Abstract
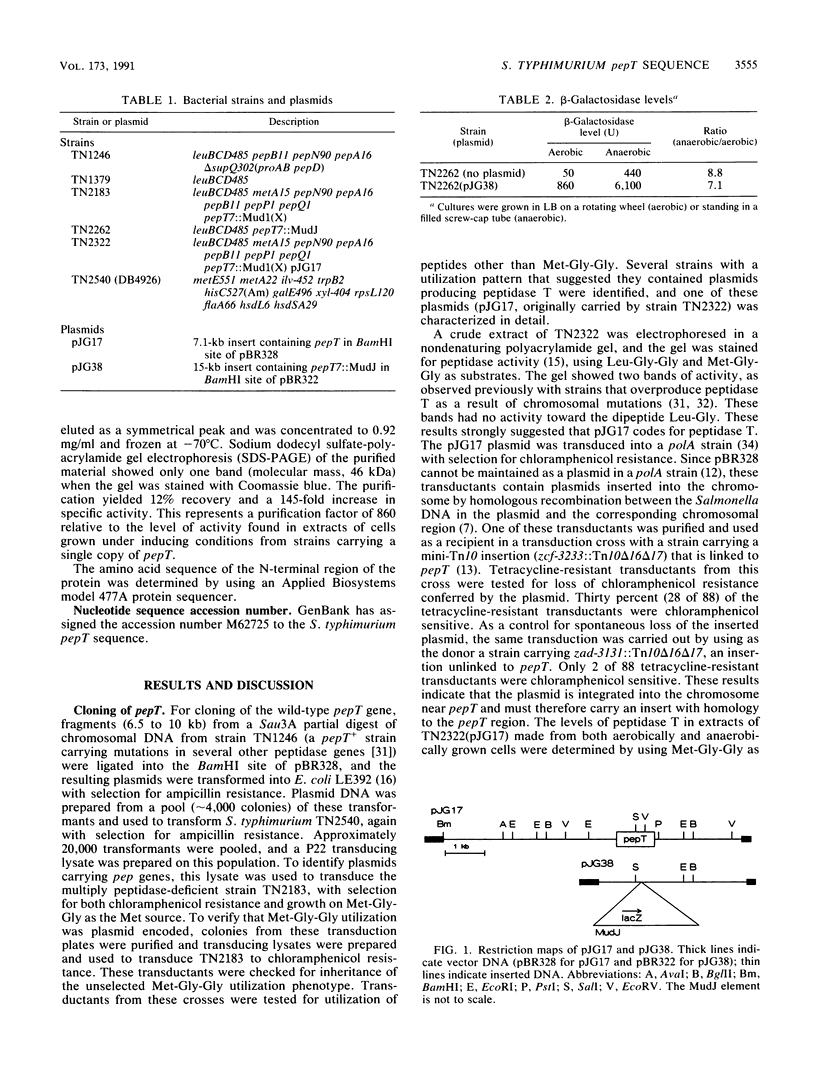
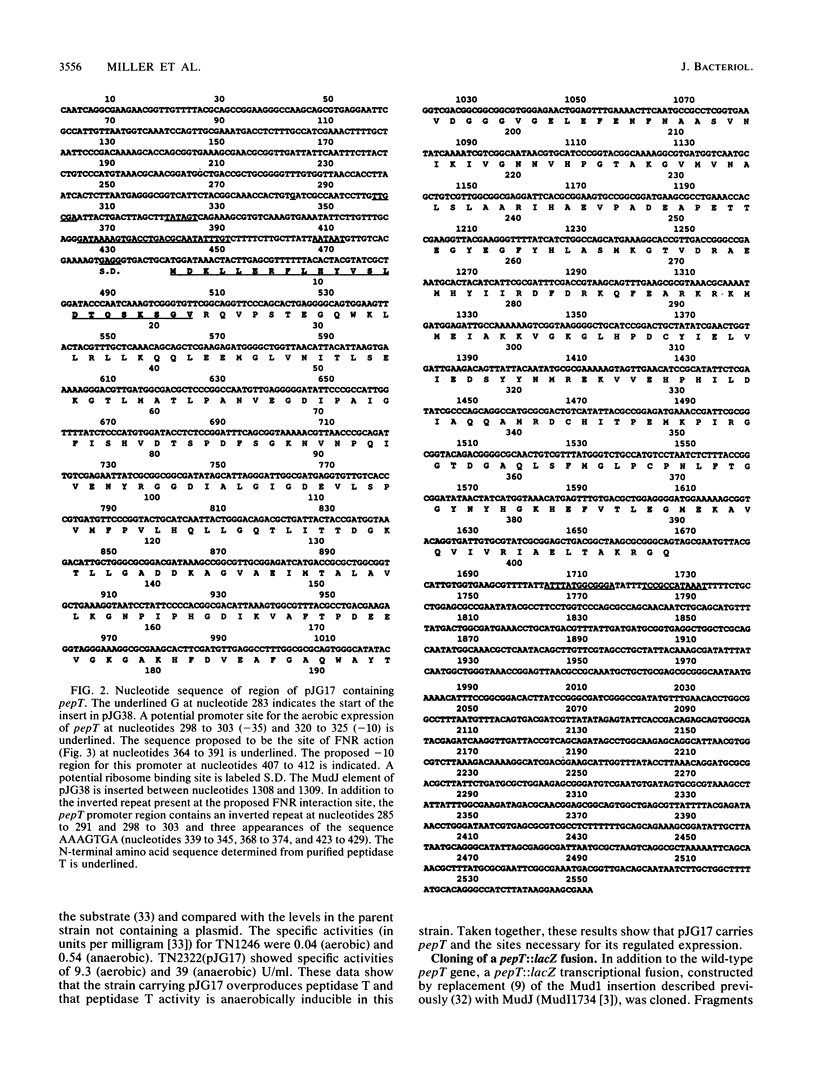
The anaerobically regulated pepT gene of Salmonella typhimurium has been cloned in pBR328. Strains carrying the pepT plasmid, pJG17, overproduce peptidase T by approximately 70-fold. The nucleotide sequence of a 2.5-kb region including pepT has been determined. The sequence codes for a protein of 44,855 Da, consistent with a molecular weight of approximately 46,000 for peptidase T (as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and gel filtration). The N-terminal amino acid sequence of peptidase T purified from a pJG17-containing strain matches that predicted by the nucleotide sequence. A plasmid carrying an anaerobically regulated pepT::lacZ transcriptional fusion contains only 165 bp 5' to the start of translation. This region contains a sequence highly homologous to that identified in Escherichia coli as the site of action of the FNR protein, a positive regulator of anaerobic gene expression. A region of the deduced amino acid sequence of peptidase T is similar to segments of Pseudomonas carboxypeptidase G2, the E. coli peptidase encoded by the iap gene, and E. coli peptidase D.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bally M., Foglino M., Bruschi M., Murgier M., Lazdunski A. Nucleotide sequence of the promoter and amino-terminal encoding region of the Escherichia coli pepN gene. Eur J Biochem. 1986 Mar 17;155(3):565–569. doi: 10.1111/j.1432-1033.1986.tb09525.x. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat A., Bauer K., Chang S. Y., Myambo K., Boosman A., Chang S. Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol. 1987 Feb;169(2):751–757. doi: 10.1128/jb.169.2.751-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiglmeier K., Honoré N., Iuchi S., Lin E. C., Cole S. T. Molecular genetic analysis of FNR-dependent promoters. Mol Microbiol. 1989 Jul;3(7):869–878. doi: 10.1111/j.1365-2958.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Gutterson N. I., Koshland D. E., Jr Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4894–4898. doi: 10.1073/pnas.80.16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich B., Monnerjahn U., Plapp R. Peptidase D gene (pepD) of Escherichia coli K-12: nucleotide sequence, transcript mapping, and comparison with other peptidase genes. J Bacteriol. 1990 Aug;172(8):4641–4651. doi: 10.1128/jb.172.8.4641-4651.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino Y., Shinagawa H., Makino K., Amemura M., Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987 Dec;169(12):5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kukral A. M., Strauch K. L., Maurer R. A., Miller C. G. Genetic analysis in Salmonella typhimurium with a small collection of randomly spaced insertions of transposon Tn10 delta 16 delta 17. J Bacteriol. 1987 May;169(5):1787–1793. doi: 10.1128/jb.169.5.1787-1793.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Harris H. Human red cell peptidases. Nature. 1967 Jul 22;215(5099):351–355. doi: 10.1038/215351a0. [DOI] [PubMed] [Google Scholar]
- McCaman M. T., Gabe J. D. The nucleotide sequence of the pepN gene and its over-expression in Escherichia coli. Gene. 1986;48(1):145–153. doi: 10.1016/0378-1119(86)90360-4. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Minton N. P., Atkinson T., Bruton C. J., Sherwood R. F. The complete nucleotide sequence of the Pseudomonas gene coding for carboxypeptidase G2. Gene. 1984 Nov;31(1-3):31–38. doi: 10.1016/0378-1119(84)90192-6. [DOI] [PubMed] [Google Scholar]
- Movva N. R., Semon D., Meyer C., Kawashima E., Wingfield P., Miller J. L., Miller C. G. Cloning and nucleotide sequence of the Salmonella typhimurium pepM gene. Mol Gen Genet. 1990 Sep;223(2):345–348. doi: 10.1007/BF00265075. [DOI] [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. doi: 10.1099/00221287-106-1-1. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R. Amplification and product identification of the fnr gene of Escherichia coli. J Gen Microbiol. 1982 Oct;128(10):2221–2228. doi: 10.1099/00221287-128-10-2221. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990 Aug;6(4):399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988 Jun;52(2):190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling C. J., Colloms S. D., Collins J. F., Szatmari G., Sherratt D. J. xerB, an Escherichia coli gene required for plasmid ColE1 site-specific recombination, is identical to pepA, encoding aminopeptidase A, a protein with substantial similarity to bovine lens leucine aminopeptidase. EMBO J. 1989 May;8(5):1623–1627. doi: 10.1002/j.1460-2075.1989.tb03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Carter T. H., Miller C. G. Overproduction of Salmonella typhimurium peptidase T. J Bacteriol. 1983 Nov;156(2):743–751. doi: 10.1128/jb.156.2.743-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Miller C. G. Isolation and characterization Salmonella typhimurium mutants lacking a tripeptidase (peptidase T). J Bacteriol. 1983 May;154(2):763–771. doi: 10.1128/jb.154.2.763-771.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield H. J., Levine G. Isolation and characterization of a mutant of Salmonella typhimurium deficient in a major deoxyribonucleic acid polymerase activity. J Bacteriol. 1973 Oct;116(1):54–58. doi: 10.1128/jb.116.1.54-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Tone H., Honda T., Osatomi K., Kobayashi R., Tsuru D. Sequencing and high expression of aminopeptidase P gene from Escherichia coli HB101. J Biochem. 1989 Mar;105(3):412–416. doi: 10.1093/oxfordjournals.jbchem.a122678. [DOI] [PubMed] [Google Scholar]



