritic NFs is visible in expressing cells (E and F, arrows) whose dendrites stain strongly for PHF-1 (D, arrows). (Bar = 50 μm.)
DISCUSSION
Chronic Expression of htau After Plasmid Microinjection into Mature Neurons in Situ.
We show here that intracellular injection of eukaryotic expression plasmids can induce exogenous gene expression in identified neurons in a differentiated vertebrate central nervous system. Because the expressing neurons can be injected and maintained in situ, the lamprey ABC system offers the opportunity to study the effects of exogenous proteins in identified mature neurons in a normal environment. It thus provides technical advantages complementary to those offered by murine transgenics and cell culture systems for studying cytological mechanisms underlying both normal and pathological events in central neurons. A particular advantage of this approach is its power as a technique for studying complex neuronal phenomena in situ, as it permits the analysis of the effects of the expression (and coexpression) of different types of constructs (deletion and substitution mutants, antisense sequences, etc.) in the neuron of choice in combination with other experimental manipulations.
Our results suggest that the increase in protein levels and in the proportion of injected cells expressing exogenous protein over time is the result of protein accumulation. This is inferred from the findings that the levels of full-length htau were consistently higher than those of the N-terminal fragments and that only cells microinjected with the full-length construct showed an increasing percentage of expressing cells over time (Table 1). The higher levels of full-length htau can result either from relatively higher levels of expression of the full-length protein and/or a greater relative rate of degradation of htau fragments. The latter appears to be more likely, especially because both cDNAs were cloned into the same plasmid and the 5′ sequences of the N-terminal and full-length constructs are identical. Similar results have been described in transfected cell cultures, where it has been shown that the level of protein expression of full-length constructs is consistently higher than that of truncated constructs (24, 25). It is unclear if the failure of GFP-tagged htau to induce neurodegeneration is due to lower levels of expression than those of untagged htau. Because GFP was fused to the N terminus of htau, the 5′ end of the coding sequence is different from the untagged N-terminal fragment and full-length htau constructs, preventing direct comparisons of expression efficiency. However, taken together, our results suggest that full-length htau, but not htau fragments, may accumulate to toxic levels in ABCs that express it heavily.
Distribution and Phosphorylation of htau in Lamprey ABCs Compared with That of tau Protein in Mammalian Systems.
Full-length htau was found in all compartments of ABCs at all times p.i. This distribution is unlike the predominantly axonal localization of tau in most mammalian species in situ (2, 3, 26, 27). However, some somatodendritic tau has been reported in both rat and monkey adult central nervous system in situ (28), and it occurs with htau expression in mice in situ (29) and with the transfection (25) and microinjection (30) of htau in neuronal primary cultures. The somatodendritic tau described here and in all of the situations listed above is phosphorylated at the PHF-1 and/or TAU-1 sites. Somatodendritic accumulation of phosphorylated tau is accentuated by a variety of cellular stresses such as heat shock (5), glutamate/Ca2+ exposure (31), β-amyloid treatment (32), and traumatic or ischemic injury (33, 34), and it occurs in the neuropathology of AD (4, 5, 35, 36), suggesting that overexpression of htau in ABCs might serve as an in situ model of cellular mechanisms underlying htau phosphorylation and localization.
The similarity of the distribution pattern of N-terminal htau protein fragments to that of htau mRNA suggests that the somatofugal transport of htau is (i) not mediated at the mRNA level and (ii) controlled by elements on the C-terminal domain of htau protein. The selective distribution of tau mRNA to the initial segment of the axon has been proposed to contribute to the predominantly axonal distribution of tau in situ (37). The failure of htau mRNA to show a polarized distribution in ABCs is consistent with such a role for endogenous tau mRNA, especially as the axonal targeting is thought to be mediated by an untranslated sequence that was not present in the constructs used in this study (38). By contrast, the appearance of the htau C-terminal fragment in dendritic tips indicates that this part of htau is sufficient for somatofugal transport and suggests a possible mechanism for somatofugal htau distribution in ABCs; i.e., that the MT-binding domain of htau can bind to both axonal and dendritic MTs, which can then in turn mediate htau transport. Our finding is consistent with results obtained by Hirokawa et al. (30), who showed a similar initial ubiquitous distribution of microinjected porcine tau in cultured mouse spinal neurons. However, detailed kinetic experiments involving the transport, MT binding, and turnover of htau will be needed to clarify the contributions of these mechanisms to tau sorting in ABCs.
htau Filament Formation with htau-GFP Expression in ABCs.
Although htau protein can assemble into various types of filaments under in vitro (39, 40) conditions, this is the first time to our knowledge that htau incorporation into such filaments has been demonstrated experimentally in vivo. The 10- to 15-nm filaments containing htau that we observed in ABCs were clearly different from PHFs, although they resembled in some respects the “straight” tau filaments seen in AD brain (41) and some of the tau filaments generated in vitro (40). Many of the filaments described here in ABCs resembled NFs ultrastructurally, making it unclear whether all of them were composed solely of htau. However, the observations that (i) htau immunostaining was much more intense than NF staining, even in heavily expressing ABCs, and (ii) NF staining was excluded from dendrites that contained heavy htau staining (Fig. 4), whereas htau filaments were found throughout the dendrites, indicate that most htau-positive filaments in ABCs were not NFs.
The Cytopathological Consequences of Hyperphosphorylated htau Accumulation.
Phosphorylation of tau at multiple sites (hyperposphorylation) reduces its affinity for MTs (22, 42–44) and is correlated with the formation of NFTs and neuronal death in AD (6, 45). Although this loss in MT stabilization resulting from tau hyperphosphorylation may indirectly injure neurons in AD, the accumulations of phosphorylated tau may also have direct deleterious effects on neurons, because NFTs often occupy most of the space within the somata and dendrites of neurons affected by AD and may themselves induce oxidative stress (46, 47). Our results are consistent with such a direct role for htau accumulations in neurodegeneration, because they show a correlation between heavy deposition of hyperphosphorylated htau, severe cytopathological changes, and a reduction in the survival rate of plasmid-injected ABCs over time (Table 1). In addition, the fact that the expression of all three constructs used the same parent vector and the fact that the 5′ ends of the coding sequences of the full-length and N-terminal fragments were identical suggests that the observed cytodegeneration is not a nonspecific effect of virally driven protein expression but is rather a toxic effect of the htau accumulations themselves.
The possibility that tau accumulation might itself cause neurofibrillary pathology and degeneration has not been tested directly by overexpressing htau in situ until recently, when overexpression of htau was achieved in transgenic mice (29). However, this did not result in dense fibrillar tau deposits in the expressing cells, although it did result in the accumulation of somatodendritic, PHF-1 positive tau. The differences between these results and ours may be due to higher rates of htau degradation or, conversely, lower rates of exogenous tau expression in mouse central neurons than in lamprey ABCs. In particular, the injection of plasmids in high concentrations into ABCs might be expected to drive htau synthesis more strongly than that in a transgenic mouse. We conclude that the ability to induce tau accumulation, hyperphosphorylation, and filament formation by htau overexpression should make lamprey ABCs a useful model system for studying the cellular mechanisms governing these events in human neuropathological conditions.
Acknowledgments
We thank Dr. Peter Davies for providing PHF-1 and Dr. Virginia Lee for providing RMO62 and RMO44. We would also like to thank the staff at the Ludington and Hammond Bay Biological Stations in Michigan for providing lampreys and Drs. Virginia Lee and Tom Shea for their critical reading of the manuscript. This work was supported by National Institutes of Health Grants NS29281 and AG13919, Paralyzed Veterans of America Grant SC1322, and a pilot grant from the American Foundation for Aging Research to G.F.H.
ABBREVIATIONS
- htau
human tau
- AD
Alzheimer disease
- PHF
paired helical filament
- NFT
neurofibrillary tangle
- ABC
anterior bulbar cell
- AP
alkaline phosphatase
- GFP
green fluorescent protein
- CMV
cytomegalovirus
- p.i.
postinjection
- MT
microtubule
- NF
neurofilament
References
- 1.Goedert M. Trends Neurosci. 1993;16:460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- 2.Lee G. Curr Opin Cell Biol. 1993;5:88–94. doi: 10.1016/s0955-0674(05)80013-4. [DOI] [PubMed] [Google Scholar]
- 3.Craig A M, Banker G A. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 4.Kowall N W, Kosik K S. Ann Neurol. 1987;22:639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- 5.Papasozomenos S, Su Y. Proc Natl Acad Sci USA. 1991;88:4543–4547. doi: 10.1073/pnas.88.10.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.McKee A C, Kosik K S, Kowall N W. Ann Neurol. 1991;30:156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- 8.Dickson D W, Crystal H A, Mattiace L A, Masur D M, Blau A D, Davies P, Yen S-H, Aronson M. Neurobiol Aging. 1991;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 9.Arriagada P A, Growdon J H, Hedley-White E T, Hyman B T. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 10.Hall G F, Cohen M J. Science. 1983;222:518–521. doi: 10.1126/science.6623092. [DOI] [PubMed] [Google Scholar]
- 11.Hall G F, Cohen M J. J Neurosci. 1988;8:3584–3597. doi: 10.1523/JNEUROSCI.08-10-03584.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall G F, Poulos A, Cohen M J. J Neurosci. 1989;9:588–599. doi: 10.1523/JNEUROSCI.09-02-00588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall G F, Lee V M-Y, Kosik K S. Proc Natl Acad Sci USA. 1991;88:5016–5020. doi: 10.1073/pnas.88.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall G F, Kosik K S. Neuron. 1993;10:613–625. doi: 10.1016/0896-6273(93)90164-m. [DOI] [PubMed] [Google Scholar]
- 15.Hall G F. Ann NY Acad Sci. 1993;679:43–64. doi: 10.1111/j.1749-6632.1993.tb18288.x. [DOI] [PubMed] [Google Scholar]
- 16.Wickelgren W O. J Physiol (London) 1977;270:89–116. doi: 10.1113/jphysiol.1977.sp011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis L, Clauser E, Morgan D O, Edery M, Roth R A, Rutter W J. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Spillatini M G, Jakes R, Rutherford D, Crowther R A. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 19.Leger J, Brandt R, Lee G. J Cell Sci. 1994;107:3403–3412. doi: 10.1242/jcs.107.12.3403. [DOI] [PubMed] [Google Scholar]
- 20.Swain G P, Jacobs A J, Frei E, Selzer M E. Exp Neurol. 1994;126:256–269. doi: 10.1006/exnr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 21.Goedert M, Jakes R, Crowther R A, Six J, Lobke U, Vandermeeren M, Cras P, Trojanowski J Q, Lee V M-Y. Proc Natl Acad Sci USA. 1993;90:5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bramblett G T, Goedert M, Jakes R, Merrick S E, Trojanowski J Q, Lee V M-Y. Neuron. 1993;10:1–20. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- 23.Brandt R, Lee G. J Biol Chem. 1993;268:3414–3419. [PubMed] [Google Scholar]
- 24.Lee G, Rook S L. J Cell Sci. 1992;102:227–237. doi: 10.1242/jcs.102.2.227. [DOI] [PubMed] [Google Scholar]
- 25.Kanai Y, Hirokawa N. Neuron. 1995;14:421–432. doi: 10.1016/0896-6273(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 26.Binder L, Frankfurter A, Rehbun L. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brion J P, Guilleminot J, Couchie D, Flament D J, Nunez J. Neuroscience. 1988;25:139–146. doi: 10.1016/0306-4522(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 28.Papasozomenos S, Binder L I. Cell Motil Cytoskeleton. 1987;8:210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- 29.Götz J, Probst A, Spillatini M G, Schäfer T, Jakes R, Bürki K, Goedert M. EMBO J. 1995;14:1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirokawa N, Funakoshi T, Sato-Harada R, Kanai Y. J Cell Biol. 1996;132:667–679. doi: 10.1083/jcb.132.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattson M P. Neuron. 1990;4:105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- 32.Busciglio J, Lorenzo A, Yeh J, Yankner B A. Neuron. 1995;14:879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 33.Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner G G. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- 34.Geddes J W, Schwab C, Craddock S, Wilson J L, Pettigrew L C. J Cereb Blood Flow Metab. 1994;14:554–564. doi: 10.1038/jcbfm.1994.69. [DOI] [PubMed] [Google Scholar]
- 35.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski H M. Brain Res. 1989;77:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- 36.Braak E, Braak H, Mandelkow E-M. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- 37.Litman P, Barg J, Rindzoonski L, Ginsburg I. Neuron. 1993;10:627–638. doi: 10.1016/0896-6273(93)90165-n. [DOI] [PubMed] [Google Scholar]
- 38.Behar L, Marx R, Barg J, Ginzburg I. Int J Dev Neurosci. 1995;13:113–127. doi: 10.1016/0736-5748(95)00001-w. [DOI] [PubMed] [Google Scholar]
- 39.Wille H, Drewes G, Biernat J, Mandelkow E M, Mandelkow E. J Cell Biol. 1992;118:573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson D M, Binder L I. J Biol Chem. 1995;270:24306–24314. doi: 10.1074/jbc.270.41.24306. [DOI] [PubMed] [Google Scholar]
- 41.Perry G, Kawai M, Tabaton M, Onorato M, Mulvihill P, Richey P, Morandi A, Connolly J, Gambetti P. J Neurosci. 1991;11:1748–1755. doi: 10.1523/JNEUROSCI.11-06-01748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindwall G, Cole D R. J Biol Chem. 1984;259:12241–12248. [PubMed] [Google Scholar]
- 43.Correas I, Diaz J, Avila J. J Biol Chem. 1992;267:15721–15728. [PubMed] [Google Scholar]
- 44.Biernat J, Gustke G, Dewes G, Mandelkow E-M, Mandelkow E. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- 45.Bramblett G T, Trojanowski J Q, Lee V M-Y. Lab Invest. 1992;66:212–221. [PubMed] [Google Scholar]
- 46.Smith M A, Sayre L M, Monnier V M, Perry G. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- 47.Trojanowski J Q, Lee V M-Y. Am J Pathol. 1994;144:449–453. [PMC free article] [PubMed] [Google Scholar]