Abstract
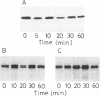
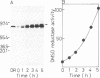
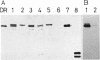
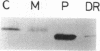
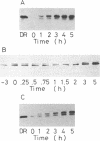
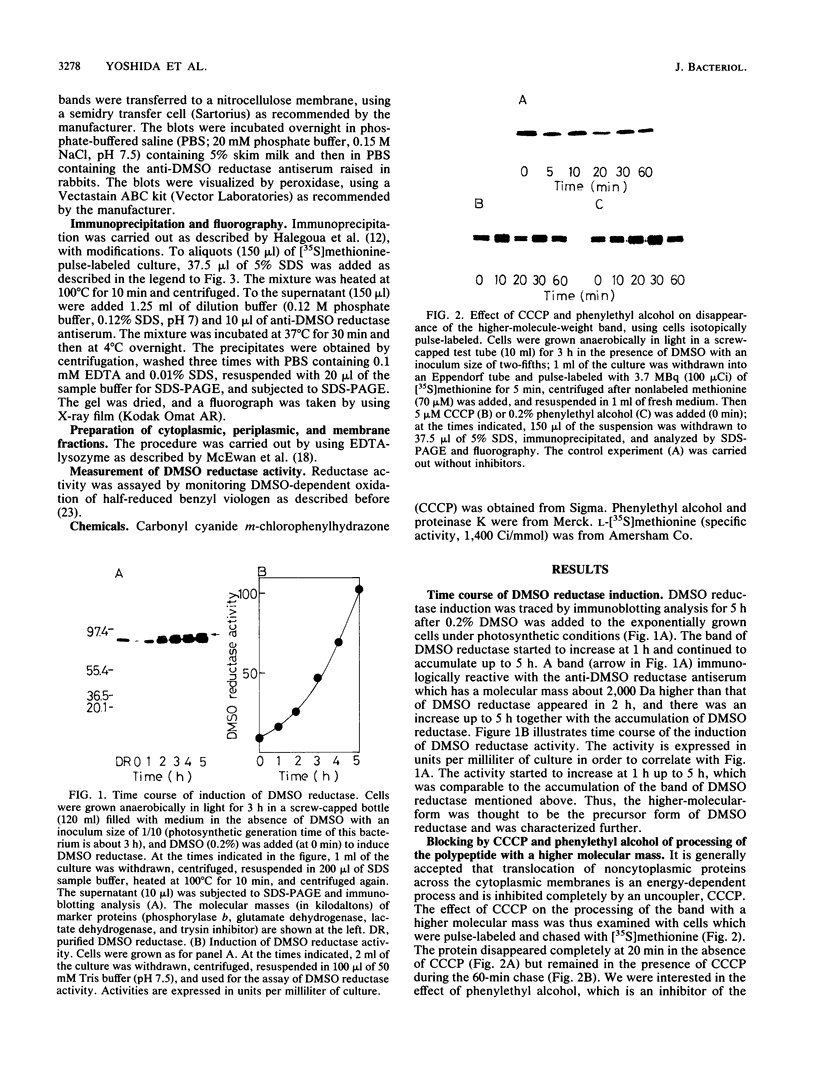
Translocation of dimethyl sulfoxide (DMSO) reductase to the periplasmic space was studied in vivo with a photodenitrifier, Rhodobacter sphaeroides f. sp. denitrificans, using immunoblotting analysis and radioactive labeling. A polypeptide with an apparent molecular mass about 2,000 Da higher than that of DMSO reductase accumulated during induction of the reductase with DMSO. An uncoupler, carbonyl cyanide-m-chlorophenylhydrazone, inhibited the processing of the polypeptide after cells had been radioactively pulse-labeled with [35S]methionine. These results indicated that the higher-molecular-mass polypeptide was the precursor form of DMSO reductase. The precursor form accumulated in either the cytoplasm or the membrane, whereas the mature form accumulated in the periplasmic space. The membrane-bound precursor was sensitive to proteinase K treatment from both the cytoplasmic and periplasmic sides of the membrane, indicating that the polypeptide binds to the membrane, exposing it to both the outer and inner surfaces of the cytoplasmic membrane. Processing of the precursor was hampered by removal of molybdate from the medium and was restored by its readdition. It was also inhibited by the addition of tungstate in the medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Crooke E., Brundage L., Rice M., Wickner W. ProOmpA spontaneously folds in a membrane assembly competent state which trigger factor stabilizes. EMBO J. 1988 Jun;7(6):1831–1835. doi: 10.1002/j.1460-2075.1988.tb03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J Biol Chem. 1985 Dec 15;260(29):15925–15931. [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Zwizinski C., Ludmerer S., Wickner W. Mechanisms of membrane assembly: effects of energy poisons on the conversion of soluble M13 coliphage procoat to membrane-bound coat protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):827–831. doi: 10.1073/pnas.77.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enequist H. G., Hirst T. R., Harayama S., Hardy S. J., Randall L. L. Energy is required for maturation of exported proteins in Escherichia coli. Eur J Biochem. 1981 May 15;116(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05323.x. [DOI] [PubMed] [Google Scholar]
- Fandl J. P., Tai P. C. Biochemical evidence for the secY24 defect in Escherichia coli protein translocation and its suppression by soluble cytoplasmic factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7448–7452. doi: 10.1073/pnas.84.21.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Hirashima A., Inouye M. Existence of a free form of a specific membrane lipoprotein in gram-negative bacteria. J Bacteriol. 1974 Dec;120(3):1204–1208. doi: 10.1128/jb.120.3.1204-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pages J. M., Lazdunski C. Maturation of exported proteins in Escherichia coli. Requirement for energy, site and kinetics of processing. Eur J Biochem. 1982 Jun;124(3):561–566. [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Ryan J. P., Bassford P. J., Jr Post-translational export of maltose-binding protein in Escherichia coli strains harboring malE signal sequence mutations and either prl+ or prl suppressor alleles. J Biol Chem. 1985 Nov 25;260(27):14832–14837. [PubMed] [Google Scholar]
- Satoh T., Hoshino Y., Kitamura H. Rhodopseudomonas sphaeroides forma sp. denitrificans, a denitrifying strain as a subspecies of Rhodopseudomonas sphaeroides. Arch Microbiol. 1976 Jul;108(3):265–269. doi: 10.1007/BF00454851. [DOI] [PubMed] [Google Scholar]
- Satoh T., Kurihara F. N. Purification and properties of dimethylsulfoxide reductase containing a molybdenum cofactor from a photodenitrifier, Rhodopseudomonas sphaeroides f.s. denitrificans. J Biochem. 1987 Jul;102(1):191–197. doi: 10.1093/oxfordjournals.jbchem.a122032. [DOI] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]