Abstract
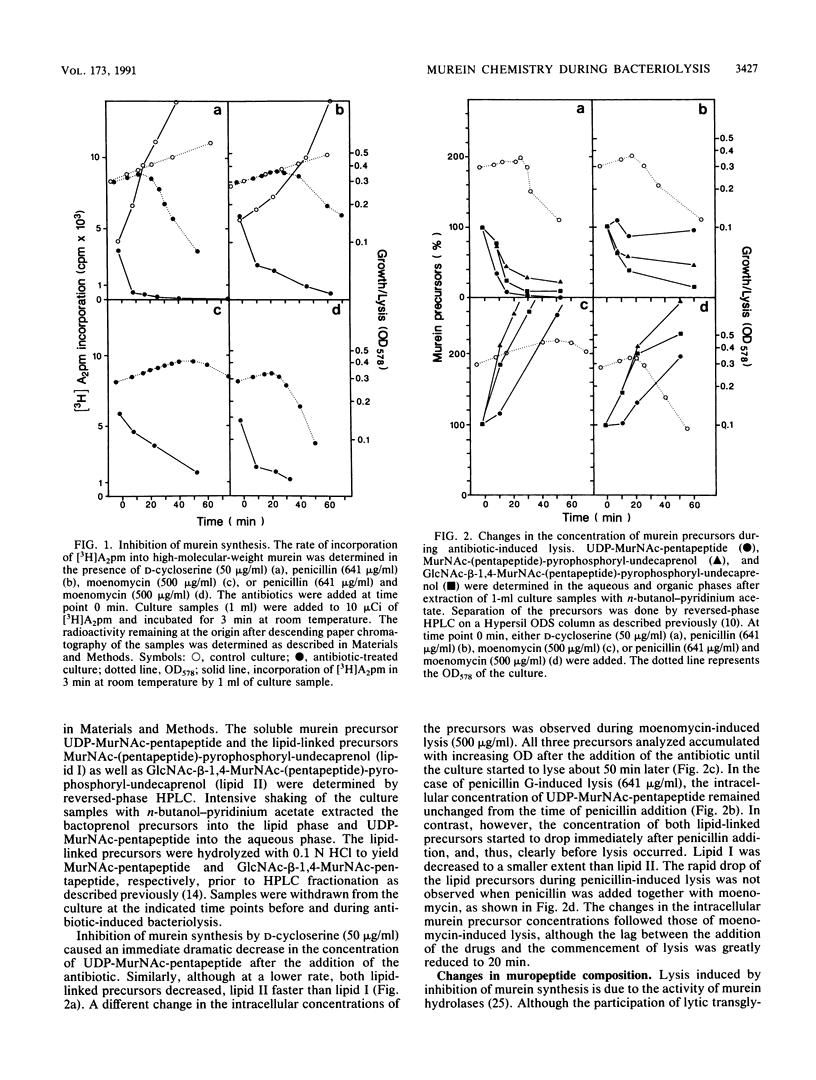
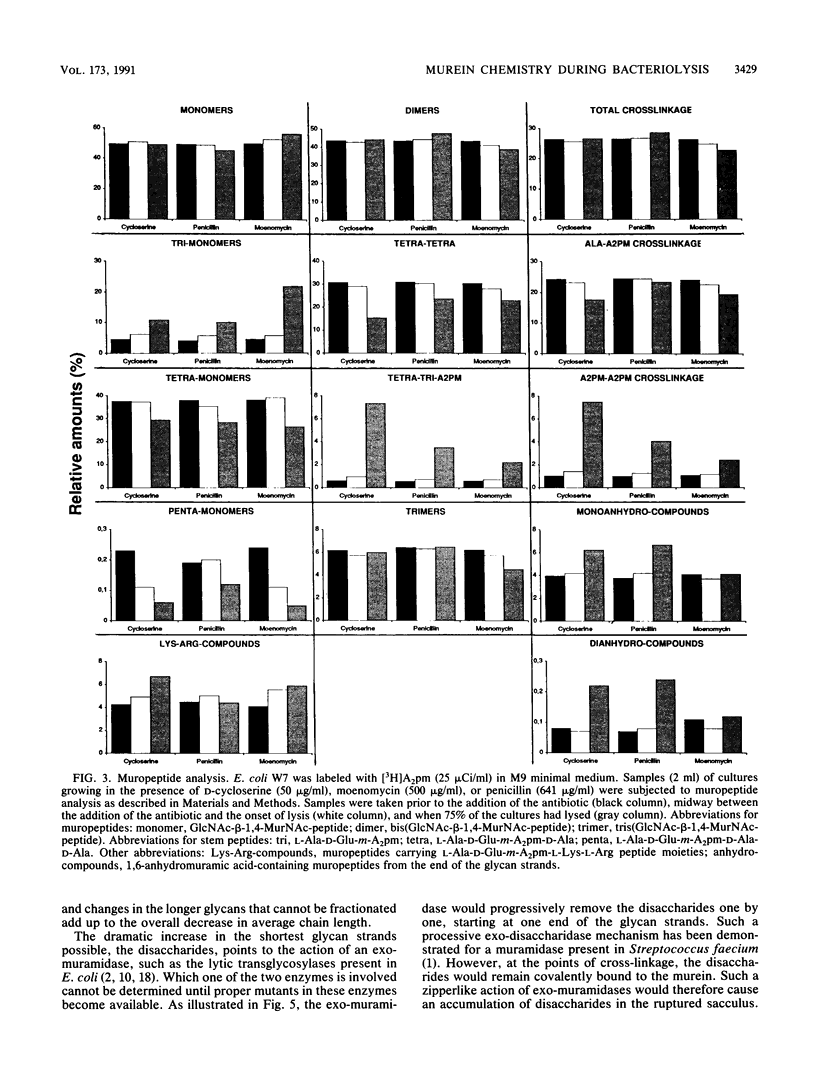
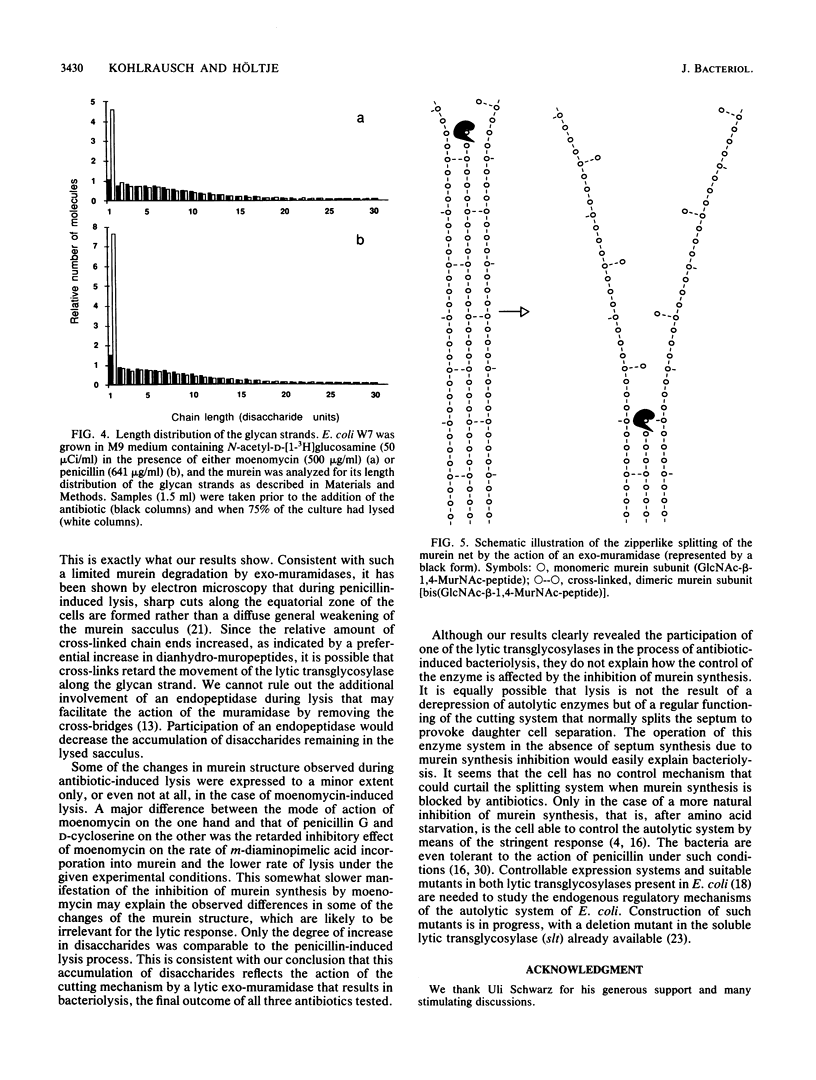
Lysis of Escherichia coli induced by either D-cycloserine, moenomycin, or penicillin G was monitored by studying murein metabolism. The levels of the soluble murein precursor UDP-N-acetylmuramyl-L-alanyl-D-glutamyl-m-diaminopimelyl-D-alanyl- D-alanine (UDP-MurNAc-pentapeptide) and the carrier-linked MurNAc-(pentapeptide)-pyrophosphoryl-undecaprenol as well as N-acetylglucosamine-beta-1,4-MurNAc-(pentapeptide)-pyrophosphoryl- undecaprenol varied in a specific way. In the presence of penicillin, which is known to interfere with the cross-linking of murein, the concentration of the lipid-linked precursors unexpectedly decreased before the onset of lysis, although the level of UDP-MurNAc-pentapeptide remained normal. In the case of moenomycin, which specifically blocks the formation of the murein polysaccharide strands, the lipid-linked precursors as well as UDP-MurNAc-pentapeptide accumulated as was expected. D-Cycloserine, which inhibits the biosynthesis of UDP-MurNAc-pentapeptide, consequently caused a decrease in all three precursors. The muropeptide composition of the murein showed general changes such as an increase in the unusual DL-cross bridge between two neighboring meso-diaminopimelic acid residues and, as a result of uncontrolled DL- and DD-carboxypeptidase activity, an increase in tripeptidyl and a decrease in tetrapeptidyl and pentapeptidyl moieties. The average length of the glycan strands decreased. When the glycan strands were fractionated according to length, a dramatic increase in the amount of single disaccharide units was observed not only in the presence of penicillin but also in the presence of moenomycin. This result is explained by the action of an exo-muramidase, such as the lytic transglycosylases present in E. coli. It is proposed that antibiotic-induced bacteriolysis is the result of a zipperlike splitting of the murein net by exo-muramidases locally restricted to the equatorial zone of the cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. F., Dolinger D. L., Schramm V. L., Shockman G. D. The mechanism of soluble peptidoglycan hydrolysis by an autolytic muramidase. A processive exodisaccharidase. J Biol Chem. 1984 Oct 10;259(19):11818–11827. [PubMed] [Google Scholar]
- Beachey E. H., Keck W., de Pedro M. A., Schwarz U. Exoenzymatic activity of transglycosylase isolated from Escherichia coli. Eur J Biochem. 1981 May 15;116(2):355–358. doi: 10.1111/j.1432-1033.1981.tb05342.x. [DOI] [PubMed] [Google Scholar]
- Beck B. D., Park J. T. Basis for the observed fluctuation of carboxypeptidase II activity during the cell cycle in BUG 6, a temperature-sensitive division mutant of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1292–1302. doi: 10.1128/jb.130.3.1292-1302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzner A. S., Ferreira L. C., Höltje J. V., Keck W. Control of the activity of the soluble lytic transglycosylase by the stringent response in Escherichia coli. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):161–164. doi: 10.1016/0378-1097(90)90187-u. [DOI] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18988–18996. [PubMed] [Google Scholar]
- Glauner B., Höltje J. V., Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10088–10095. [PubMed] [Google Scholar]
- Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988 Aug 1;172(2):451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- Harz H., Burgdorf K., Höltje J. V. Isolation and separation of the glycan strands from murein of Escherichia coli by reversed-phase high-performance liquid chromatography. Anal Biochem. 1990 Oct;190(1):120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Glauner B. Structure and metabolism of the murein sacculus. Res Microbiol. 1990 Jan;141(1):75–89. doi: 10.1016/0923-2508(90)90100-5. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tuomanen E. I. The murein hydrolases of Escherichia coli: properties, functions and impact on the course of infections in vivo. J Gen Microbiol. 1991 Mar;137(3):441–454. doi: 10.1099/00221287-137-3-441. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Kitano K., Tuomanen E., Tomasz A. Transglycosylase and endopeptidase participate in the degradation of murein during autolysis of Escherichia coli. J Bacteriol. 1986 Sep;167(3):759–765. doi: 10.1128/jb.167.3.759-765.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlrausch U., Wientjes F. B., Höltje J. V. Determination of murein precursors during the cell cycle of Escherichia coli. J Gen Microbiol. 1989 Jun;135(6):1499–1506. doi: 10.1099/00221287-135-6-1499. [DOI] [PubMed] [Google Scholar]
- Kusser W., Ishiguro E. E. Involvement of the relA gene in the autolysis of Escherichia coli induced by inhibitors of peptidoglycan biosynthesis. J Bacteriol. 1985 Nov;164(2):861–865. doi: 10.1128/jb.164.2.861-865.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Wachi M., Ishino F. Machinery for cell growth and division: penicillin-binding proteins and other proteins. Res Microbiol. 1990 Jan;141(1):89–103. doi: 10.1016/0923-2508(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Mett H., Keck W., Funk A., Schwarz U. Two different species of murein transglycosylase in Escherichia coli. J Bacteriol. 1980 Oct;144(1):45–52. doi: 10.1128/jb.144.1.45-52.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Enzyme replacement in a bacterium: phenotypic correction by the experimental introduction of the wild type enzyme into a live enzyme defective mutant pneumococcus. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1311–1319. doi: 10.1016/s0006-291x(75)80373-1. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Tomasz A. Induction of autolysis in nongrowing Escherichia coli. J Bacteriol. 1986 Sep;167(3):1077–1080. doi: 10.1128/jb.167.3.1077-1080.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heijenoort Y., Derrien M., Van Heijenoort J. Polymerization by transglycosylation in the biosynthesis of the peptidoglycan of Escherichia coli K 12 and its inhibition by antibiotics. FEBS Lett. 1978 May 1;89(1):141–144. doi: 10.1016/0014-5793(78)80540-7. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Walderich B., Ursinus-Wössner A., van Duin J., Höltje J. V. Induction of the autolytic system of Escherichia coli by specific insertion of bacteriophage MS2 lysis protein into the bacterial cell envelope. J Bacteriol. 1988 Nov;170(11):5027–5033. doi: 10.1128/jb.170.11.5027-5033.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




