Abstract
A barrier (seal) must form at the cut ends of a severed axon if a neuron is to survive and eventually regenerate. Following severance of crayfish medial giant axons in physiological saline, vesicles accumulate at the cut end and form a barrier (seal) to ion and dye diffusion. In contrast, squid giant axons do not seal, even though injury-induced vesicles form after axonal transection and accumulate at cut axonal ends. Neither axon seals in Ca2+-free salines. The addition of calpain to the bath saline induces the sealing of squid giant axons, whereas the addition of inhibitors of calpain activity inhibits the sealing of crayfish medial giant axons. These complementary effects involving calpain in two different axons suggest that endogenous calpain activity promotes plasmalemmal repair by vesicles or other membranes which form a plug or a continuous membrane barrier to seal cut axonal ends.
Keywords: axotomy, crayfish, medial giant axon, squid giant axons, plasmalemmal repair
The rapid (within 60 min) repair of unmyelinated crayfish medial giant axons (MGAs) (1) and myelinated earthworm MGAs (2) is associated with the accumulation of vesicles and other membranous material at the cut axonal ends. In contrast, squid giant axons (GAs) do not seal, even though injury-induced vesicles form after transection and accumulate at cut axonal ends (3). Calpain (4) is a calcium-activated neutral protease endogenous to squid GAs (5), other axons (4), and other cell types (4, 6) that could enhance or inhibit one or more stages (vesicle formation, movement, aggregation, membrane fusion, etc.) of the sealing process. Calpain and other proteases degrade cytoskeletal proteins such as neurofilaments (4, 6, 7, 8), which help maintain axonal diameter and shape (9). Such degradation might produce a complete or partial collapse of the cut end (10, 11), thereby inducing sealing by enhancing the interactions between vesicles that form a seal by themselves and/or that form a continuous membrane at the cut end. (In this paper, the term “complete constriction or complete collapse” is used to describe 100% closure of a cut axonal end; the term “partial constriction or partial collapse” is used to describe any constriction that leaves an opening at the cut end.) Alternatively, calpain or other proteases might inhibit sealing by repressing the formation of vesicles or a continuous membrane or by degrading cytoskeletal proteins (microtubules, actin, molecular motors, etc.), which might affect the movement of injury-induced or other vesicles to the cut axonal end.
We examined the effects of calpain and other proteases on the sealing of two giant axons: the crayfish MGA, which normally seals after severance in physiological saline (see preceding paper, ref. 1), and the squid GA, which normally does not seal after severance in physiological saline (2). [GAs (2) and MGAs (1) transected in Ca2+-free salines do not seal.] According to our electrophysiological measures (decay of injury current, Ii, and resting membrane potential, Vm) and optical measures (dye exclusion), we find that exogenous calpain, but not chymotrypsin, induces sealing of transected squid GAs when added to physiological salines. Calpain does not appear to enhance vesicle formation in squid GAs, but rather enables existing vesicles to form a seal. According to these same measures, crayfish MGAs normally seal in physiological salines, but crayfish MGAs do not seal when transected in physiological salines containing exogenous inhibitors of calpain activity (leupeptin, human calpastatin peptide, calpeptin, endogenous rabbit calpastatin, iodoacetamide, and monoclonal antibodies to calpain). However, crayfish MGAs will seal in Ca2+-free solutions containing exogenous proteases (bromelian, papain, dispase, trypsin, chymotrypsin), which do not require Ca2+ for activation and which (unlike calpain) are not normally found in axons. These and other data (11) suggest that endogenous calpain enhances the sealing of severed invertebrate and vertebrate (including mammalian) axons primarily by inducing junctional complexes in an accumulation of vesicles or by enhancing the formation of a continuous membrane, rather than by enhancing vesicle formation, vesicle movement, or axonal closure by degrading cytoskeletal proteins.
MATERIALS AND METHODS
As previously described (2), squid GAs dissected from Loligo pealei or Sepioteuthis lessoniana were placed in artificial sea water (physiological saline for squid): 430 mM NaCl, 10 mM KCl, 10 mM CaCl2, 50 mM MgCl2, and 5 mM Tris⋅Cl (pH 7.4), 974 mOsm (osmolality). Divalent-cation-free salines consisted of 426 mM NaCl, 10 mM KCl, 110 mM tetramethylammonium chloride, 5 mM Tris⋅Cl, 1 mM EDTA, and 1 mM EGTA (pH 8), 972 mOsm. MGAs were dissected from crayfish (Procambaris clarkii) and placed in various salines, as described in the preceding paper (1).
Use of a vibrating probe, intracellular electrodes, differential interference contrast (DIC) optics, and fluorescence and confocal fluorescence imaging were as described (1). In this paper, relative measures of injury current density (Ii) and control current density (Ic), measured for the intact axon prior to its transection, are denoted by the primed symbols I′i and I′c; I′i and I′c are normalized with respect to the first value of Ii acquired 3–5 min after transection. According to this definition, the initial value of I′i always equals 100%. An axon was considered to seal electrically when Ii decayed to the upper end of the range of values recorded for Ic (25 μA/cm2 for GAs and 15 μA/cm2 for MGAs). The largest Ic values were more than 20 times smaller than the smallest Ii values recorded after transection.
Dyes and other substances were obtained as follows: α-chymotrypsin, bromelian, calpain, cytochalasin E, iodoacetamide, leupeptin, papain, protease inhibitor (endogenous rabbit calpastatin), tetramethylammonium chloride, and trypsin (Sigma); calpeptin and taxol (LC Laboratories, Woburn, MA); fluorescein isothiocyanate (FITC)–dextran, Lucifer Yellow, Oregon Green-dextran, pyrene, Texas Red–dextran, tetramethylrhodamine–dextran (Molecular Probes); calpeptin (Calbiochem); human calpastatin peptide (Research Biochemicals, Natick, MA); dispase (Boehringer Mannheim); calpain antibody (N. Banik, Medical University of South Carolina, Charleston).
RESULTS AND DISCUSSION
We measured the Ic and membrane potential (Vm) of control (intact) squid GAs in physiological saline (Fig. 1, Table 1). Intact GAs, 300–600 μm in diameter, were surrounded by a nonmyelinated glial sheath 5–10 μm wide consisting of glial and collagen layers (Fig. 2B Inset) (12, 13). The axoplasm of intact GAs contained neurofilaments, microtubules, smooth endoplasmic reticulum, mitochondria, and some vesicles <1 μm in diameter. Ic of intact GAs (n > 10) ranged from 4 to 12 μA/cm2 and was directed outwardly (2). Ic did not change for 120 min unless the GA was accidentally damaged, in which case Ic increased 20–200 times as rapidly as the new measurement could be made. Intact GAs (n = 14) always excluded a hydrophilic dye (FITC–dextran, Lucifer Yellow, Oregon Green–dextran, pyrene, Texas Red–dextran, or tetramethylrhodamine–dextran) when placed in physiological saline for 30–100 min and maintained rapid orthograde and retrograde vesicular transport for at least 2–3 h in physiological saline (n = 4).
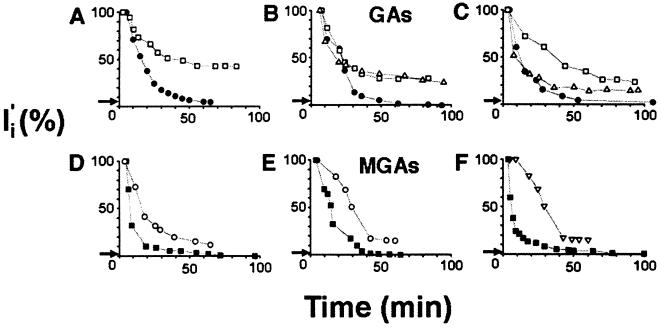
Figure 1.
Injury current density (Ii) determined by vibrating probe measurements from cut ends of three GAs and three MGAs. I′i plotted as percent of initial Ii obtained within 3–5 min after transection. Arrow on each ordinate denotes relative value of background current (I′c) of intact axon prior to transection. (A–C) Time course (min) of I′i decay from squid GAs transected in physiological saline (open squares) and physiological saline containing 0.02 unit/ml calpain (• or 10 μg/ml chymotrypsin (▵). (D–F) Time course (min) of I′i decay from crayfish MGAs transected in physiological saline (▪) or physiological saline containing 200 μg/ml leupeptin (○) or 100 μg/ml calpeptin (▿).
Table 1.
Comparison of Ic, Ii, and Vm in intact and transected GAs in physiological saline with and without added calpain (0.02 activity unit/ml)
| GA | Pretransection measure
|
Posttransection measure
|
||
|---|---|---|---|---|
| Ic, μA/cm2 | Vm, mV | Ii, μA/cm2 | Vm, mV | |
| Intact | ||||
| Physiological saline | 9.2 ± 1.4 (4) | 59.6 ± 1.9 (6) | ||
| Physiological saline | 8.8 ± 3.7 (5) | 58.9 ± 1.7 (6) | ||
| plus calpain | ||||
| Transected | ||||
| Physiological saline | ||||
| 10 min | 947 ± 73.2 (5)* | 37.9 ± 5.2 (5)* | ||
| 90 min | 355 ± 99.0 (5)* | 39.8 ± 8.0 (6)* | ||
| Physiological saline | ||||
| plus calpain | ||||
| 10 min | 646 ± 109 (6)* | 27.4 ± 7.2 (3)* | ||
| 90 min | 18.8 ± 2.1 (6)* | 58.1 ± 2.1 (6) | ||
Data are given as the average ± SEM for sample size (n).
Significantly (P < 0.05) different from control (pretransection) values according to Student’s t test.
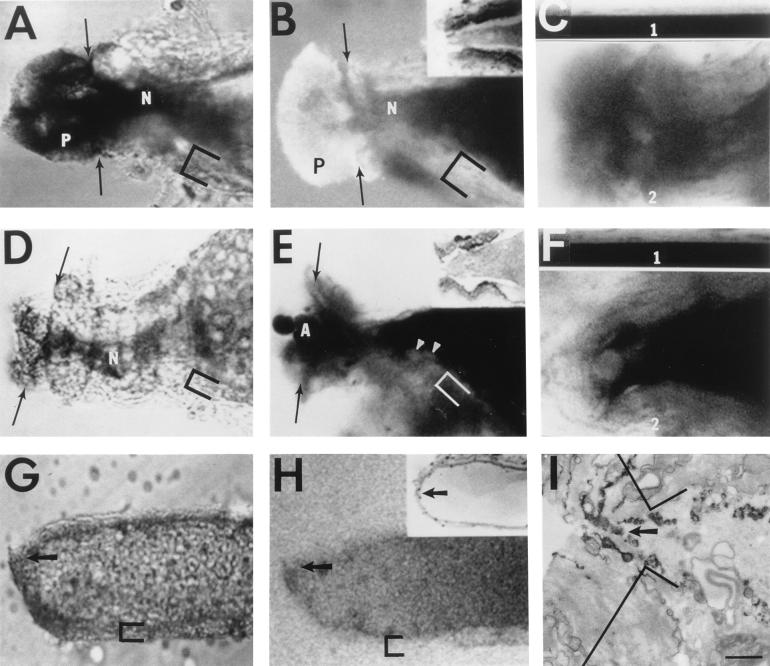
Figure 2.
DIC (A, D, and G) images, confocal images (B, C, E, F, and H), and electron micrograph (EM) (I) of transected squid GAs (A–F) or crayfish MGAs (G–I). Cut ends (arrows) of transected axons oriented to the left. Confocal plane (<5 μm thick) in B, C, E, F, and H parallel to, and through, midsection of long axis of GA or MGA. Bath contains dye and exhibits fluorescence, as does glial sheath (brackets). N, neck of the cut end of the GA. (A) DIC of cut end of GA 60 min after transection in physiological saline. (B) Confocal fluorescence image showing uptake of dye (0.1% Texas Red–dextran in physiological saline) 92 min postseverance and 32 min after adding dye. P, plume of axoplasm containing vesicles when viewed at higher magnification. (B Inset) Low resolution EM of the cut end of a GA in physiological saline. (C) Comparison of fluorescence images of paired segments (C1, intact; C2, transected) from the same GA placed in the same physiological saline. (C1) Control (intact) segment imaged simultaneously with cut end of the transected segment in C2. To allow quantitative comparison between C1 and C2, contrast and brightness settings were the same for both images. (C2) Cut end of a severed GA imaged 100 min posttransection and 40 min after 0.1% FITC–dextran was added to the bath saline showing the uptake of dye in the neck region (compare the greyness within the neck region to the blackness of the axoplasm in the intact axon segment in C1). (D) DIC of GA imaged 60 min after transection in physiological saline containing calpain (0.02 activity unit/ml). Note longer length of neck (N) compared with GA in A. (E) Confocal fluorescence image of GA 94 min after transection in physiological saline containing calpain (0.02 activity unit/ml). Texas Red–dextran (0.1% in physiological saline) added 60 min after transection. (E Inset) Low-resolution EM of the cut end of GA transected in physiological saline containing calpain. (F) Comparison of fluorescence images of paired segments (F1, intact; F2, transected) of the same GA in physiological saline containing calpain (0.02 activity unit/ml). (F1) Control (intact) segment imaged simultaneously with the cut end of the transected segment in F2 and with the same contrast and brightness settings for quantitative comparison. (F2) Cut end of a severed GA imaged 165 min posttransection and 105 min after 0.1% FITC–dextran was added to the physiological saline containing calpain showing dye exclusion (compare the blackness within the cut end to the blackness of the axoplasm in the intact axon segment in F1). (G) DIC of cut end of MGA 60 min after transection in physiological saline containing leupeptin (200 μg/ml). Arrow indicates small-diameter (3–5 μm) pore at cut end. (H) Confocal fluorescence image of MGA transected in physiological saline containing leupeptin (200 μg/ml) and imaged 87 min after transection and 27 min after adding 0.1% Texas Red–dextran. (H Inset) Photomicrograph of MGA 1 hr after transection in physiological saline containing 200 μg/ml leupeptin. Arrow points to pore-like opening filled with vesicles when imaged at higher magnification. (I) EM of cut end of MGA fixed 60 min after transection in physiological saline containing 200 μg/ml leupeptin. Arrow points to 3- to 5-μm diameter pore at apex of cut end. (Bar = 165 μm for A, B, D, and E; 1 μm for C, F, and I; 75 μm for G and H; 130 μm for B and E Insets; and 150 μm for H Inset.)
After Ic was measured, squid GAs (n > 10) were transected in physiological saline. The initial I′i, measured 3–5 min after transection, was always directed into the cut end and was significantly (P < 0.0001; 20- to 200-fold) greater than I′c (2). I′i typically decayed rapidly for 20–30 min followed by a slower prolonged decay during which I′i remained 30–50% greater than I′c (Fig. 1 A–C). Vm, measured 100–300 μm from the cut end, usually recovered to 75% of Vm in intact GAs at 60 min after transection (2). Vm then began to decline steadily for 60–120 min after transection (Fig. 1, Table 1), almost certainly due to degenerative changes in the GA (2, 12). As previously reported (2), Ii and Vm at 90–150 min after transection were significantly different (P < 0.001) from control values (Table 1). According to these combined measures, GAs transected in physiological saline did not seal within 90–120 min (Table 2).
Table 2.
The percentage of squid GAs and crayfish MGAs that seal within 90–120 min in physiological salines containing calpain, various calpain inhibitors, or other substances, as measured by the percent of axons in which Ii decayed to Ic or by the percent of axons that excluded dye as assessed by fluorescence imaging of pyrene or confocal fluorescence imaging of other hydrophilic dyes
| Experimental conditions, substances added | Measures of sealing
|
||
|---|---|---|---|
| Electrophysiological, % decay of Ii to Ic(n) | Optical, % dye exclusion (n)
|
||
| Fluorescence | Confocal | ||
| Squid GAs | |||
| None, control | 0 (14) | 0% (14) | 0 (11) |
| Calpain, 0.02 au/ml | 100 (5)* | 100% (4)* | 88 (8)* |
| Chymotrypsin, 0.01 au/ml | 0 (5) | 20% (5) | 20 (5) |
| Crayfish MGAs | |||
| None, control | 100 (6) | 93 (42) | 95 (37) |
| Leupeptin | |||
| 200 μg/ml | 0 (2)* | 0 (30)* | |
| 100 μg/ml | 14 (14)* | 20 (30)* | 8 (12)* |
| 50 μg/ml | 25 (4)* | ||
| Calpeptin, 100 μg/ml | 0 (3)* | 0 (8)* | 0 (5)* |
| Human calpastatin, 100 μM | 0 (16)* | ||
| Rabbit calpastatin, 0.04 au/ml | 0 (3)* | 25 (16)* | 0 (5)* |
| Calpain antibody, 1:50 dilution | 17 (28)* | ||
| Iodoacetamide, 10 mM | 0 (9)*; P | ||
| Taxol, 20 mM | 93 (14) | ||
| Cytochalasin E, 6 μg/ml | 92 (12) | ||
| OCa2+ saline | 21 (43)* | ||
| Plus bromelian, 1 mg/ml | 100 (4) | ||
| Plus papain, 140 μg/ml | 100 (4) | ||
| Plus trypsin, 2.5 mg/ml | 100 (6) | ||
| Plus dispase, 340 μg/ml | 100 (12) | ||
| Plus chymotrypsin, 1 mg/ml | 100 (4) | ||
| Divalent-free saline | 17 (12)* | ||
| Plus bromelian, 1 mg/ml | 88 (8) | ||
| Plus papain, 140 μg/ml | 100 (8) | ||
| Plus trypsin, 2.5 mg/ml | 100 (4) | ||
| Plus dispase, 340 μg/ml | 50 (10)* | ||
| Plus chymotrypsin, 0.01 au/ml | 75 (4) | ||
n, Number of proximal and distal cut ends examined, unless only data from proximal cut ends are shown (marked by a P). All substances added to physiological saline, unless otherwise noted. au, Activity units.
Significally different (P < 0.05, χ2 test) from controls in physiological saline.
DIC (Fig. 2A) or confocal fluorescence (Fig. 2B) images of GAs in vitro and photomicrographs of thick sections (Fig. 2B Inset) or electron micrographs of thin sections of fixed GAs showed that cut ends transected in physiological salines were not completely closed and contained loosely packed vesicles 0.1–50 μm in diameter at 60–120 min post-severance. As previously reported (2), inspection of electron micrographs from over 10 GAs showed that the axolemma and glialemma were disrupted for 50–200 μm from the cut end. When transected and maintained in physiological saline for 60–90 min before adding hydrophilic dyes to the bath, GAs did not exclude hydrophilic dye (did not seal) at 90–120 min postseverance (Fig. 2 B and C; Table 2). Orthograde and retrograde axonal transport ceased in GAs (n = 3) at 60 min posttransection. I′c of intact GAs (n = 5) measured for up to 120 min was not significantly (P > 0.05) different in physiological saline compared with that in saline containing calpain (0.02 activity unit/ml). Intact GAs (n = 3) excluded hydrophilic fluorescent dye and exhibited orthograde and retrograde axonal transport for up to 120 min after adding calpain.
For 3–20 min posttransection, the rates of decay of I′i for GAs transected in physiological saline containing calpain were not obviously different from the rates of decay measured for GAs transected in physiological saline [Fig. 1 A–C: physiological saline (□), physiological saline with calpain (•)]. In contrast to GAs transected in physiological saline, I′i for GAs transected in calpain continued to decay from ≈20 to 90 min posttransection until I′i was <25 μA/cm2 (see Materials and Methods). Vm for GAs transected in calpain was significantly (P < 0.0001) lower than control values at 10 min posttransection; Vm recovered to levels at 90 min posttransection that did not differ significantly (P > 0.05) from control values (Table 1). That is, GAs did seal when transected in calpain-containing saline (Table 2).
Analyses of DIC images (Fig. 2D), confocal fluorescence images (Fig. 2E), and photomicrographs (Fig. 2E Inset) showed that squid GAs transected and maintained in calpain for 90–120 min contained larger vesicles at or near the cut end, compared with GAs transected in physiological saline (Fig. 2 A–C). Once formed in the presence of a hydrophilic dye added to the bath saline, dye-filled vesicles in calpain-treated GAs migrated to the cut end, as did vesicles in GAs transected in physiological salines viewed with time-lapse confocal microscopy. As reported for crayfish MGAs, which seal within 60 min after transection in physiological saline, ultrastructural observations showed that the axolemma and glialemma of GAs was disrupted for 50–200 μm from the cut end. When transected GAs were maintained in physiological saline containing calpain for 60–90 min, at which time a hydrophilic dye was added to the bath saline, confocal fluorescence images when compared with controls (intact GAs) showed that the cut ends of these transected GAs excluded dye (sealed) (Fig. 2 E and F) and that the outer border of the barrier to diffusion of hydrophilic dyes was no more than 1 μm wide. When DIC and confocal fluorescence images were superimposed and examined at higher magnification, the dye-diffusion barrier coincided with the accumulation of vesicles that filled the incompletely closed cut end of the GA. All calpain-treated GAs sealed according to our electrophysiological measure, and 94% sealed when all our optical measures of dye exclusion were taken into account (Table 2).
According to any of our electrical or dye-exclusion measures (Fig. 1, Table 2), chymotrypsin or low-activity calpain did not induce sealing in GAs. The initial decay of I′i and the final value of Ii for squid GAs transected in exogenous chymotrypsin (Fig. 1 A–C, ▵) was comparable to that for squid GAs transected in physiological saline. Vesicles were obviously present in the axoplasm and at the cut end. Dye was not excluded at the cut ends of transected GAs exposed to chymotrypsin (Table 2). As observed for calpain-treated GAs, chymotrypsin-treated GAs often had a longer length of constricted axon proximal to the cut end than did GAs cut in physiological saline. In contrast to calpain-treated GAs, which had a very gel-like axoplasm at the cut end, the axoplasm of chymotrypsin-treated GAs was not viscous and flowed out the cut end.
Similar to squid GAs, intact crayfish MGAs (80–150 μm in diameter) are surrounded by a nonmyelinated glial sheath 3–6 μm wide consisting of glial and collagen layers (1). When crayfish MGAs were severed in physiological saline, I′i and Vm recovered to near-control levels within 60 min posttransection. The extent and pattern of decay of I′i for crayfish MGAs (n = 6) transected in physiological saline (Fig. 1 D–F, ▪) was similar to that observed for squid GAs transected in physiological saline with exogenous calpain (Fig. 1 A–C, •), as were DIC, fluorescence, confocal fluorescence, and observations and measures of dye exclusion (Table 2; also see ref. 1). The time course (60 min) of sealing of crayfish MGAs transected in physiological saline was somewhat faster than the time course (90 min) of sealing of squid GAs transected in calpain (Fig. 1). The time course (60 min) of sealing of squid GAs injected with calpain prior to transection and maintained in physiological saline was very similar to that of crayfish MGAs transected in physiological saline, suggesting that increased endogenous calpain activity in the axoplasm expedites sealing.
When crayfish MGAs were severed in divalent-cation-free saline (Table 2) or in physiological saline containing inhibitors of calpain (Fig. 1 D–F) such as 200 μg/ml leupeptin (Fig. 1D; n = 12) or 100 μg/ml calpeptin (Fig. 1E; n = 3), I′i did not decline to control levels within 60–120 min posttransection. The decay of I′i to 10–15% (50–100 μA/cm2) of the initial I′i (500–700 μA/cm2) for crayfish MGAs transected for 60–120 min in divalent-cation-free salines or in physiological salines containing calpain inhibitors [Fig. 1 D–F, leupeptin (○; calpeptin (▿)] was somewhat greater than the decay of I′i to 30–50% of initial I′i observed for squid GAs transected for 60–120 min in divalent-cation-free salines or in physiological salines lacking calpain (Fig. 1 A–C, □). However, an I′i at 60 min posttransection equal to 10% of the initial I′i was much greater than the range of Ic values (3–15 μA/cm2) and represented a very incomplete seal—i.e., a substantial opening at the cut end. I′i rarely decayed to I′c within 120 min posttransection for MGAs transected in 100 μg/ml leupeptin, 100 μg/ml calpeptin or 0.04 unit/ml endogenous rabbit calpastatin (Table 2).
The conclusion that inhibitors of calpain activity prevent sealing based on electrophysiological data was confirmed by our optical measures (Table 2). Crayfish MGAs were transected in physiological saline containing a calpain inhibitor and maintained in this inhibitor-containing saline for 60 min, at which time pyrene or other hydrophilic dye was added to the bath saline for 15 min before imaging with fluorescence or confocal fluorescence optics. Dye was rarely excluded by MGAs placed in leupeptin (50–200 μg), calpeptin, endogenous rabbit calpastatin, iodoacetamide, or a monoclonal antibody to calpain (Table 2). Inspection of DIC, confocal, photomicrographic, and electron micrographic images showed that the cut ends of leupeptin-treated MGAs (n > 10) incompletely closed to form a pore-like structure which was filled with vesicles (Fig. 2 G–I) and vesicles accumulated in the cortical axoplasm for several hundred micrometers on either side of the pore similar to MGAs severed in physiological salines. Furthermore, the rate of vesicle migration to the cut end in leupeptin-treated crayfish MGAs was not obviously less than MGAs transected in physiological saline. That is, leupeptin did not inhibit vesicle formation, vesicle accumulation, or pore formation. However, leupeptin-treated MGAs that did not seal differed in several ways from MGAs which did seal within 60 min when transected in physiological salines. Specifically, in leupeptin-treated MGAs, many of the vesicles were very large, the diameter of the MGA was greatly reduced, and hydrophilic dyes were not excluded at the outer boundary of these vesicles.
When other proteases (which are not as dependent on Ca2+ as is calpain for activation) were added to divalent-free salines or Ca2+-free salines, crayfish MGAs usually sealed within 60 min when transected and maintained for 20 min in salines containing these proteases (Table 2). All these proteases (bromelian, papain, trypsin, dispase, and chymotryspin) behaved similarly in Ca2+-free vs. divalent-free salines, except for dispase which requires Mg2+ for activation. According to our measure of dye exclusion (Table 2), dispase always induced sealing in Ca2+-free salines containing Mg2+, but dispase did not always induce sealing in divalent-free salines containing EDTA (a chelator of Mg2+).
Our observations are in agreement with a previous report (11) that several proteases (papain, dispase, and trypsin) enhanced sealing in Ca2+-free salines of severed mammalian septal axons in tissue culture. This previous study (11) suggested that calpain and several exogenous proteases enhanced sealing by promoting the complete collapse of the axon and fusion of the apposed axolemmal leaflets at the cut end. However, our observations show that the cut axonal ends are not completely closed when transected squid GAs seal in exogenous calpain (Fig. 2 A and B) or when transected crayfish MGAs seal in physiological salines. Furthermore, inhibitors of calpain activity (e.g., leupeptin) did not inhibit sealing in crayfish MGAs by preventing pore formation or partial constriction of the cut end. Finally, various stabilizers of microtubules (20 mM taxol) or destabilizers of F-actin (6 μg/ml cytochalasin E)—compounds that should stabilize or reduce axonal diameter—did not significantly (P > 0.05) affect the sealing of crayfish MGAs (Table 2).
Various data suggest that endogenous calpain helps induce plasmalemmal sealing in squid GAs, crayfish MGAs, and other cells by enhancing interactions of vesicles with other vesicles, the plasmalemma, and/or other membranes, perhaps by promotion of membrane fusion (14–16) or activation of various kinases (17–19). (i) Calpain induces the formation of a dye diffusion barrier at a region of vesicle accumulation and a barrier to ionic movements at the cut end of squid GAs. (ii) Inhibitors of protease activity some of which are specific for calpain (e.g., calpain antibody, human calpastatin peptide, endogenous rabbit calpastatin) inhibit seal formation in crayfish MGAs. (iii) Calpain is an endogenous protease in squid GAs (5), other axons (including the crayfish MGA) (4, 6, 8, 10, 20), and many other cell types (14–19). (While other proteases may induce sealing in Ca2+-free salines in crayfish MGAs (this report) and in mammalian axons (11), these proteases are not known to be endogenous to these axons.) (iv) Leupeptin (an inhibitor of calpain) did not inhibit vesicle production or movement in crayfish MGAs. Specifically, leupeptin induced the formation of many large vesicles that filled, but did not seal, the cut end. These data suggest that calpain may enhance the fusion of vesicles with the plasmalemma. (v) We did not detect other possible actions of calpain (or inhibitors of calpain) on vesicle formation, vesicle movement, or constriction of the cut end of squid GAs or crayfish MGAs.
In summary, whatever its cellular/molecular mechanism(s) of action, exogenous calpain induces sealing in squid GAs which otherwise do not seal. In fact, it appears that GAs possess all the necessary components of the multistage sealing process except for a sufficient level of calpain activity [squid GAs contain endogenous calpain (5)]. In contrast, nonspecific and specific inhibitors of calpain activity inhibit sealing in transected crayfish MGAs which otherwise appear to possess sufficient calpain activity to seal. Other proteases can induce sealing of crayfish MGAs transected in Ca2+-free salines that should inhibit calpain activity. Given these data and a report (11) that other proteases enhance the sealing of severed mammalian axons and that nonspecific inhibitors of calpain activity inhibit their sealing, we propose that calpain promotes the close interactions among vesicles and the axolemma necessary to seal the severed ends of invertebrate and vertebrate axons.
Acknowledgments
We thank Mr. Alan Shipley and Mr. Louis Kerr for technical assistance and the Marine Biomedical Institute in Galveston for supplying cephalopods (NIH RR01024). We thank Dr. Claire Hulsebosch, Dr. Todd Krause, Dr. Sandra Tanner, and Dr. Guy Thompson for a careful reading of drafts of this manuscript, and David Bobb and Loc Nguyen for assistance with dye exclusion studies of crayfish MGAs. We thank Carl Zeiss Inc., the Central Microscope and the National Vibrating Probe Facilities at the Marine Biological Laboratory (Woods Hole, MA) for use of their equipment. This work was supported by National Institutes of Health Grant NS31256 and an Advanced Technology Project grant.
ABBREVIATIONS
- DIC
differential interference contrast
- GA
giant axon
- MGA
medial giant axon
- Vm
membrane potential
- Ii
injury current density
- Ic
control current density
- FITC
fluorescein isothiocyanate
References
- 1.Eddleman C S, Ballinger M L, Smyers M E, Godell C M, Fishman H M, Bittner G D. Proc Natl Acad Sci USA. 1997;94:4759–4764. doi: 10.1073/pnas.94.9.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krause T L, Fishman H M, Ballinger M L, Bittner G D. J Neurosci. 1994;14:6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman H M, Tewari K P, Stein P G. Biochim Biophys Acta. 1990;1023:421–435. doi: 10.1016/0005-2736(90)90135-b. [DOI] [PubMed] [Google Scholar]
- 4.Pontremoli S, Melloni E. Trends Neurosci. 1989;12:438–442. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- 5.Pant G C, Gainer H. J Neurobiol. 1980;11:1–12. doi: 10.1002/neu.480110102. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman U J, Schlaepfer W W. Prog Neurobiol. 1984;23:63–78. doi: 10.1016/0301-0082(84)90012-1. [DOI] [PubMed] [Google Scholar]
- 7.Pant H C. Biochem J. 1988;256:665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaepfer W W. Exp Cell Res. 1971;67:173–80. doi: 10.1016/0014-4827(71)90622-7. [DOI] [PubMed] [Google Scholar]
- 9.de Waegh S M, Lee V M-Y, Brady S T. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- 10.Raabe T D, Nguyen T, Archer C, Bittner G D. J Neurosci. 1996;16:1605–1613. doi: 10.1523/JNEUROSCI.16-05-01605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X, Barrett J N. J Neurosci. 1991;11:3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown A, Lasek R J. In: Squid as Experimental Animals. Gilbert D L, Adelman W J Jr, Arnold J M, editors. New York: Plenum; 1990. p. 235. [Google Scholar]
- 13.Villegas G M, Villegas R. Curr Top Membr Transp. 1984;22:3–37. [Google Scholar]
- 14.Balcerak D, Poussard S, Brustis J J, Elamrani N, Soriano M, Cottin P, Ducastaing A. J Cell Sci. 1995;108:2077–2082. doi: 10.1242/jcs.108.5.2077. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Saito Y, Kawashima S. Biochem Biophys Res Commun. 1992;182:939–946. doi: 10.1016/0006-291x(92)91822-8. [DOI] [PubMed] [Google Scholar]
- 16.Kwak K B, Kambayashi J, Kang M, Ha D B, Chung C H. FEBS Lett. 1993;323:151–154. doi: 10.1016/0014-5793(93)81468-f. [DOI] [PubMed] [Google Scholar]
- 17.Eto A, Akita Y, Saido J C, Suzuki K, Kawashima S. J Biol Chem. 1995;270:25115–25120. doi: 10.1074/jbc.270.42.25115. [DOI] [PubMed] [Google Scholar]
- 18.Shea T B, Beermann M L, Griffin W R, Leli U. FEBS Lett. 1994;350:223–229. doi: 10.1016/0014-5793(94)00769-1. [DOI] [PubMed] [Google Scholar]
- 19.Shea T B, Cressman C M, Spencer M J, Beermann M L, Nixon R A. J Neurochem. 1995;65:517–527. doi: 10.1046/j.1471-4159.1995.65020517.x. [DOI] [PubMed] [Google Scholar]
- 20.Raabe T D, Nguyen T, Bittner G D. J Neurobiol. 1995;26:253–261. doi: 10.1002/neu.480260209. [DOI] [PubMed] [Google Scholar]