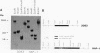Abstract
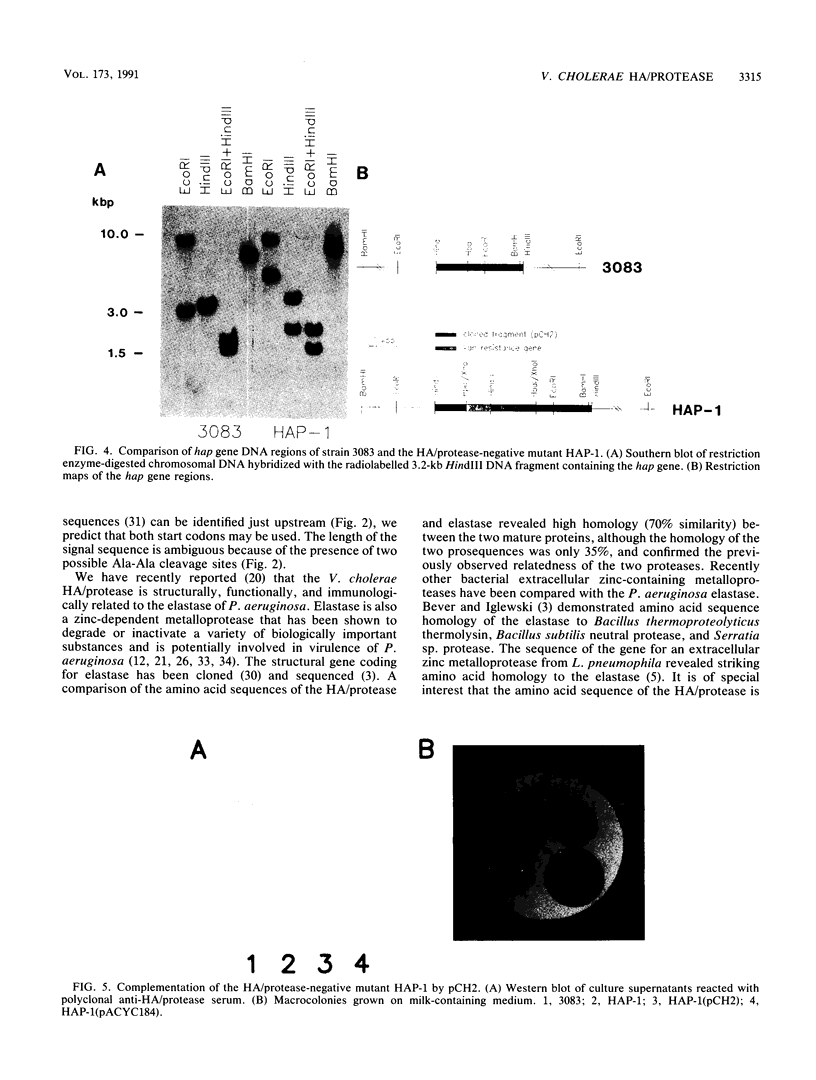
The structural gene hap for the extracellular hemagglutinin/protease (HA/protease) of Vibrio cholerae was cloned and sequenced. The cloned DNA fragment contained a 1,827-bp open reading frame potentially encoding a 609-amino-acid polypeptide. The deduced protein contains a putative signal sequence followed by a large propeptide. The extracellular HA/protease consists of 414 amino acids with a computed molecular weight of 46,700. In the absence of protease inhibitors, this is processed to the 32-kDa form which is usually isolated. The deduced amino acid sequence of the mature HA/protease showed 61.5% identity with the Pseudomonas aeruginosa elastase. The cloned hap gene was inactivated and introduced into the chromosome of V. cholerae by recombination to construct the HA/protease-negative strain HAP-1. The cloned fragment containing the hap gene was then shown to complement the mutant strain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Quinn F. D., Tompkins L. S. Legionella pneumophila zinc metalloprotease is structurally and functionally homologous to Pseudomonas aeruginosa elastase. J Bacteriol. 1990 May;172(5):2608–2613. doi: 10.1128/jb.172.5.2608-2613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. A., Boesman-Finkelstein M., Finkelstein R. A. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect Immun. 1984 Sep;45(3):558–560. doi: 10.1128/iai.45.3.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. A., Boesman-Finkelstein M., Finkelstein R. A. Vibrio cholerae soluble hemagglutinin/protease is a metalloenzyme. Infect Immun. 1983 Nov;42(2):639–644. doi: 10.1128/iai.42.2.639-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. A., Finkelstein R. A. Presence of hemagglutinin/protease and other potential virulence factors in O1 and non-O1 Vibrio cholerae. J Infect Dis. 1986 Jul;154(1):183–186. doi: 10.1093/infdis/154.1.183. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. A., Miyoshi S., Shinoda S. Purification and characterization of a protease produced by Vibrio mimicus. Infect Immun. 1990 Dec;58(12):4159–4162. doi: 10.1128/iai.58.12.4159-4162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahler G. S., Barras F., Keen N. T. Cloning of genes encoding extracellular metalloproteases from Erwinia chrysanthemi EC16. J Bacteriol. 1990 Oct;172(10):5803–5815. doi: 10.1128/jb.172.10.5803-5815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Leimeister-Wächter M., Goebel W., Chakraborty T. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect Immun. 1991 Jan;59(1):65–72. doi: 10.1128/iai.59.1.65-72.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Sobocinski P. Z., Atthasampunna P., Charunmethee P. Pathogenesis of experimental cholera: identification of choleragen (procholeragen A) by disc immunoelectrophoresis and its differentiation from cholera mucinase. J Immunol. 1966 Jul;97(1):25–33. [PubMed] [Google Scholar]
- Franzon V. L., Manning P. A. Molecular cloning and expression in Escherichia coli K-12 of the gene for a hemagglutinin from Vibrio cholerae. Infect Immun. 1986 Apr;52(1):279–284. doi: 10.1128/iai.52.1.279-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Booth B. A., Boesman-Finkelstein M., Finkelstein R. A. Comparative study of Vibrio cholerae non-O1 protease and soluble hemagglutinin with those of Vibrio cholerae O1. Infect Immun. 1987 Feb;55(2):451–454. doi: 10.1128/iai.55.2.451-454.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Comparison of the Vibrio cholerae hemagglutinin/protease and the Pseudomonas aeruginosa elastase. Infect Immun. 1990 Dec;58(12):4011–4015. doi: 10.1128/iai.58.12.4011-4015.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M. H., Kreger A. S. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J Gen Microbiol. 1987 Jul;133(7):1783–1791. doi: 10.1099/00221287-133-7-1783. [DOI] [PubMed] [Google Scholar]
- McKevitt A. I., Bajaksouzian S., Klinger J. D., Woods D. E. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect Immun. 1989 Mar;57(3):771–778. doi: 10.1128/iai.57.3.771-778.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahama K., Yoshimura K., Marumoto R., Kikuchi M., Lee I. S., Hase T., Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986 Jul 25;14(14):5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norqvist A., Norrman B., Wolf-Watz H. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect Immun. 1990 Nov;58(11):3731–3736. doi: 10.1128/iai.58.11.3731-3736.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad P. A., Bever R. A., Nicas T. I., Leduc F., Hanne L. F., Iglewski B. H. Cloning and characterization of elastase genes from Pseudomonas aeruginosa. J Bacteriol. 1987 Jun;169(6):2691–2696. doi: 10.1128/jb.169.6.2691-2696.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B., Pavlovskis O. R. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S998–1004. doi: 10.1093/clinids/5.supplement_5.s998. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young D. B., Broadbent D. A. Biochemical characterization of extracellular proteases from Vibrio cholerae. Infect Immun. 1982 Sep;37(3):875–883. doi: 10.1128/iai.37.3.875-883.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]