Abstract
femA is a chromosomally encoded factor, occurring naturally in Staphylococcus aureus, which is essential for the expression of high-level methicillin resistance in this organism. The production of a low-affinity penicillin-binding protein, PBP2a or PBP2', which is intimately involved with methicillin resistance in S. aureus, is not influenced by femA. To elucidate a possible physiological function of the 48-kDa protein encoded by femA, several related methicillin-resistant, methicillin-susceptible, and Tn551 insertionally inactivated femA mutants were analyzed for possible changes in cell wall structure and metabolism. Independent of the presence of mec, the methicillin resistance determinant, all femA mutants had a reduced peptidoglycan (PG) glycine content (up to 60% in the molar ratio of glycine/glutamic acid) compared to that of related femA+ parent strains. Additional effects of femA inactivation and the subsequent decrease in PG-associated glycine were (i) reduced digestion of PG by recombinant lysostaphin, (ii) unaltered digestion of PG by Chalaropsis B-muramidase, (iii) reduced cell wall turnover, (iv) reduced whole-cell autolysis, and (v) increased sensitivity towards beta-lactam antibiotics. Also, the PG-associated glycine content of a femA::Tn551 methicillin-susceptible strain was restored concomitantly with the methicillin resistance to a level almost equal to that of its femA+ methicillin-resistant parent strain by introduction of plasmid pBBB31, encoding femA.
Full text
PDF

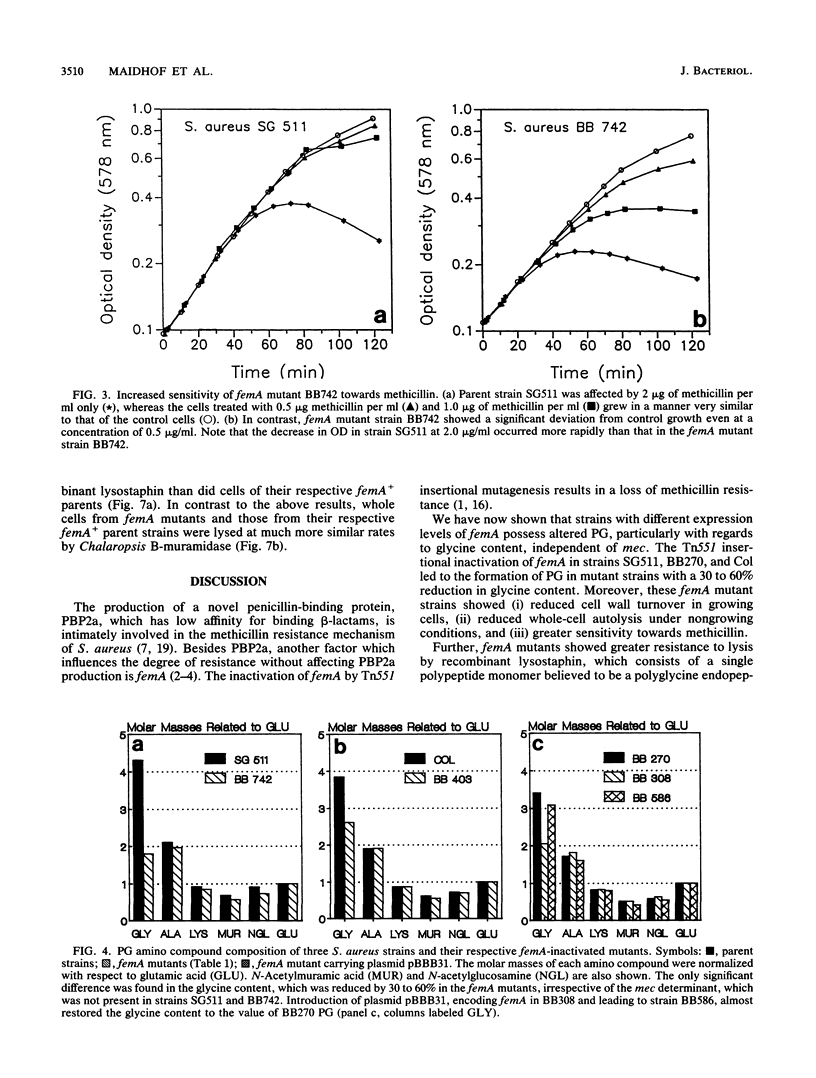
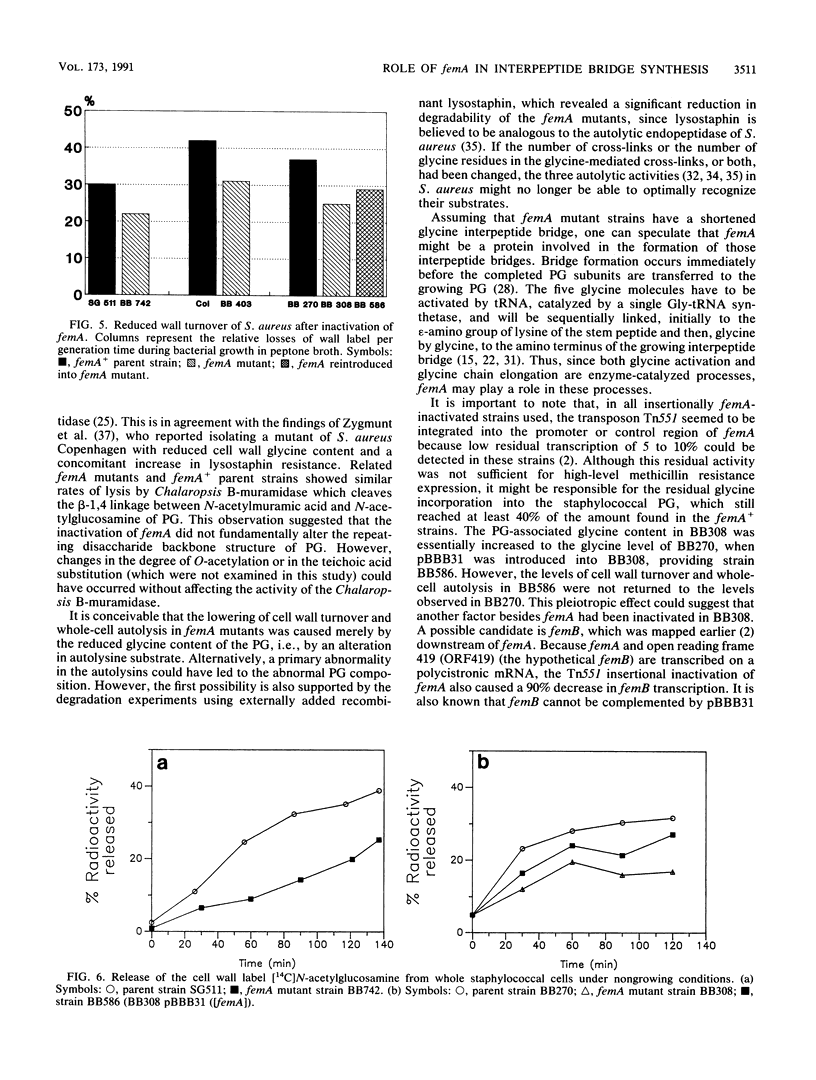

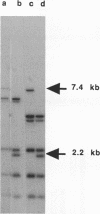
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B., Barberis-Maino L., Strässle A., Kayser F. H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989 Oct;219(1-2):263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Blümel P., Uecker W., Giesbrecht P. Zero order kinetics of cell wall turnover in Staphylococcus aureus. Arch Microbiol. 1979 May;121(2):103–110. doi: 10.1007/BF00689972. [DOI] [PubMed] [Google Scholar]
- Chambers H. F. Coagulase-negative staphylococci resistant to beta-lactam antibiotics in vivo produce penicillin-binding protein 2a. Antimicrob Agents Chemother. 1987 Dec;31(12):1919–1924. doi: 10.1128/aac.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Hackbarth C. J. Effect of NaCl and nafcillin on penicillin-binding protein 2a and heterogeneous expression of methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Dec;31(12):1982–1988. doi: 10.1128/aac.31.12.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F. Methicillin-resistant staphylococci. Clin Microbiol Rev. 1988 Apr;1(2):173–186. doi: 10.1128/cmr.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Sachdeva M. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990 Jun;161(6):1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisford W. C., Reynolds P. E. Methicillin resistance in Staphylococcus epidermidis. Relationship between the additional penicillin-binding protein and an attachment transpeptidase. Eur J Biochem. 1989 Oct 20;185(1):211–218. doi: 10.1111/j.1432-1033.1989.tb15104.x. [DOI] [PubMed] [Google Scholar]
- Giesbrecht P., Labischinski H., Wecke J. A special morphogenetic wall defect and the subsequent activity of "murosomes" as the very reason for penicillin-induced bacteriolysis in staphylococci. Arch Microbiol. 1985 May;141(4):315–324. doi: 10.1007/BF00428843. [DOI] [PubMed] [Google Scholar]
- HASH J. H. PURIFICATION AND PROPERTIES OF STAPHYLOLYTIC ENZYMES FROM CHALAROPSIS SP. Arch Biochem Biophys. 1963 Sep;102:379–388. doi: 10.1016/0003-9861(63)90245-5. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Matsuhashi M. The biosynthesis of the cross-linking peptides in the cell wall peptidoglycan of Staphylococcus aureus. J Biol Chem. 1972 Oct 10;247(19):6306–6311. [PubMed] [Google Scholar]
- Kornblum J., Hartman B. J., Novick R. P., Tomasz A. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol. 1986 Dec;5(6):714–718. doi: 10.1007/BF02013311. [DOI] [PubMed] [Google Scholar]
- Kuhl S. A., Pattee P. A., Baldwin J. N. Chromosomal map location of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1978 Aug;135(2):460–465. doi: 10.1128/jb.135.2.460-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju M. V., Brunner D. P., Wilkinson B. J. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Nov;31(11):1727–1733. doi: 10.1128/aac.31.11.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Tomasz A. Involvement of multiple genetic determinants in high-level methicillin resistance in Staphylococcus aureus. J Bacteriol. 1989 Feb;171(2):874–879. doi: 10.1128/jb.171.2.874-879.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Recsei P. A., Gruss A. D., Novick R. P. Cloning, sequence, and expression of the lysostaphin gene from Staphylococcus simulans. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1127–1131. doi: 10.1073/pnas.84.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Sidow T., Johannsen L., Labischinski H. Penicillin-induced changes in the cell wall composition of Staphylococcus aureus before the onset of bacteriolysis. Arch Microbiol. 1990;154(1):73–81. doi: 10.1007/BF00249181. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Rosenblum E. D. Genetic behavior of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1980 Dec;144(3):1200–1202. doi: 10.1128/jb.144.3.1200-1202.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike J., Park J. T. A method for demonstrating the stepwise addition of glycine from transfer RNA into the murein precursor of Staphylococcus aureus. Biochem Biophys Res Commun. 1969 Jun 6;35(5):642–647. doi: 10.1016/0006-291x(69)90452-5. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Nonoguchi R., Matsuhashi M., Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989 May;171(5):2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadstrom T., Vesterberg O. Studies on endo-beta-acetylglucosaminidase, staphylolytic peptidase, and N-acetylmuramyl-L-alanine amidase in lysostaphin and from Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):248–264. doi: 10.1111/j.1699-0463.1971.tb02152.x. [DOI] [PubMed] [Google Scholar]
- Wadström T., Hisatsune K. Bacteriolytic enzymes from Staphylococcus aureus. Purification of an endo-beta-N-acetylglucosaminidase. Biochem J. 1970 Dec;120(4):725–734. doi: 10.1042/bj1200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt W. A., Browder H. P., Tavormina P. A. Lytic action of lysostaphin on susceptible and resistant strains of Staphylococcus aureus. Can J Microbiol. 1967 Jul;13(7):845–853. doi: 10.1139/m67-111. [DOI] [PubMed] [Google Scholar]