Abstract
The speA gene of Escherichia coli encodes biosynthetic arginine decarboxylase (ADC), the first of two enzymes in a putrescine biosynthetic pathway. The activity of ADC is negatively regulated by mechanisms requiring cyclic AMP (cAMP) and cAMP receptor protein (CRP) or putrescine. A 2.1-kb BamHI fragment containing the speA-metK intergenic region, speA promoter, and 1,389 bp of the 5' end of the speA coding sequence was used to construct transcriptional and translational speA-lacZ fusion plasmids. A single copy of either type of speA-lacZ fusion was transferred into the chromosomes of Escherichia coli KC14-1, CB806, and MC4100, using bacteriophage lambda. The speA gene in lysogenized strains remained intact and served as a control. Addition of 5 mM cAMP to lysogenic strains resulted in 10 to 37% inhibition of ADC activity, depending on the strain used. In contrast, the addition of 5 or 10 mM cAMP to these strains did not inhibit the activity of beta-galactosidase (i.e., ADC::beta-galactosidase). Addition of 10 mM putrescine to lysogenized strains resulted in 24 to 31% repression of ADC activity and 41 to 47% repression of beta-galactosidase activity. E. coli strains grown in 5 mM cAMP and 10 mM putrescine produced 46 to 61% less ADC activity and 41 to 52% less beta-galactosidase activity. cAMP (0.1 to 10 mM) did not inhibit ADC activity assayed in vitro. The effects of cAMP and putrescine on ADC activity were additive, indicating the use of independent regulatory mechanisms. These results show that cAMP acts indirectly to inhibit ADC activity and that putrescine causes repression of speA transcription.
Full text
PDF

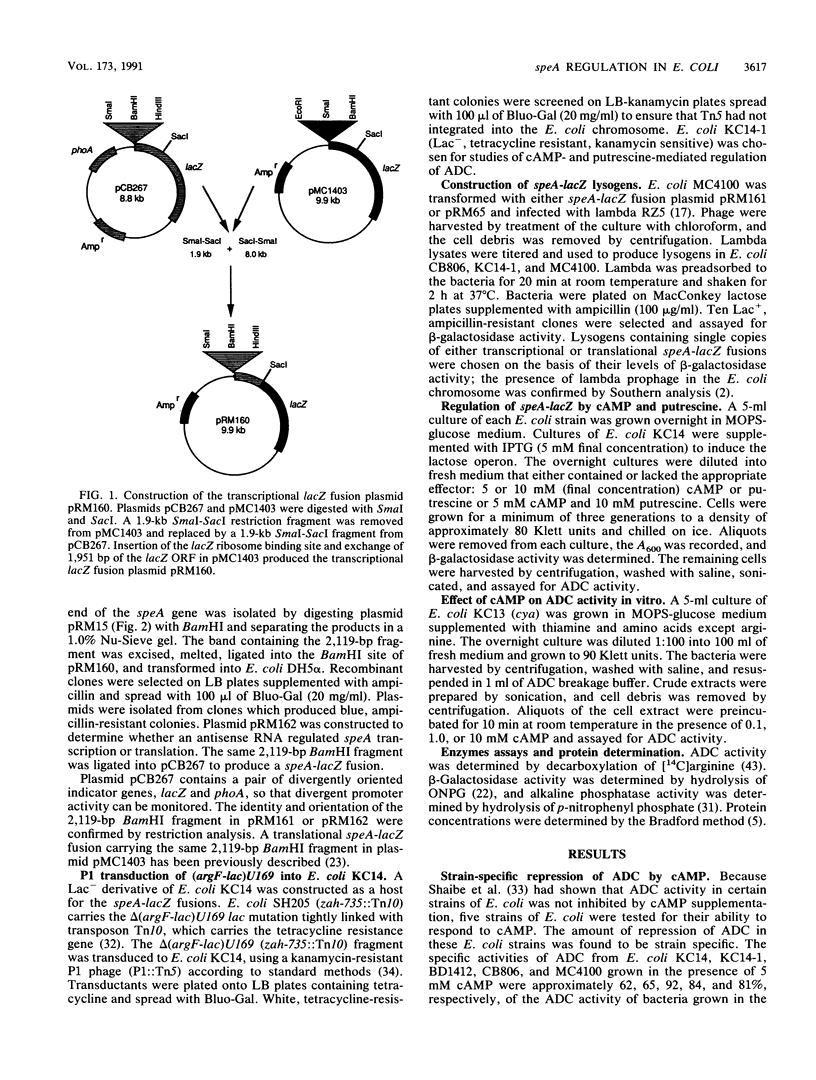

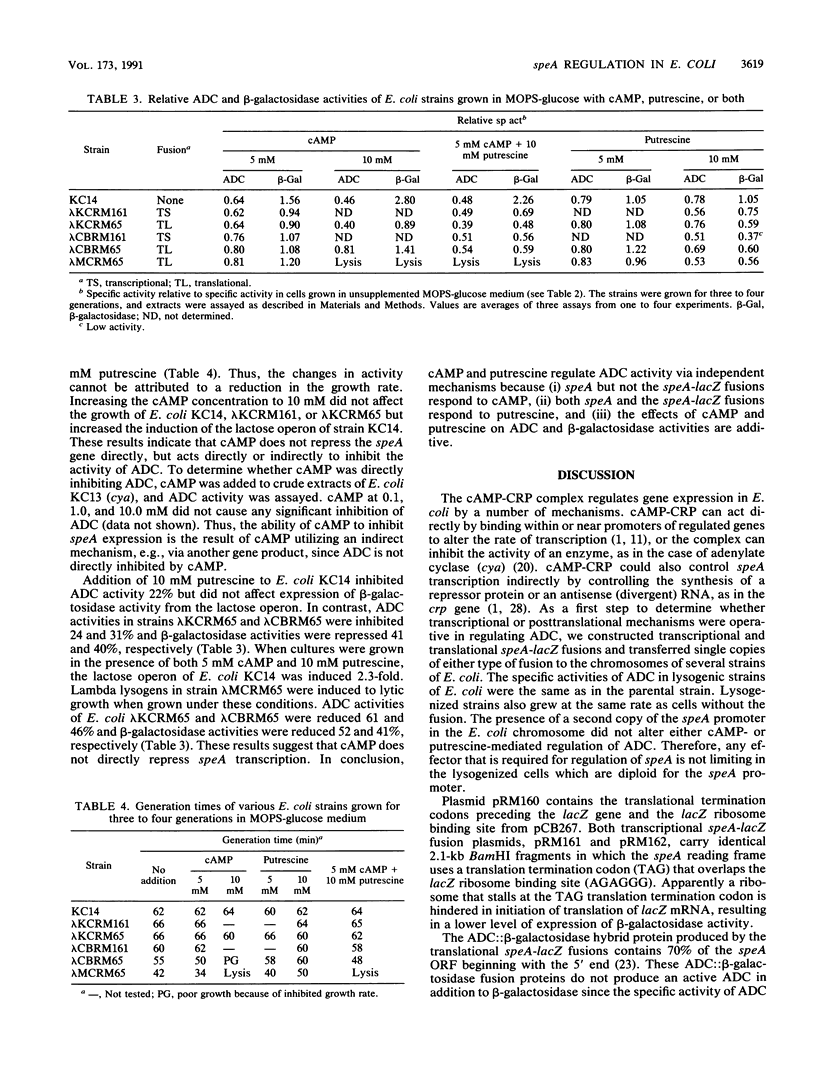


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H. Autoregulation of the Escherichia coli crp gene: CRP is a transcriptional repressor for its own gene. Cell. 1983 Jan;32(1):141–149. doi: 10.1016/0092-8674(83)90504-4. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., MacIntyre M. F., Sells B. H. Polyamine levels in Escherichia coli during nutritional shiftup and exponential growth. Biochim Biophys Acta. 1977 Aug 2;477(3):221–227. doi: 10.1016/0005-2787(77)90047-8. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Markham G. D., Hafner E. W., Wright J. M., Tabor H., Tabor C. W. Expression of the cloned genes encoding the putrescine biosynthetic enzymes and methionine adenosyltransferase of Escherichia coli (speA, speB, speC and metK). Gene. 1984 Oct;30(1-3):129–136. doi: 10.1016/0378-1119(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buch J. K., Boyle S. M. Biosynthetic arginine decarboxylase in Escherichia coli is synthesized as a precursor and located in the cell envelope. J Bacteriol. 1985 Aug;163(2):522–527. doi: 10.1128/jb.163.2.522-527.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Jaffé A., Bouloc P., Robin A. Cyclic AMP and cell division in Escherichia coli. J Bacteriol. 1988 Jan;170(1):65–70. doi: 10.1128/jb.170.1.65-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Amino acid sequence of beta-galactosidase. XI. Peptide ordering procedures and the complete sequence. J Biol Chem. 1978 Aug 10;253(15):5521–5525. [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C., Gronenborn A. M., Clore G. M. The binding of the cyclic AMP receptor protein to synthetic DNA sites containing permutations in the consensus sequence TGTGA. Biochem J. 1987 Aug 15;246(1):227–232. doi: 10.1042/bj2460227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987 Jul;169(7):3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K., Igarashi K. Nonspecific inhibition of Escherichia coli ornithine decarboxylase by various ribosomal proteins: detection of a new ribosomal protein possessing strong antizyme activity. Biochim Biophys Acta. 1987 Jan 30;911(2):180–190. doi: 10.1016/0167-4838(87)90007-0. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Bankaitis V., Brown C. S., Montefiori D. Imidazole acetic acid as a substitute for cAMP. Biochem Biophys Res Commun. 1979 Mar 30;87(2):566–574. doi: 10.1016/0006-291x(79)91832-1. [DOI] [PubMed] [Google Scholar]
- Majerfeld I. H., Miller D., Spitz E., Rickenberg H. V. Regulation of the synthesis of adenylate cyclase in Escherichia coli by the cAMP -- cAMP receptor protein complex. Mol Gen Genet. 1981;181(4):470–475. doi: 10.1007/BF00428738. [DOI] [PubMed] [Google Scholar]
- Moore R. C., Boyle S. M. Nucleotide sequence and analysis of the speA gene encoding biosynthetic arginine decarboxylase in Escherichia coli. J Bacteriol. 1990 Aug;172(8):4631–4640. doi: 10.1128/jb.172.8.4631-4640.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Putrescine biosynthesis in Escherichia coli. Regulation through pathway selection. J Biol Chem. 1969 Nov 25;244(22):6094–6099. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Freundlich M. Mechanism for the autogenous control of the crp operon: transcriptional inhibition by a divergent RNA transcript. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5000–5004. doi: 10.1073/pnas.83.14.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotidis C. A., Canellakis E. S. Comparison of the basic Escherichia coli antizyme 1 and antizyme 2 with the ribosomal proteins S20/L26 and L34. J Biol Chem. 1984 Dec 25;259(24):15025–15027. [PubMed] [Google Scholar]
- Satishchandran C., Boyle S. M. Antagonistic transcriptional regulation of the putrescine biosynthetic enzyme agmatine ureohydrolase by cyclic AMP and agmatine in Escherichia coli. J Bacteriol. 1984 Feb;157(2):552–559. doi: 10.1128/jb.157.2.552-559.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Beck C. F. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene. 1986;42(1):37–48. doi: 10.1016/0378-1119(86)90148-4. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Transfer of the delta (argF-lac)U169 mutation between Escherichia coli strains by selection for a closely linked Tn10 insertion. Mol Gen Genet. 1983;192(1-2):293–294. doi: 10.1007/BF00327683. [DOI] [PubMed] [Google Scholar]
- Shaibe E., Metzer E., Halpern Y. S. Control of utilization of L-arginine, L-ornithine, agmatine, and putrescine as nitrogen sources in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):938–942. doi: 10.1128/jb.163.3.938-942.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Szumanski M. B., Boyle S. M. Analysis and sequence of the speB gene encoding agmatine ureohydrolase, a putrescine biosynthetic enzyme in Escherichia coli. J Bacteriol. 1990 Feb;172(2):538–547. doi: 10.1128/jb.172.2.538-547.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W., Markham G. D., Boyle S. M. Cloning of the Escherichia coli genes for the biosynthetic enzymes for polyamines. Methods Enzymol. 1983;94:117–121. doi: 10.1016/s0076-6879(83)94019-3. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- Wright J. M., Boyle S. M. Negative control of ornithine decarboxylase and arginine decarboxylase by adenosine-3':5'-cyclic monophosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):482–487. doi: 10.1007/BF00337952. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]


