Abstract
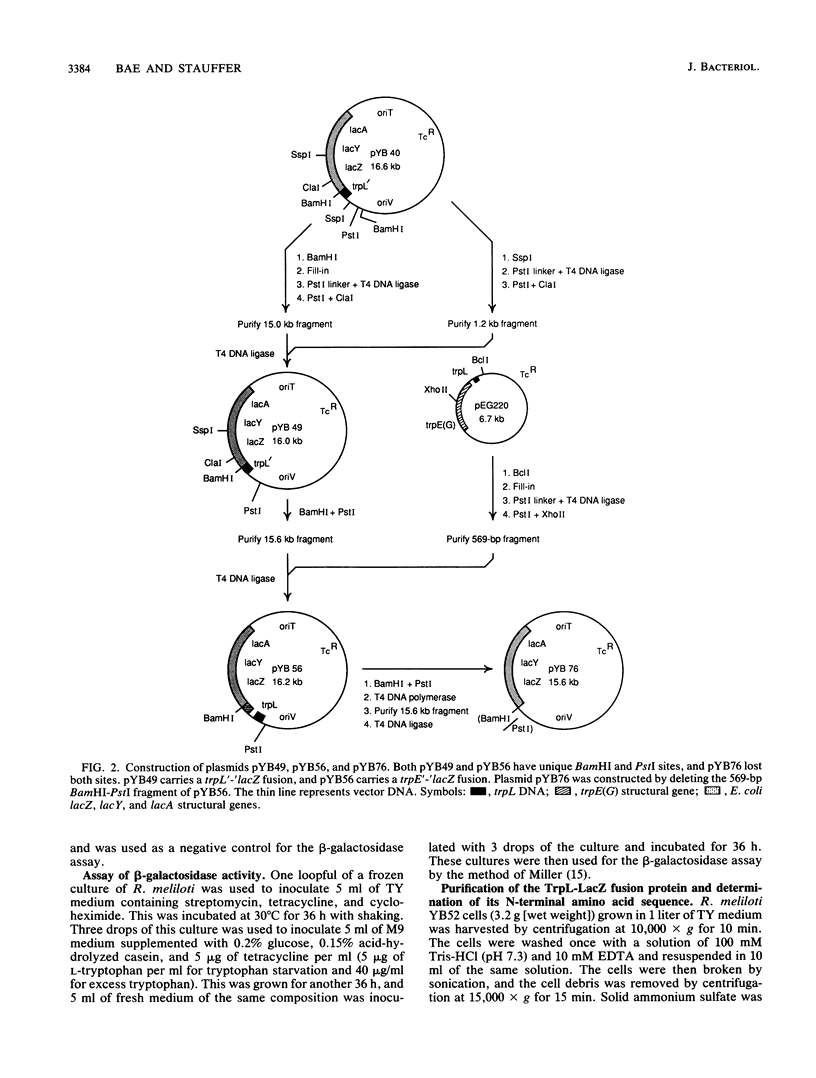
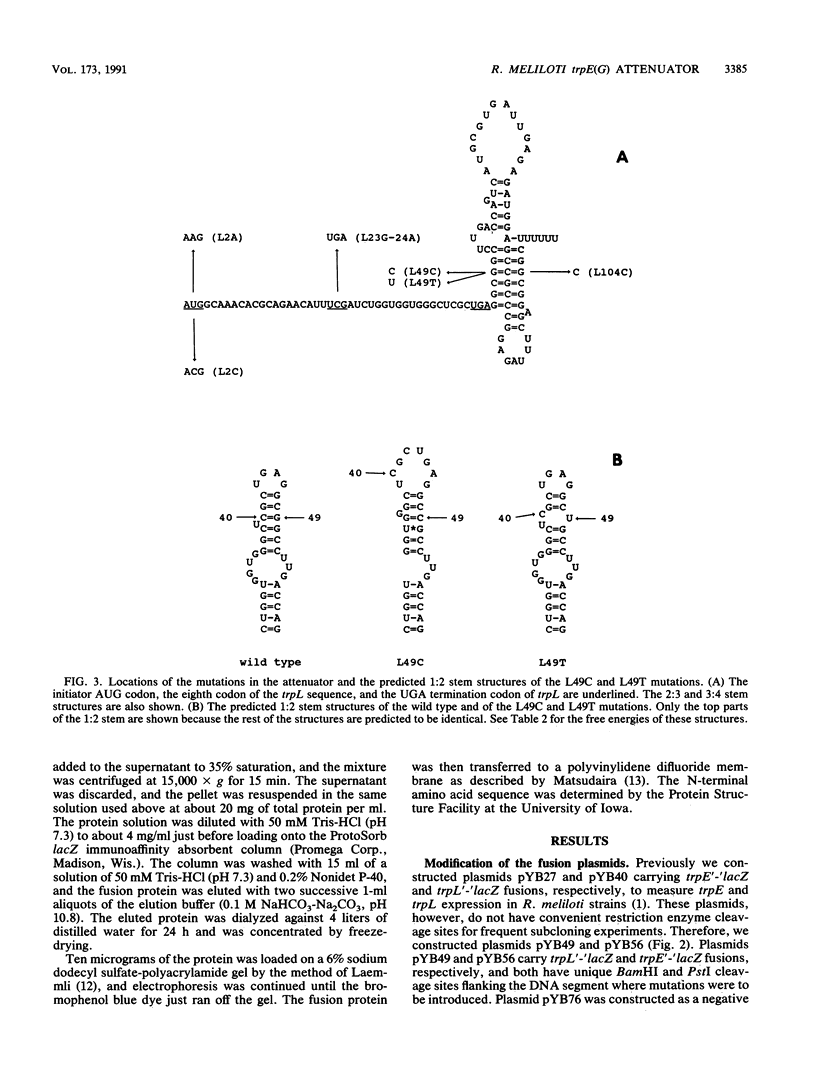
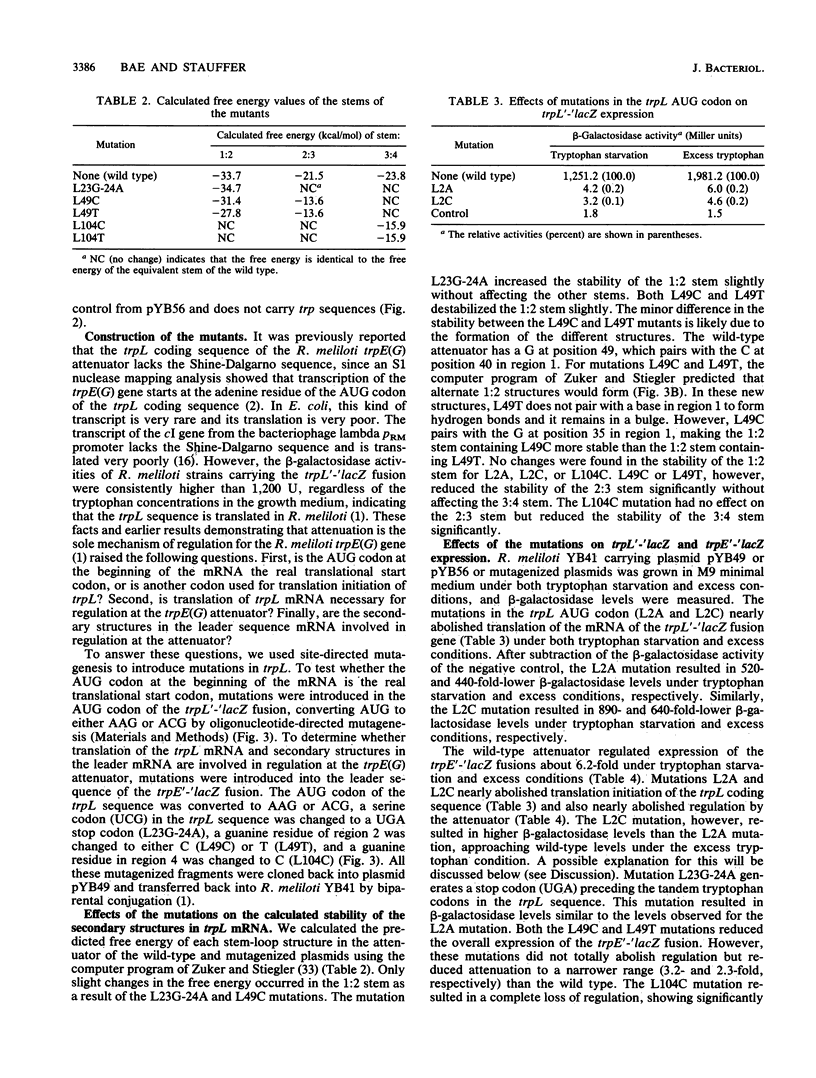
It was previously reported that transcription of the Rhizobium meliloti trpE(G) gene starts at the adenine residue of the AUG codon of the leader peptide coding sequence (trpL), suggesting that translation of the trpL sequence starts without the Shine-Dalgarno sequence. We constructed mutations replacing the AUG codon of the trpL sequence with AAG or ACG. These mutations reduced the expression of a trpL'-'lacZ fusion gene to 0.1 and 0.2% of the wild-type level, respectively, indicating that the AUG codon is the translation initiation codon for the trpL coding sequence. In addition, these mutations, as well as a mutation converting the eighth codon (UCG) of the trpL sequence to UGA, abolished regulation by attenuation when introduced upstream of the tandem tryptophan codons in a trpE'-'lacZ fusion. Mutations affecting the stability of the probable antiterminator and terminator secondary structures in trpL mRNA were also constructed. Studies using these mutations indicate that the attenuator of R. meliloti functions in a way analogous to that of the Escherichia coli trp attenuator.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae Y. M., Crawford I. P. The Rhizobium meliloti trpE(G) gene is regulated by attenuation, and its product, anthranilate synthase, is regulated by feedback inhibition. J Bacteriol. 1990 Jun;172(6):3318–3327. doi: 10.1128/jb.172.6.3318-3327.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y. M., Holmgren E., Crawford I. P. Rhizobium meliloti anthranilate synthase gene: cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3471–3478. doi: 10.1128/jb.171.6.3471-3478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- Holmgren E., Crawford I. P. Regulation of tryptophan genes in Rhizobium leguminosarum. J Bacteriol. 1982 Mar;149(3):1135–1137. doi: 10.1128/jb.149.3.1135-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. W., Bibb M. J., Beringer J. E. Tryptophan genes in Rhizobium--their organization and their transfer to other bacterial genera. Mol Gen Genet. 1978 Oct 24;165(3):323–330. doi: 10.1007/BF00332533. [DOI] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Genetic analysis of the tryptophan operon regulatory region using site-directed mutagenesis. J Mol Biol. 1984 May 25;175(3):299–312. doi: 10.1016/0022-2836(84)90350-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M. I., Shimotsu H., Henner D. J., Yanofsky C. Regulatory elements common to the Bacillus pumilus and Bacillus subtilis trp operons. J Bacteriol. 1986 Sep;167(3):792–798. doi: 10.1128/jb.167.3.792-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M. I., Yanofsky C. Evidence for the transcript secondary structures predicted to regulate transcription attenuation in the trp operon. J Biol Chem. 1984 Oct 25;259(20):12838–12843. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesser J. R., Nakamura Y., Yanofsky C. Regulation of basal level expression of the tryptophan operon of Escherichia coli. J Biol Chem. 1989 Jul 25;264(21):12284–12288. [PubMed] [Google Scholar]
- Roesser J. R., Yanofsky C. Ribosome release modulates basal level expression of the trp operon of Escherichia coli. J Biol Chem. 1988 Oct 5;263(28):14251–14255. [PubMed] [Google Scholar]
- Roesser J. R., Yanofsky C. The RNA antiterminator causes transcription pausing in the leader region of the tryptophan operon. J Biol Chem. 1990 Apr 15;265(11):6055–6060. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotsu H., Kuroda M. I., Yanofsky C., Henner D. J. Novel form of transcription attenuation regulates expression the Bacillus subtilis tryptophan operon. J Bacteriol. 1986 May;166(2):461–471. doi: 10.1128/jb.166.2.461-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Zurawski G., Yanofsky C. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4833–4837. doi: 10.1073/pnas.75.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Comparison of regulatory and structural regions of genes of tryptophan metabolism. Mol Biol Evol. 1984 Feb;1(2):143–161. doi: 10.1093/oxfordjournals.molbev.a040307. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Transcription attenuation. J Biol Chem. 1988 Jan 15;263(2):609–612. [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]


