Abstract
Valproic acid and lithium are effective antibipolar drugs. We recently showed that lithium stimulated the release of glutamate in monkey and mouse cerebral cortex slices, which, through activation of the N-methyl-d-aspartate receptor, increased accumulation of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3]. We show here that valproate behaves similarly to lithium in that at therapeutic concentrations it stimulates glutamate release and Ins(1,4,5)P3 accumulation in mouse cerebral cortex slices. The fact that these two effects are a common denominator for two structurally unrelated antibipolar drugs suggests that these effects are important in their antibipolar action. The effects of maximal concentrations of lithium and valproate on glutamate release are additive, suggesting different mechanisms for release, which are discussed. The additivity of the two drugs on glutamate release is consistent with the clinical benefit of combining the two drugs in the treatment of subsets of bipolar patients, e.g., in rapid cycling manic-depression. Unlike lithium, valproate does not increase accumulation of inositol monophosphates, inositol bisphosphates, or inositol 1,3,4-trisphosphate. This is additional evidence against the “inositol depletion” hypothesis, which states that, by trapping inositol in the form of inositol monophosphates and certain inositol polyphosphates, lithium exerts its antimanic action by inhibiting resynthesis of phosphoinositides with resultant blunting of Ins(1,4,5)P3 signaling.
Keywords: N-methyl-d-aspartate receptor, bipolar disorder, manic-depression, signaling
Bipolar disorder, or manic-depression, is a major medical problem. One percent of the population in the United States is afflicted with this disease, and one in five people with the disease commits suicide. The two FDA-approved antibipolar drugs, lithium and valproic acid, are equally effective in stabilizing mood in people with bipolar disorder, in spite of the drugs’ totally different structures. Numerous biochemical effects of lithium have been reported, e.g., modulation of sodium, potassium-activated ATPase, increase in cAMP, modulation of G proteins, down-regulation of protein kinase C, and changes in phosphoinositide metabolism (for a review, see ref. 1). We previously showed that lithium stimulated accumulation of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] in cerebral cortex slices of guinea pig and rabbit (2) and rhesus monkey (3, 4) in the presence or absence of cholinergic-muscarinic agonists such as acetylcholine or carbamylcholine and in human neuroblastoma cells (5) in the presence of acetylcholine. However, as reported earlier (6), lithium inhibited accumulation of Ins(1,4,5)P3 in mouse or rat cerebral cortex slices in the presence of a cholinergic agonist (2), but we found that if the incubation medium was supplemented with inositol, lithium also stimulated accumulation of Ins(1,4,5)P3 (2). If a cholinergic agonist was omitted, lithium increased accumulation of Ins(1,4,5)P3 in mouse or rat cerebral cortex slices in the absence of supplemental inositol.
In the absence of cholinergic agonists, the increased accumulation of Ins(1,4,5)P3 was due to lithium-induced presynaptic release of the neurotransmitter glutamate, which in turn activated the N-methyl-d-aspartate (NMDA) receptor, as evidenced by the fact that antagonists to the NMDA receptor–channel complex selectively blocked the lithium-induced increase in Ins(1,4,5)P3 (4). Antagonists to other receptors were ineffective. Activation of the NMDA receptor is known to increase Ins(1,4,5)P3 accumulation in cerebral cortex (7) by increased influx of Ca2+, which activates phospholipase C.
Although valproate has been used for years in the treatment of epilepsy, the drug has only recently been used in the treatment of manic-depression. It has been known for some time that valproate increases γ-aminobutyric acid levels in many cerebral structures, and this has been suggested as a mechanism for valproate’s antiseizure activity (for reviews, see refs. 8 and 9). On the other hand, there are no convincing data on valproate’s possible effects on glutamate release. We felt that if the release of glutamate were a common denominator in the action of lithium and valproate, this would support the view that glutamate release and activation of the NMDA receptor would have therapeutic relevance. We have, therefore, compared the effects of lithium and valproate on glutamate release and accumulation of various inositol phosphates in cerebral cortex.
EXPERIMENTAL PROCEDURES
White male adult ICR mice (Harlan Sprague–Dawley) were killed by cervical dislocation. The brain was rapidly removed and cross-chopped cerebral cortex slices were prepared and incubated in batch as previously described (2). Nominally Ca2+-free Krebs–Henseleit bicarbonate saline (KHBS) was supplemented with 10 mM MgCl2 for slicing and restorative incubations because this buffer has been shown to prevent damage that occurs when slices are incubated in normal physiological buffer immediately after slicing (10). After the first 45 min of incubation, MgCl2 was returned to normal (1 mM).
Slices were then incubated with 0.25 mCi of myo-[3H]inositol (1 Ci = 37 GBq) in 5 ml for 60 min. At the end of this incubation, free label was removed by four sequential washes in 25 ml of KHBS. Slices were then divided into aliquots (1–2 mg average protein as measured by the bichichoninic acid method) and incubated in 3.2 ml of KHBS for 30 min. This incubation was repeated three times, with complete medium replacement between incubations, before treatment with valproic acid. Samples were then preincubated for 60 min with or without various concentrations of valproate. Ca2+ was added (1.3 mM final concentration), and the reaction was stopped after 20 min. Removal of medium for glutamate measurement, quenching with perchloric acid, extraction of inositol phosphates, and separation by HPLC were as previously described (2, 4). Data were normalized to total tritium disintegrations per minute.
Samples were prepared for enzymatic assay of glutamate as previously described (4). The glutamate assay described in ref. 4 was changed to improve the signal-to-noise ratio (permitting higher amplification of the fluorescence signal and therefore higher sensitivity) and also changed to increase the number of samples that could be assayed. We adopted the glutamate dehydrogenase assay described by Passonneau and Lowry (11) with the following modifications. The reaction was carried out entirely in disposable methacrylate cuvettes (4 ml) in a volume of 1 ml containing 0.2–0.5 ml of sample (water was added to bring all samples to 0.5 ml), 50 mM Tris⋅acetate, pH 8.4 [the blank fluorescence of reagent grade Tris is more than an order of magnitude lower than that of “Trizma” grade (Sigma)]/0.15 mM NAD+/0.1 mM ADP/0.1 mM EDTA/1 unit/ml glutamate dehydrogenase. Blank fluorescence of each sample and standard was measured 2 min after the start of the reaction (excitation wavelength = 340 nm; emission wavelength = 453 nm). The samples were then kept for 1 hr at room temperature before the final fluorescence reading. The new normal range of the assay was 0–0.1 nmol of l-glutamate. All values are the means ± SEM of triplicate or quadruplicate slice incubations. All experiments were repeated at least three times, and the values are either presented as an averaged percent effect from several experiments or presented as absolute values from representative experiments.
RESULTS AND DISCUSSION
Effects of Valproate on Accumulation of Extracellular Glutamate and Intracellular Ins(1,4,5)P3 in Mouse Cerebral Cortex Slices.
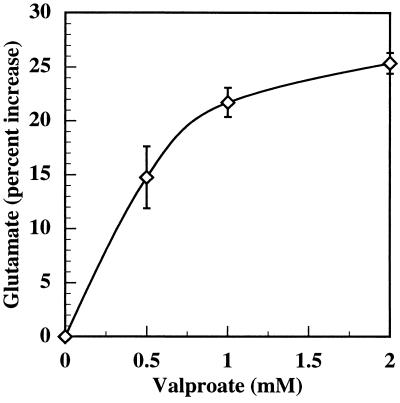
Increasing concentrations of valproate progressively increased glutamate release up to 1 mM, followed by a plateau (Fig. 1). The therapeutic concentration of valproate in the blood is as high as 0.7 mM (12). The stimulation of glutamate accumulation by valproate was greater at 2 hr than at 1 hr (data not shown).
Figure 1.
Effect of valproate on glutamate release from mouse cerebral cortex slices. Slices were prepared, restored, and prelabeled as described in Experimental Procedures. Slices (average protein = 1.5 mg) were washed by incubating four times for 30 min in fresh buffer (3.2 ml). After washing, the slices were preincubated in the absence of Ca2+ with the indicated concentrations of sodium valproate for 60 min. The incubations were continued for 20 min with 1.2 mM Ca2+. Aliquots of the medium free of slices were analyzed enzymatically for glutamate as described under Experimental Procedures. 3H disintegrations per minute in the medium and tissue were measured, and the values were normalized to total disintegrations per minute to correct for differences in amounts of tissue. This figure combines three separate experiments.
Table 1 shows in a separate experiment the effect of valproate on glutamate release and Ins(1,4,5)P3 accumulation. Valproate increased glutamate release by 31% and Ins(1,4,5)P3 accumulation by 22%. Both effects were highly significant. In these two respects, lithium and valproate act similarly. The fact that lithium and valproate, two structurally unrelated drugs, enhance glutamate release and Ins(1,4,5)P3 accumulation suggests that these effects may be involved in their antibipolar activity.
Table 1.
Effect of valproate on glutamate release and [3H]Ins(1,4,5)P3 levels
| Glutamate | [3H]Ins(1,4,5)P3 | |
|---|---|---|
| Control | 139 ± 5 nM | 1,340 ± 62 dpm |
| Valproate (2 mM) | 183 ± 11 nM | 1,640 ± 28 dpm |
| Increase | 31.4% | 22% |
| P value for increase | 0.008 | 0.003 |
Mouse cerebral cortex slices were prepared, prelabeled, and incubated as described in Experimental Procedures and in Fig. 1 with these modifications. myo-[3H]inositol was 0.1 mCi/ml during prelabeling. After aliquots of medium were taken for enzymatic determination of glutamate, the reaction was stopped with 3% perchloric acid, and the acid soluble extract was treated as described in Experimental Procedures.
Effects of Combination of Lithium and Valproate on Glutamate Release.
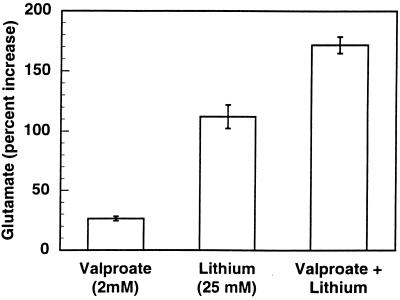
Do valproate and lithium increase extracellular glutamate by the same mechanism? To test this, we studied the effect of maximally effective concentrations of each drug on glutamate release separately and in combination (Fig. 2). If valproic acid and lithium released glutamate by separate mechanisms, one would predict that their effects on glutamate would be additive at maximally effective concentrations (over 10 times the maximal therapeutic concentration for lithium and twice the therapeutic concentration for valproate), whereas no additivity would suggest a similar mechanism for glutamate release. When combined, there was at least an additive effect of the two drugs. This result suggests that the two drugs elevate glutamate by different mechanisms. It has been reported (13) that valproate stimulates glutaminase and inhibits glutamine synthetase activities in primary cultures of rat brain astrocytes, leading to increased intracellular glutamate, which could account for the elevated level of extracellular glutamate in cerebral cortex slices in the presence of valproate. It has also been reported that valproate lowered the Km and the Vmax for glutamate uptake in rat astroglial primary cultures in acute but not chronic experiments (14). We were unable to find any acute effects of valproate on these kinetic parameters in cerebral cortex slices (unpublished observations). On the other hand, we were able to show an inhibition by lithium of glutamate uptake in cortical slices and synaptosomes (unpublished observations). These separate mechanisms for elevating extracellular glutamate could account for the additivity of the two drugs on glutamate release. With regard to the clinical significance of the additive effect of valproate and lithium on glutamate release, it has been reported that the combination of valproate and lithium is more effective than either drug alone in some patients showing inadequate response to monotherapy, e.g., in rapid cycling bipolar disorder (15).
Figure 2.
Effects of high valproate (2 mM) and high lithium (25 mM) concentrations separately and in combination on extracellular glutamate. Mouse cerebral cortex slices were prepared, restored, prelabeled, and incubated as described in Experimental Procedures and in Fig. 1. Glutamate levels in the media of drug-treated slices all differed significantly from the control (P < 0.001). The percent increase above the control is shown (control, glutamate = 106 ± 1 nM).
Comparison of the Effects of Lithium and Valproate on Accumulation of Inositol Monophosphates, Inositol Bisphosphates, and Inositol 1,3,4-Trisphosphate.
Lithium, through its inhibition of inositol monophosphatase and inositol 1,4-bisphosphate- and inositol 1,3,4-trisphosphate 1-phosphomonoesterases, causes accumulation of the respective inositol phosphates (16, 17). As shown in Table 2, in the presence of carbachol, lithium exerted a strong enhancing effect on accumulation of inositol mono- and bisphosphates as well as inositol 1,3,4-trisphosphate. On the other hand, in the same experiment, valproate did not increase accumulation of these inositol phosphates. Similar results were found if carbachol was omitted (data not shown). Recently, it has been shown that valproate does not inhibit inositol monophosphatase in cell-free enzyme preparations from brain (18). Thus, inhibition of inositol monophosphatase and selective inositol polyphosphate 1-phosphatases with enhancement of accumulation of their respective inositol phosphates does not appear to be essential for antibipolar activity, at least in the case of valproic acid.
Table 2.
Effects of valproate and lithium on [3H]inositol phosphates in carbachol-stimulated mouse cerebral cortical slices
| Inositol phosphates | Carbachol | Valproate + carbachol | Lithium + carbachol |
|---|---|---|---|
| Monophosphates | 53,000 ± 1,320 | 50,700 ± 917 | 128,400 ± 4,300 |
| Ins(1,3)P2 | 495 ± 13 | 455 ± 34 | 1,340 ± 46 |
| Ins(1,4)P2 | 10,700 ± 132 | 11,800 ± 132 | 85,200 ± 2,480 |
| Ins(1,3,4)P3 | 201 ± 19 | 234 ± 10 | 402 ± 18 |
Values represent 3H disintegrations per minute in inositol phosphates. Prelabeled mouse brain cortical slices were preincubated for 12 min with or without sodium valproate (2 mM) or LiCl (10 mM) in KHBS (1.2 mM in CaCl2). The reaction was continued for 12 min with carbamylcholine chloride (1 mM), then quenched with perchloric acid. The [3H]inositol phosphates in the neutralized extract were separated by HPLC. In each of three experiments, brains from six mice were used, and 0.4 mM myo-[3H]inositol was present during prelabeling. See Experimental Procedures for detailed methods.
A prevalent hypothesis for the antimanic action of lithium (the mechanism of its antidepressive effect is not explained) has been the “inositol depletion” hypothesis, which states that accumulation of the above nonmessenger inositol phosphates traps enough inositol in the phosphorylated form to inhibit phosphatidylinositol synthesis sufficiently to lower Ins(1,4,5)P3 signaling (19). This has been postulated to exert an antimanic effect via inhibition of Ins(1,4,5)P3 postsynaptic signaling, which is coupled to the neurotransmitter, acetylcholine, and possibly to other neurotransmitters which lead to activation of phospholipase C. Although inhibitions of Ins(1,4,5)P3 and inositol 1,3,4,5-tetrakisphosphate accumulations by lithium have been shown in rat and mouse cerebral cortex slices incubated in the presence of high concentrations of cholinergic agonists (2, 6), it is important to note that if cholinergic agonists were omitted, lithium increased Ins(1,4,5)P3 accumulation in mouse cerebral cortex slices (4). In the higher species, including man and lower primates, lithium increased Ins(1,4,5)P3 in the presence or absence of cholinergic agonists. The high concentrations of cholinergic agonists, which have been routinely used, stimulate a large breakdown of phosphatidylinositol, trapping considerable inositol as inositol phosphates in the presence of lithium. Also, in astrocytoma cells, cholinergic agonists inhibit the uptake of inositol (20). Thus, under conditions of high concentrations of cholinergic agonists and in the presence of lithium, rat and mouse cerebral cortex slices, unlike slices in higher species (2–4), are incapable of retaining sufficient inositol to maintain normal levels of Ins(1,4,5)P3.
Lithium produces dramatic morphogenic defects in early development in numerous organisms, and it has been suggested that inositol depletion through inhibition of inositol monophosphatase is responsible (19). However, Klein and Melton (21) recently showed that inositol monophosphatase could be completely inhibited by a specific inhibitor, L-690.330, without producing any developmental defects. Rather, evidence is presented that the selective inhibition of glycogen synthetase kinase-3 β is involved in lithium’s teratogenic effects. This is additional evidence that inhibition of inositol monophosphatase may not be important in lithium’s biological effects.
It is not difficult to visualize how increased release of the excitatory neurotransmitter glutamate might exert an antidepressive effect. How might elevated glutamate exert an antimanic effect? It is generally believed that antidepressants exert their mood-elevating effect, not by the immediate increase in synaptic serotonin and norepinephrine, but by the gradual down-regulation of selective receptors. This is consonant with their delayed antidepressive effect. It is possible that chronic elevation of glutamate and Ins(1,4,5)P3 might down-regulate their respective receptors, which could exert an antimanic effect by decreasing intracellular calcium. We have observed a small but statistically significant down-regulation of the Ins(1,4,5)P3 receptor in the cerebral cortices of mice chronically treated with lithium (G. V. Los and L.E.H., unpublished observations).
Activation of the NMDA receptor affords some central nervous system selectivity because NMDA receptors are largely, but not exclusively, confined to the central nervous system. Stimulation of this receptor would have widespread ramifications in the brain through increased influx of Ca2+ in NMDA-bearing neurons and their known crosstalk with other neurotransmitter systems.
Acknowledgments
We are grateful to Terry Hanlon for typing, Karen Wipperfurth for proofreading and editing, and Connie Bowes for technical assistance. This work was supported by a grant from the National Institutes of Health (HL 16318) and an Established Investigatorship to L.E.H. from the National Alliance for Research on Schizophrenia and Depression.
ABBREVIATIONS
- Ins(1
4,5)P3, inositol 1,4,5-trisphosphate
- KHBS
Krebs–Henseleit bicarbonate saline
- NMDA
N-methyl-d-aspartate
References
- 1.Jope R S, Williams M B. Biochem Pharmacol. 1994;47:429–441. doi: 10.1016/0006-2952(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee C H, Dixon J F, Reichman M, Moummi C, Los G V, Hokin L E. Biochem J. 1992;282:377–385. doi: 10.1042/bj2820377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon J F, Lee C H, Los G V, Hokin L E. J Neurochem. 1992;59:2332–2335. doi: 10.1111/j.1471-4159.1992.tb10129.x. [DOI] [PubMed] [Google Scholar]
- 4.Dixon J F, Los G V, Hokin L E. Proc Natl Acad Sci USA. 1994;91:8358–8362. doi: 10.1073/pnas.91.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Los G V, Artemenko P, Hokin L E. Biochem J. 1995;311:225–232. doi: 10.1042/bj3110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy E D, Challiss J R A, Nahorski S R. J Neurochem. 1989;53:1652–1655. doi: 10.1111/j.1471-4159.1989.tb08566.x. [DOI] [PubMed] [Google Scholar]
- 7.Mayer M L, Miller R J. Trends Pharmacol Sci. 1990;11:254–260. doi: 10.1016/0165-6147(90)90254-6. [DOI] [PubMed] [Google Scholar]
- 8.Cotariu D, Zaidman J L, Evans S. Prog Neurobiol. 1990;34:343–354. doi: 10.1016/0301-0082(90)90010-e. [DOI] [PubMed] [Google Scholar]
- 9.Loscher W. Neurochem Res. 1993;18:485–502. doi: 10.1007/BF00967253. [DOI] [PubMed] [Google Scholar]
- 10.Feig S, Lipton P. J Neurochem. 1990;55:473–483. doi: 10.1111/j.1471-4159.1990.tb04160.x. [DOI] [PubMed] [Google Scholar]
- 11.Passonneau J V, Lowry O H. Enzymatic Analysis: A Practical Guide. Totowa, NJ: Humana; 1993. pp. 167–170. [Google Scholar]
- 12.Baldessarini R J. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th Ed. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. p. 452. [Google Scholar]
- 13.Collins R M, Jr, Zielke H R, Woody R C. J Neurochem. 1994;62:1137–1143. doi: 10.1046/j.1471-4159.1994.62031137.x. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson M, Hansson E, Ronnback L. Neurochem Res. 1992;17:327–332. doi: 10.1007/BF00974573. [DOI] [PubMed] [Google Scholar]
- 15.Sharma V, Persad E, Mazmanian D, Karunaratne K. Can J Psychiatry. 1993;38:137–139. doi: 10.1177/070674379303800213. [DOI] [PubMed] [Google Scholar]
- 16.Sherman W R, Gish B G, Honchar M P, Munsell L Y. Fed Proc. 1986;45:2639–2646. [PubMed] [Google Scholar]
- 17.Sherman W R. In: Lithium and the Cell: Pharmacology and Biochemistry. Birch N J, editor. New York: Academic; 1991. pp. 121–157. [Google Scholar]
- 18.Vadnal R, Parthasarathy R. Neuropsychopharmacology. 1995;12:2777–2785. doi: 10.1016/0893-133X(94)00088-H. [DOI] [PubMed] [Google Scholar]
- 19.Berridge M J, Downes C P, Hanley M R. Cell. 1989;53:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 20.Batty I H, Michie A, Fennel M, Downes C P. Biochem J. 1993;294:49–55. doi: 10.1042/bj2940049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein P S, Melton D A. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]