Abstract
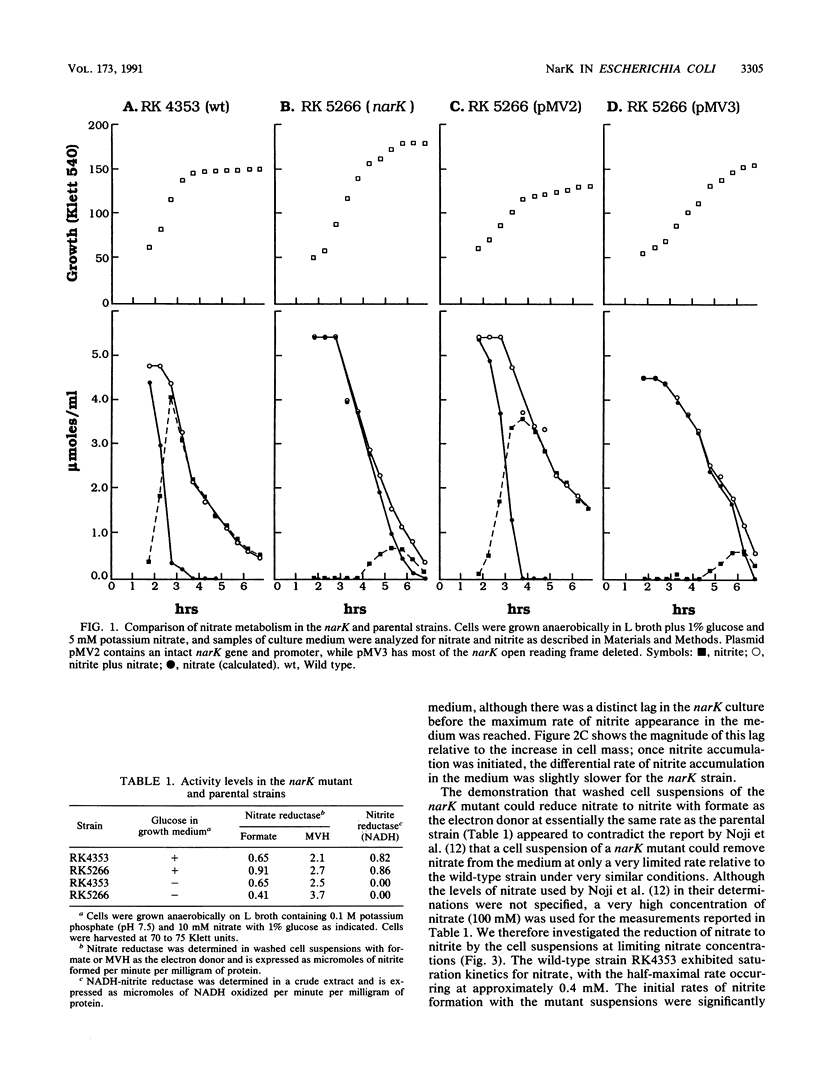
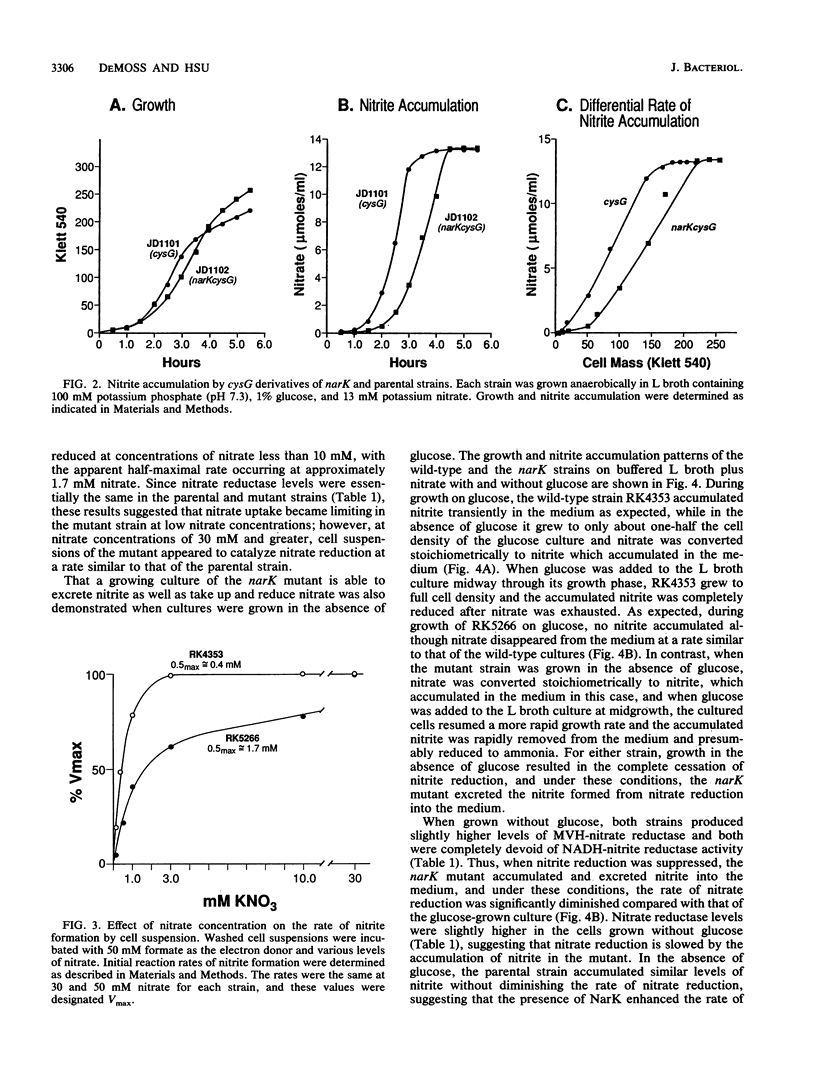
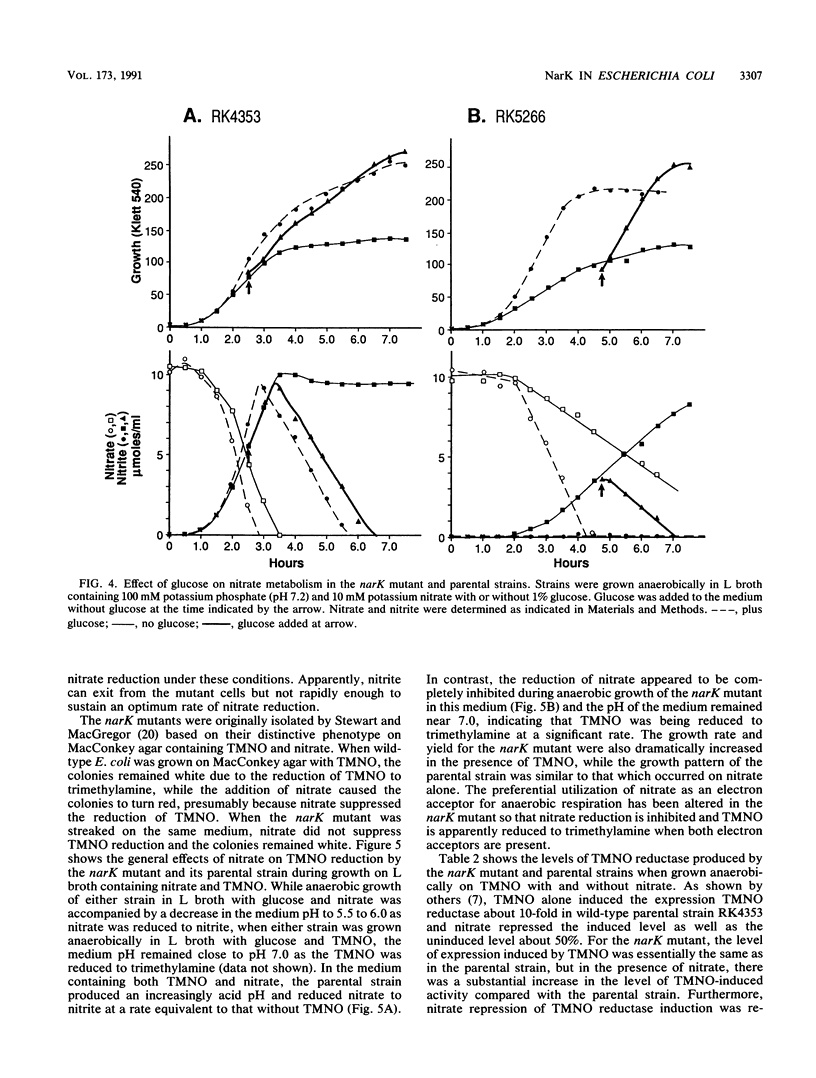
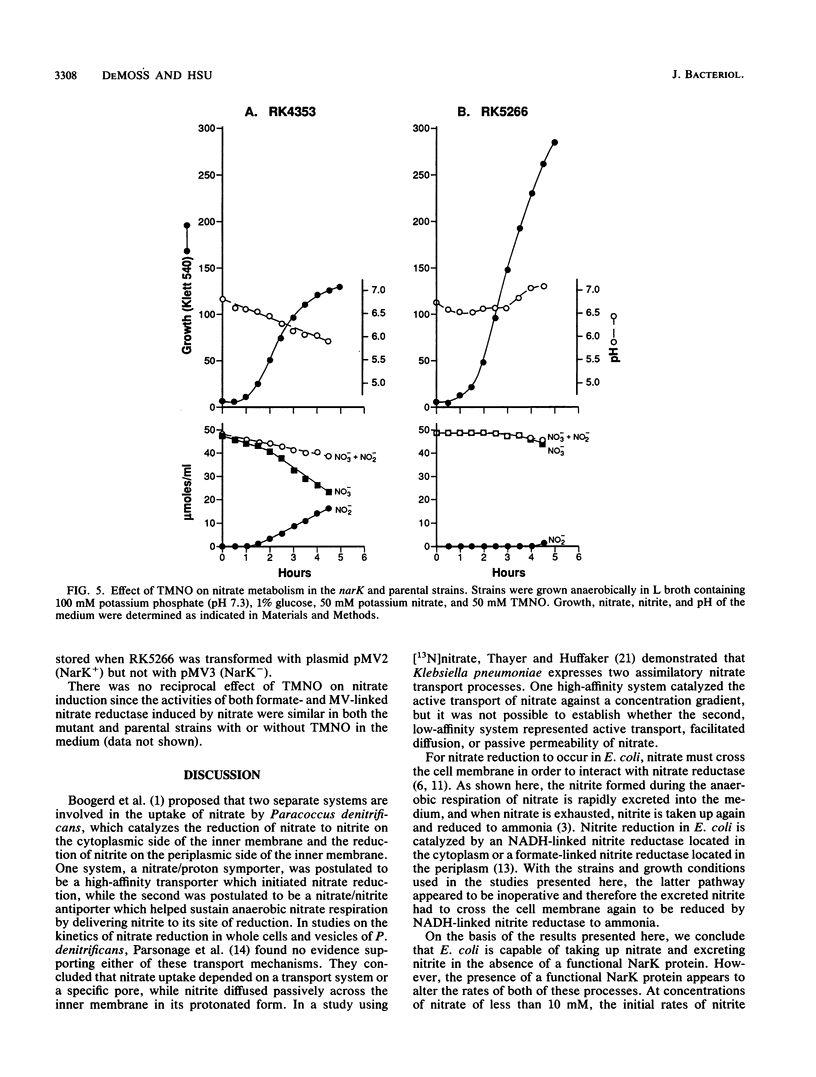
narK mutants of Escherichia coli produce wild-type levels of nitrate reductase but, unlike the wild-type strain, do not accumulate nitrite when grown anaerobically on a glucose-nitrate medium. Comparison of the rates of nitrate and nitrite metabolism in cultures growing anaerobically on glucose-nitrate medium revealed that a narK mutant reduced nitrate at a rate only slightly slower than that in the NarK+ parental strain. Although the specific activities of nitrate reductase and nitrite reductase were similar in the two strains, the parental strain accumulated nitrite in the medium in almost stoichiometric amounts before it was further reduced, while the narK mutant did not accumulate nitrite in the medium but apparently reduced it as rapidly as it was formed. Under conditions in which nitrite reductase was not produced, the narK mutant excreted the nitrite formed from nitrate into the medium; however, the rate of reduction of nitrate to nitrite was significantly slower than that of the parental strain or that which occurred when nitrite reductase was present. These results demonstrate that E. coli is capable of taking up nitrate and excreting nitrite in the absence of a functional NarK protein; however, in growing cells, a functional NarK promotes a more rapid rate of anaerobic nitrate reduction and the continuous excretion of the nitrite formed. Based on the kinetics of nitrate reduction and of nitrite reduction and excretion in growing cultures and in washed cell suspensions, it is proposed that the narK gene encodes a nitrate/nitrite antiporter which facilitates anaerobic nitrate respiration by coupling the excretion of nitrite to nitrate uptake. The failure of nitrate to suppress the reduction of trimethylamine N-oxide in narK mutants was not due to a change in the level of trimethylamine N-oxide reductase but apparently resulted from a relative decrease in the rate of anaerobic nitrate reduction caused by the loss of the antiporter system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Activation of nitrite reductase from Escherichia coli K12 by oxidized nicotinamide-adenine dinucleotide. Biochem J. 1978 Nov 1;175(2):495–499. doi: 10.1042/bj1750495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Arrangement of respiratory nitrate reductase in the cytoplasmic membrane of Escherichia coli. Location of beta subunit. FEBS Lett. 1980 Apr 21;113(1):15–20. doi: 10.1016/0014-5793(80)80484-4. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. The narL gene product activates the nitrate reductase operon and represses the fumarate reductase and trimethylamine N-oxide reductase operons in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3901–3905. doi: 10.1073/pnas.84.11.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacGregor C. H., Christopher A. R. Asymmetric distribution of nitrate reductase subunits in the cytoplasmic membrane of Escherichia coli: evidence derived from surface labeling studies with transglutaminase. Arch Biochem Biophys. 1978 Jan 15;185(1):204–213. doi: 10.1016/0003-9861(78)90160-1. [DOI] [PubMed] [Google Scholar]
- Macdonald H., Cole J. Molecular cloning and functional analysis of the cysG and nirB genes of Escherichia coli K12, two closely-linked genes required for NADH-dependent nitrite reductase activity. Mol Gen Genet. 1985;200(2):328–334. doi: 10.1007/BF00425444. [DOI] [PubMed] [Google Scholar]
- Noji S., Nohno T., Saito T., Taniguchi S. The narK gene product participates in nitrate transport induced in Escherichia coli nitrate-respiring cells. FEBS Lett. 1989 Jul 31;252(1-2):139–143. doi: 10.1016/0014-5793(89)80906-8. [DOI] [PubMed] [Google Scholar]
- Page L., Griffiths L., Cole J. A. Different physiological roles of two independent pathways for nitrite reduction to ammonia by enteric bacteria. Arch Microbiol. 1990;154(4):349–354. doi: 10.1007/BF00276530. [DOI] [PubMed] [Google Scholar]
- Rondeau S. S., Hsu P. Y., DeMoss J. A. Construction in vitro of a cloned nar operon from Escherichia coli. J Bacteriol. 1984 Jul;159(1):159–166. doi: 10.1128/jb.159.1.159-166.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Showe M. K., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: isolation and characterization of mutants unable to reduce nitrate. J Bacteriol. 1969 Mar;97(3):1291–1297. doi: 10.1128/jb.97.3.1291-1297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergren E. J., Hsu P. Y., DeMoss J. A. Roles of the narJ and narI gene products in the expression of nitrate reductase in Escherichia coli. J Biol Chem. 1988 Nov 5;263(31):16156–16162. [PubMed] [Google Scholar]
- Stewart V., Berg B. L. Influence of nar (nitrate reductase) genes on nitrate inhibition of formate-hydrogen lyase and fumarate reductase gene expression in Escherichia coli K-12. J Bacteriol. 1988 Oct;170(10):4437–4444. doi: 10.1128/jb.170.10.4437-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. R., Huffaker R. C. Kinetic evaluation, using 13N, reveals two assimilatory nitrate transport systems in Klebsiella pneumoniae. J Bacteriol. 1982 Jan;149(1):198–202. doi: 10.1128/jb.149.1.198-202.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



