Abstract
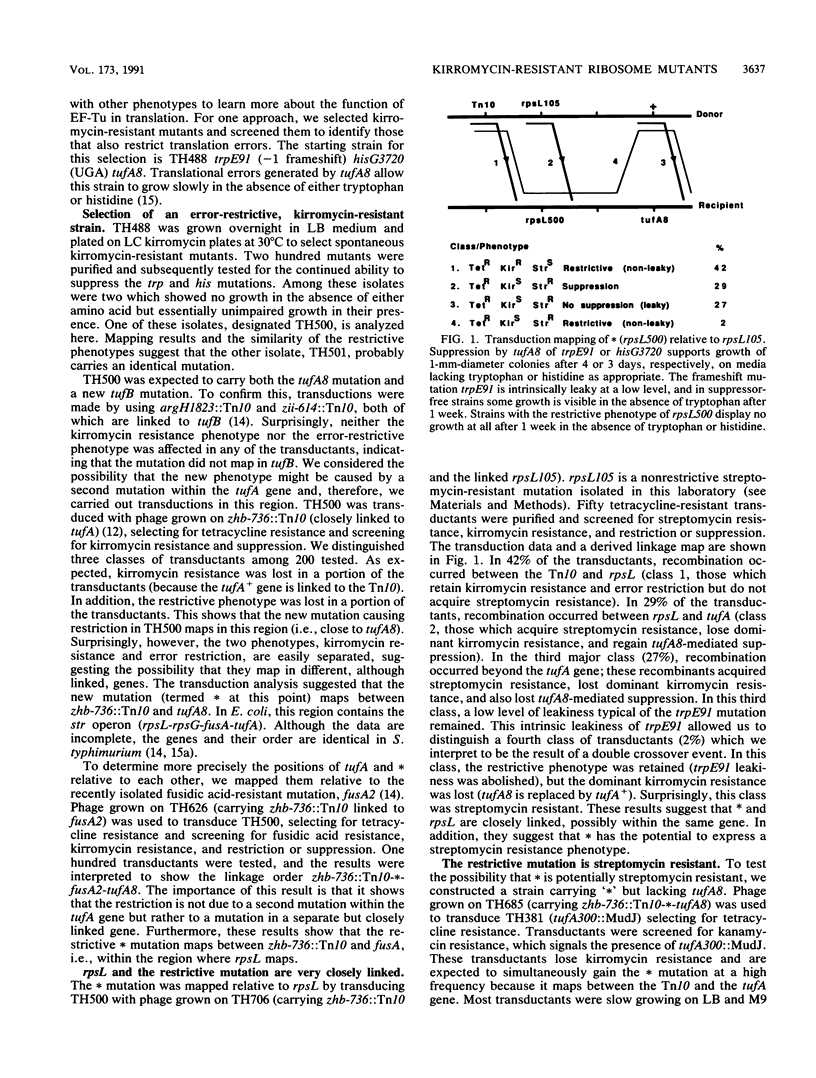
Mutations in the two genes for EF-Tu in Salmonella typhimurium and Escherichia coli, tufA and tufB, can confer resistance to the antibiotic kirromycin. Kirromycin resistance is a recessive phenotype expressed when both tuf genes are mutant. We describe a new kirromycin-resistant phenotype dominant to the effect of wild-type EF-Tu. Strains carrying a single kirromycin-resistant tuf mutation and an error-restrictive, streptomycin-resistant rpsL mutation are resistant to high levels of kirromycin, even when the other tuf gene is wild type. This phenotype is dependent on error-restrictive mutations and is not expressed with nonrestrictive streptomycin-resistant mutations. Kirromycin resistance is also expressed at a low level in the absence of any mutant EF-Tu. These novel phenotypes exist as a result of differences in the interactions of mutant and wild-type EF-Tu with the mutant ribosomes. The restrictive ribosomes have a relatively poor interaction with wild-type EF-Tu and are thus more easily saturated with mutant kirromycin-resistant EF-Tu. In addition, the mutant ribosomes are inherently kirromycin resistant and support a significantly faster EF-Tu cycle time in the presence of the antibiotic than do wild-type ribosomes. A second phenotype associated with combinations of rpsL and error-prone tuf mutations is a reduction in the level of resistance to streptomycin.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M. H., Rechenmacher A., Böck A. Interaction between aminoglycoside uptake and ribosomal resistance mutations. Antimicrob Agents Chemother. 1980 Nov;18(5):798–806. doi: 10.1128/aac.18.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D. I., van Verseveld H. W., Stouthamer A. H., Kurland C. G. Suboptimal growth with hyper-accurate ribosomes. Arch Microbiol. 1986 Feb;144(1):96–101. doi: 10.1007/BF00454963. [DOI] [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Altered ribosomal protein in streptomycin-dependent Escherichia coli. Science. 1969 Dec 5;166(3910):1282–1284. doi: 10.1126/science.166.3910.1282. [DOI] [PubMed] [Google Scholar]
- Bohman K., Ruusala T., Jelenc P. C., Kurland C. G. Kinetic impairment of restrictive streptomycin-resistant ribosomes. Mol Gen Genet. 1984;198(2):90–99. doi: 10.1007/BF00328706. [DOI] [PubMed] [Google Scholar]
- Breckenridge L., Gorini L. Genetic analysis of streptomycin resistance in Escherichia coli. Genetics. 1970 May;65(1):9–25. doi: 10.1093/genetics/65.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A plasmid cloning vector for the direct selection of strains carrying recombinant plasmids. Gene. 1981 Oct;15(1):99–102. doi: 10.1016/0378-1119(81)90108-6. [DOI] [PubMed] [Google Scholar]
- Duisterwinkel F. J., de Graaf J. M., Kraal B., Bosch L. A kirromycin resistant elongation factor EF-Tu from Escherichia coli contains a threonine instead of an alanine residue in position 375. FEBS Lett. 1981 Aug 17;131(1):89–93. doi: 10.1016/0014-5793(81)80894-0. [DOI] [PubMed] [Google Scholar]
- Funatsu G., Wittmann H. G. Ribosomal proteins. 33. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. J Mol Biol. 1972 Jul 28;68(3):547–550. doi: 10.1016/0022-2836(72)90108-8. [DOI] [PubMed] [Google Scholar]
- Gay N. J. Construction and characterization of an Escherichia coli strain with a uncI mutation. J Bacteriol. 1984 Jun;158(3):820–825. doi: 10.1128/jb.158.3.820-825.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D., Atkins J. F., Thompson S. Mutants of elongation factor Tu promote ribosomal frameshifting and nonsense readthrough. EMBO J. 1987 Dec 20;6(13):4235–4239. doi: 10.1002/j.1460-2075.1987.tb02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. Both genes for EF-Tu in Salmonella typhimurium are individually dispensable for growth. J Mol Biol. 1990 Sep 5;215(1):41–51. doi: 10.1016/S0022-2836(05)80093-2. [DOI] [PubMed] [Google Scholar]
- Hughes D. Mutant forms of tufA and tufB independently suppress nonsense mutations. J Mol Biol. 1987 Oct 20;197(4):611–615. doi: 10.1016/0022-2836(87)90467-0. [DOI] [PubMed] [Google Scholar]
- Hughes D. The isolation and mapping of EF-Tu mutations in Salmonella typhimurium. Mol Gen Genet. 1986 Jan;202(1):108–111. doi: 10.1007/BF00330525. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Moore P. B. Structure-function correlations in the small ribosomal subunit from Escherichia coli. Annu Rev Biophys Biophys Chem. 1988;17:349–367. doi: 10.1146/annurev.bb.17.060188.002025. [DOI] [PubMed] [Google Scholar]
- Piepersberg W., Noseda V., Böck A. Bacterial ribosomes with two ambiguity mutations: effects of translational fidelity, on the response to aminoglycosides and on the rate of protein synthesis. Mol Gen Genet. 1979 Mar 9;171(1):23–34. doi: 10.1007/BF00274011. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S., Kostiashkina O. E., Jonák J. Contribution of the elongation factors to resistance of ribosomes against inhibitors: comparison of the inhibitor effects on the factor-free translation systems. J Mol Biol. 1976 Mar 15;101(4):553–562. doi: 10.1016/0022-2836(76)90244-8. [DOI] [PubMed] [Google Scholar]
- Tapio S., Isaksson L. A. Antagonistic effects of mutant elongation factor Tu and ribosomal protein S12 on control of translational accuracy, suppression and cellular growth. Biochimie. 1988 Feb;70(2):273–281. doi: 10.1016/0300-9084(88)90071-5. [DOI] [PubMed] [Google Scholar]
- Tuohy T. M., Thompson S., Gesteland R. F., Hughes D., Atkins J. F. The role of EF-Tu and other translation components in determining translocation step size. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):274–278. doi: 10.1016/0167-4781(90)90180-a. [DOI] [PubMed] [Google Scholar]
- Van de Klundert J. A., Van der Meide P. H., Van de Putte P., Bosch L. Mutants of Escherichia coli altered in both genes coding for the elongation factor Tu. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4470–4473. doi: 10.1073/pnas.75.9.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgenboom E., Vink T., Kraal B., Bosch L. Mutants of the elongation factor EF-Tu, a new class of nonsense suppressors. EMBO J. 1985 Apr;4(4):1049–1052. doi: 10.1002/j.1460-2075.1985.tb03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Mechanism of the inhibition of protein synthesis by kirromycin. Role of elongation factor Tu and ribosomes. Eur J Biochem. 1977 May 2;75(1):67–75. doi: 10.1111/j.1432-1033.1977.tb11504.x. [DOI] [PubMed] [Google Scholar]
- van de Klundert J. A., den Turk E., Borman A. H., van der Meide P. H., Bosch L. Isolation and characterization of a mocimycin resistant mutant of Escherichia coli with an altered elongation factor EF-Tu. FEBS Lett. 1977 Sep 15;81(2):303–307. doi: 10.1016/0014-5793(77)80540-1. [DOI] [PubMed] [Google Scholar]



