Abstract
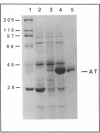
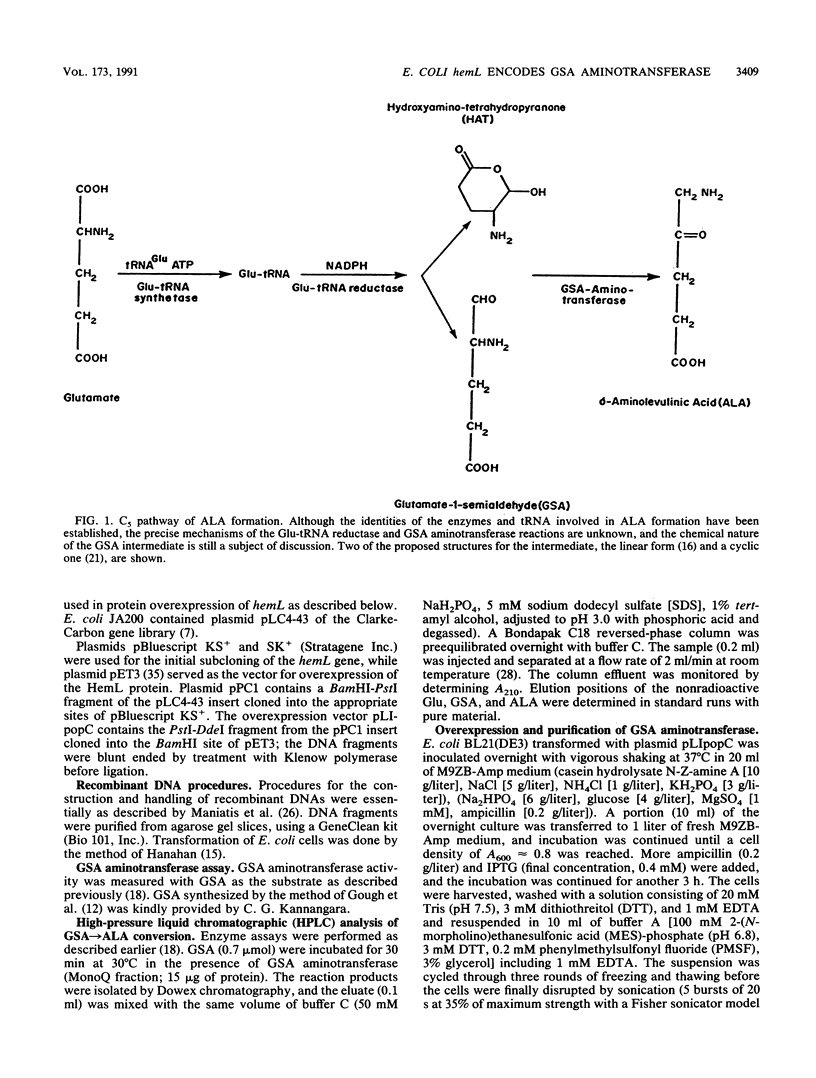
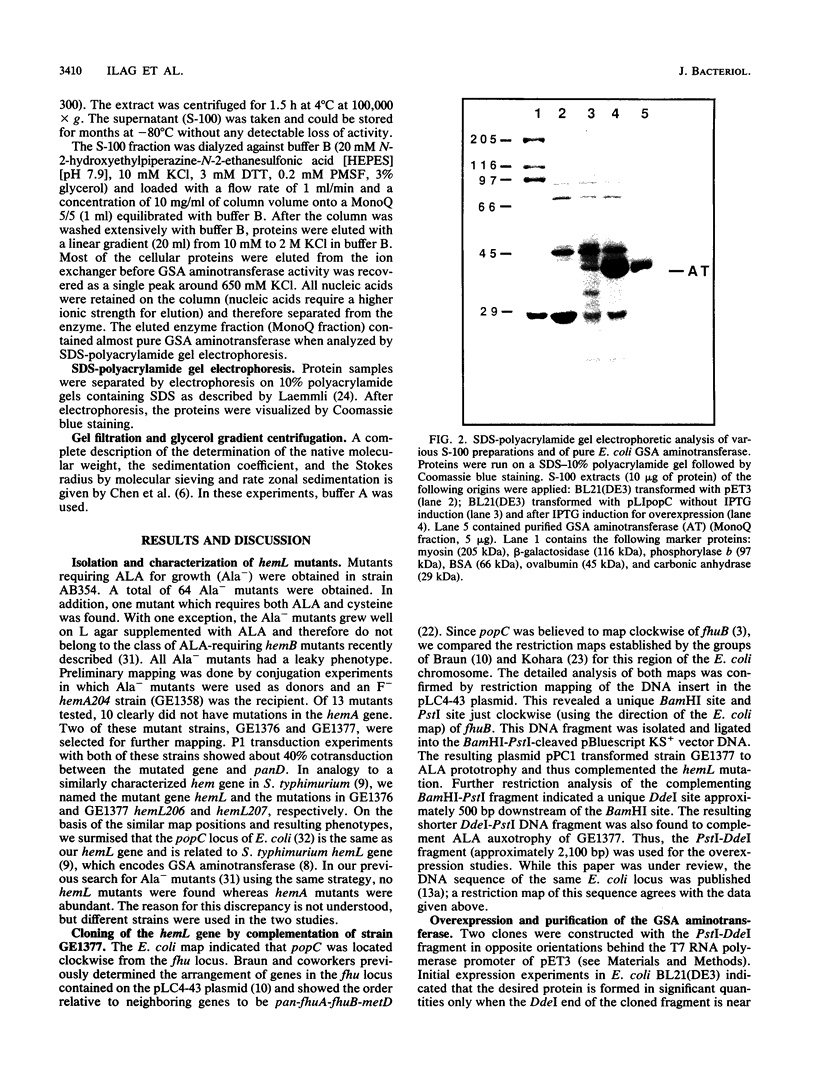
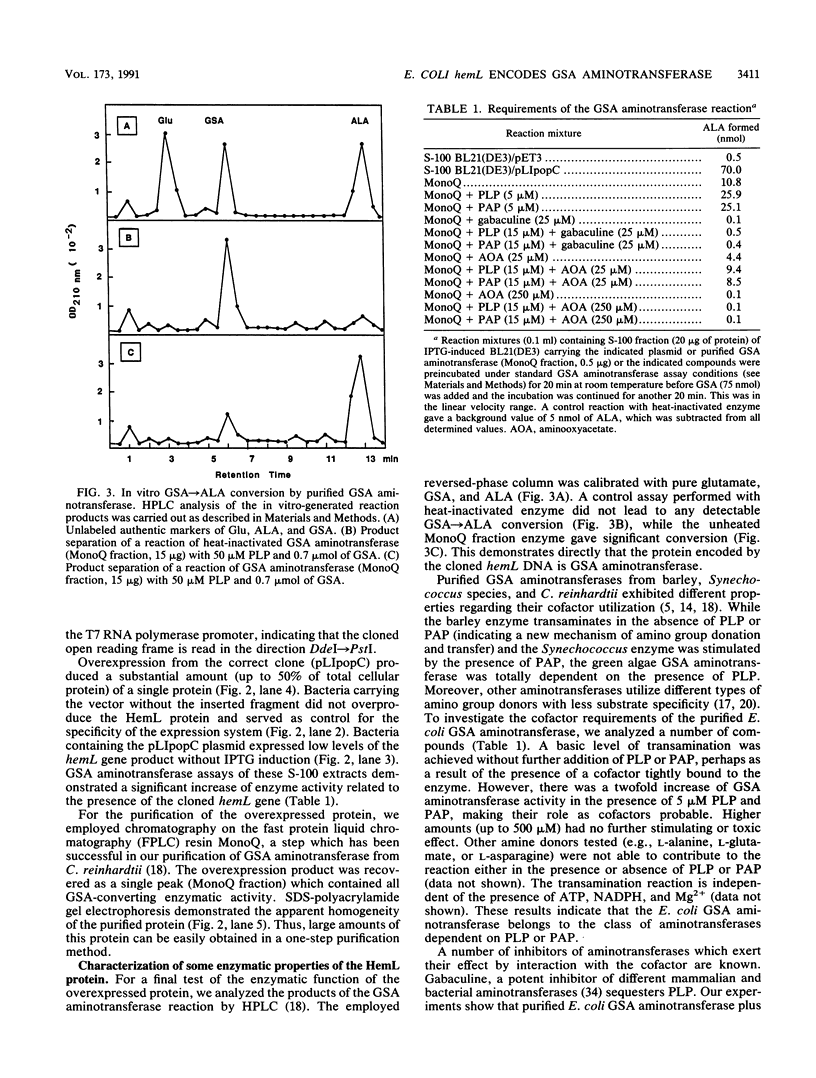
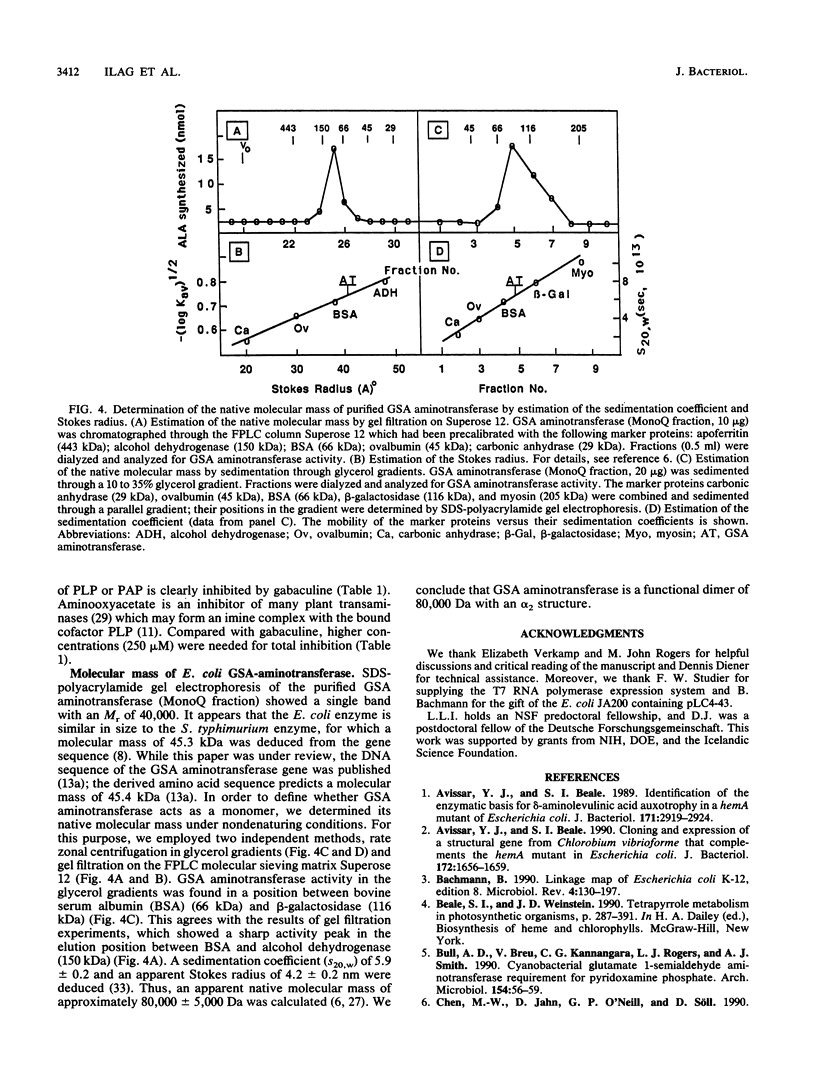
delta-Aminolevulinic acid (ALA), the first committed precursor of porphyrin biosynthesis, is formed in Escherichia coli by the C5 pathway in a three-step, tRNA-dependent transformation from glutamate. The first two enzymes of this pathway, glutamyl-tRNA synthetase and Glu-tRNA reductase, are known in E. coli (J. Lapointe and D. Söll, J. Biol. Chem. 247:4966-4974, 1972; D. Jahn, U. Michelsen, and D. Söll, J. Biol. Chem. 266:2542-2548, 1991). Here we present the mapping and cloning of the gene for the third enzyme, glutamate 1-semialdehyde (GSA) aminotransferase, and an initial characterization of the purified enzyme. Ethylmethane sulfonate-induced mutants of E. coli AB354 which required ALA for growth were isolated by selection for respiration-defective strains resistant to the aminoglycoside antibiotic kanamycin. Two mutations were mapped to min 4 at a locus named hemL. Map positions and resulting phenotypes suggest that hemL may be identical with the earlier described porphyrin biosynthesis mutation popC. Complementation of the auxotrophic phenotype by wild-type DNA from the corresponding clone pLC4-43 of the Clarke-Carbon bank (L. Clarke and J. Carbon, Cell 9:91-99, 1976) allowed the isolation of the gene. Physical mapping showed that hemL mapped clockwise next to fhuB. The hemL gene product was overexpressed and purified to apparent homogeneity. The pure protein efficiently converted GSA to ALA. The reaction was stimulated by the addition of pyridoxal 5' -phosphate or pyridoxamine 5' -phosphate and inhibited by gabaculine or aminooxyacetic acid. The molecular mass of the purified GSA aminotransferase under denaturing conditions was 40,000 Da, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The enzyme has apparent native molecular mass of approximately 80,000 Da, as determined by rate zonal sedimentation on glycerol gradients and molecular sieving through Superose 12, which indicates a homodimeric alpha2, structure of the protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Beale S. I. Cloning and expression of a structural gene from Chlorobium vibrioforme that complements the hemA mutation in Escherichia coli. J Bacteriol. 1990 Mar;172(3):1656–1659. doi: 10.1128/jb.172.3.1656-1659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. W., Jahn D., O'Neill G. P., Söll D. Purification of the glutamyl-tRNA reductase from Chlamydomonas reinhardtii involved in delta-aminolevulinic acid formation during chlorophyll biosynthesis. J Biol Chem. 1990 Mar 5;265(7):4058–4063. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Elliott T., Avissar Y. J., Rhie G. E., Beale S. I. Cloning and sequence of the Salmonella typhimurium hemL gene and identification of the missing enzyme in hemL mutants as glutamate-1-semialdehyde aminotransferase. J Bacteriol. 1990 Dec;172(12):7071–7084. doi: 10.1128/jb.172.12.7071-7084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Heme-deficient mutants of Salmonella typhimurium: two genes required for ALA synthesis. Mol Gen Genet. 1989 Apr;216(2-3):303–314. doi: 10.1007/BF00334369. [DOI] [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse P. W., Hopper S., Schatz L., Segal H. L. Further characterization of alanine aminotransferase of rat liver. J Biol Chem. 1967 May 25;242(10):2319–2324. [PubMed] [Google Scholar]
- Grimm B., Bull A., Breu V. Structural genes of glutamate 1-semialdehyde aminotransferase for porphyrin synthesis in a cyanobacterium and Escherichia coli. Mol Gen Genet. 1991 Jan;225(1):1–10. doi: 10.1007/BF00282635. [DOI] [PubMed] [Google Scholar]
- Grimm B., Bull A., Welinder K. G., Gough S. P., Kannangara C. G. Purification and partial amino acid sequence of the glutamate 1-semialdehyde aminotransferase of barley and synechococcus. Carlsberg Res Commun. 1989;54(2):67–79. doi: 10.1007/BF02907586. [DOI] [PubMed] [Google Scholar]
- Grimm B. Primary structure of a key enzyme in plant tetrapyrrole synthesis: glutamate 1-semialdehyde aminotransferase. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4169–4173. doi: 10.1073/pnas.87.11.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Jahn D., Chen M. W., Söll D. Purification and functional characterization of glutamate-1-semialdehyde aminotransferase from Chlamydomonas reinhardtii. J Biol Chem. 1991 Jan 5;266(1):161–167. [PubMed] [Google Scholar]
- Jahn D., Michelsen U., Söll D. Two glutamyl-tRNA reductase activities in Escherichia coli. J Biol Chem. 1991 Feb 5;266(4):2542–2548. [PubMed] [Google Scholar]
- Kadner R. J., Heller K., Coulton J. W., Braun V. Genetic control of hydroxamate-mediated iron uptake in Escherichia coli. J Bacteriol. 1980 Jul;143(1):256–264. doi: 10.1128/jb.143.1.256-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification and properties. J Biol Chem. 1972 Aug 25;247(16):4966–4974. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mau Y. H., Wang W. Y. Biosynthesis of delta-Aminolevulinic Acid in Chlamydomonas reinhardtii: Study of the Transamination Mechanism Using Specifically Labeled Glutamate. Plant Physiol. 1988 Mar;86(3):793–797. doi: 10.1104/pp.86.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G. P., Söll D. Transfer RNA and the formation of the heme and chlorophyll precursor, 5-aminolevulinic acid. Biofactors. 1990 Oct;2(4):227–235. [PubMed] [Google Scholar]
- O'Neill G. P., Thorbjarnardóttir S., Michelsen U., Pálsson S., Söll D., Eggertsson G. delta-Aminolevulinic acid dehydratase deficiency can cause delta-aminolevulinate auxotrophy in Escherichia coli. J Bacteriol. 1991 Jan;173(1):94–100. doi: 10.1128/jb.173.1.94-100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. A., Cox R., McConville M., Charles H. P. Mutations affecting porphyrin biosynthesis in Escherichia coli. Enzyme. 1973;16(1):65–73. doi: 10.1159/000459363. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]