Abstract
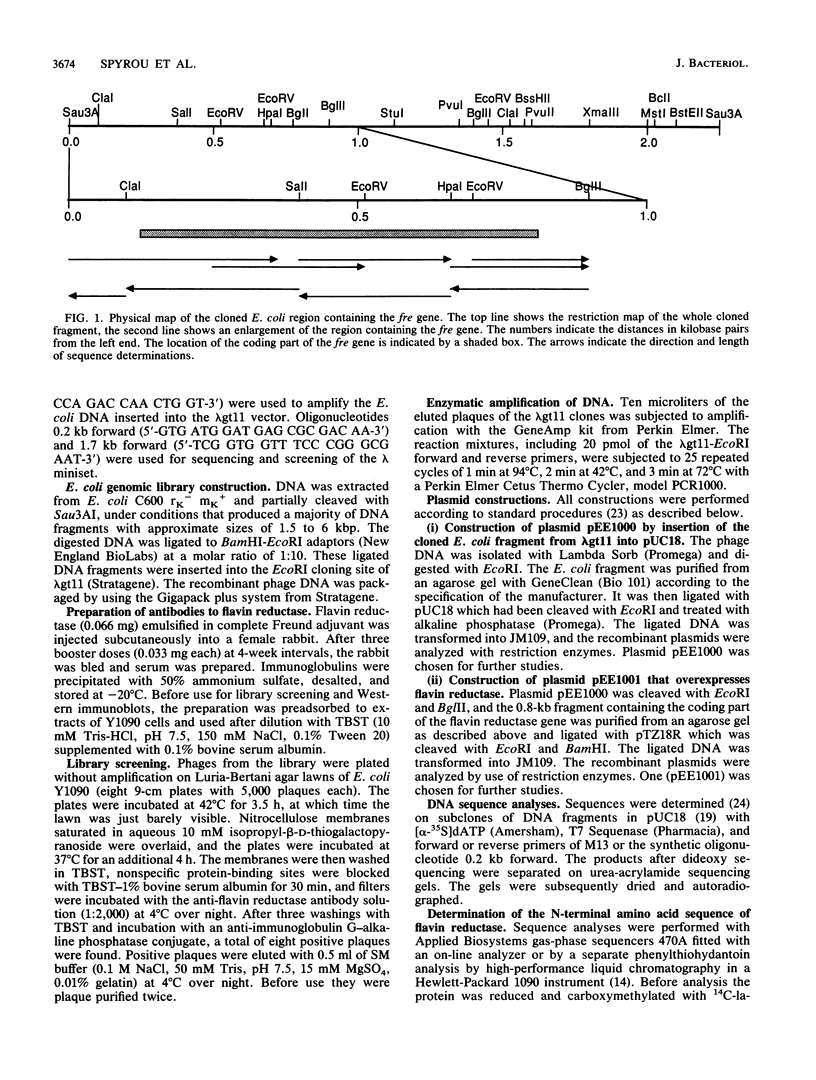
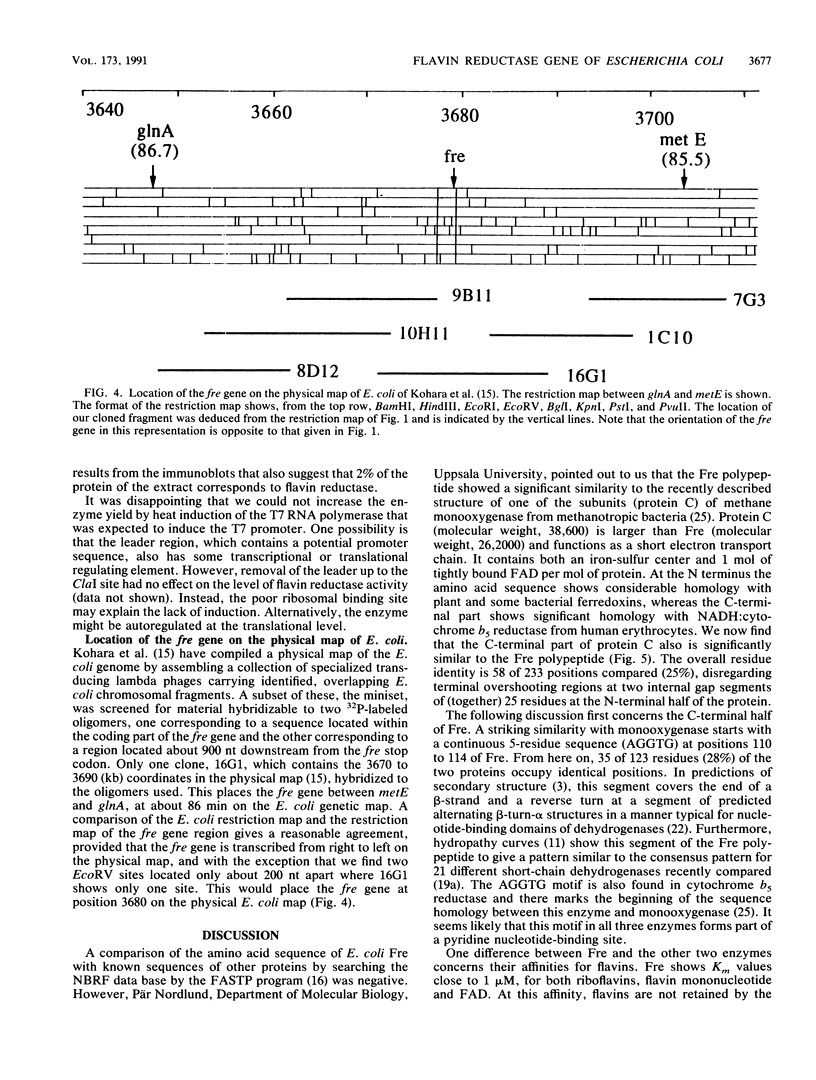
The enzyme NAD(P)H:flavin oxidoreductase (flavin reductase) catalyzes the reduction of soluble flavins by reduced pyridine nucleotides. In Escherichia coli it is part of a multienzyme system that reduces the Fe(III) center of ribonucleotide reductase to Fe(II) and thereby sets the stage for the generation by dioxygen of a free tyrosyl radical required for enzyme activity. Similar enzymes are known in other organisms and may more generally be involved in iron metabolism. We have now isolated the gene for the E. coli flavin reductase from a lambda gt11 library. After DNA sequencing we found an open reading frame coding for a polypeptide of 233 amino acids, with a molecular weight of 26,212 and with an N-terminal segment identical to that determined by direct Edman degradation. The coding sequence is preceded by a weak ribosome binding site centered 8 nucleotides from the start codon and by a promoterlike sequence centered at a distance of 83 nucleotides. In a Kohara library the gene hybridized to position 3680 on the physical map of E. coli. A bacterial strain that overproduced the enzyme approximately 100-fold was constructed. The translated amino acid sequence contained a potential pyridine nucleotide-binding site and showed 25% identity with the C-terminal part of one subunit (protein C) of methane monooxygenase from methanotropic bacteria that reduces the iron center of a second subunit (protein A) of the oxygenase by pyridine nucleotides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman T., Jörnvall H. Electroblotting of individual polypeptides from SDS/polyacrylamide gels for direct sequence analysis. Eur J Biochem. 1987 Nov 16;169(1):9–12. doi: 10.1111/j.1432-1033.1987.tb13573.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Fontecave M., Eliasson R., Reichard P. Enzymatic regulation of the radical content of the small subunit of Escherichia coli ribonucleotide reductase involving reduction of its redox centers. J Biol Chem. 1989 Jun 5;264(16):9164–9170. [PubMed] [Google Scholar]
- Fontecave M., Eliasson R., Reichard P. NAD(P)H:flavin oxidoreductase of Escherichia coli. A ferric iron reductase participating in the generation of the free radical of ribonucleotide reductase. J Biol Chem. 1987 Sep 5;262(25):12325–12331. [PubMed] [Google Scholar]
- Fontecave M., Gräslund A., Reichard P. The function of superoxide dismutase during the enzymatic formation of the free radical of ribonucleotide reductase. J Biol Chem. 1987 Sep 5;262(25):12332–12336. [PubMed] [Google Scholar]
- Gerlo E., Charlier J. Identification of NADH-specific and NADPH-specific FMN reductases in Beneckea harveyi. Eur J Biochem. 1975 Sep 15;57(2):461–467. doi: 10.1111/j.1432-1033.1975.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Hasan N., Nester E. W. Purification and characterization of NADPH-dependent flavin reductase. An enzyme required for the activation of chorismate synthase in Bacillus subtilis. J Biol Chem. 1978 Jul 25;253(14):4987–4992. [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski E., DeLuca M. Purification and properties of the NADH and NADPH specific FMN oxidoreductases from Beneckea harveyi. Biochemistry. 1977 Jun 28;16(13):2932–2936. doi: 10.1021/bi00632a020. [DOI] [PubMed] [Google Scholar]
- Jeffery J., Hobbs L., Jörnvall H. Glucose-6-phosphate dehydrogenase from Saccharomyces cerevisiae: characterization of a reactive lysine residue labeled with acetylsalicylic acid. Biochemistry. 1985 Jan 29;24(3):666–671. doi: 10.1021/bi00324a019. [DOI] [PubMed] [Google Scholar]
- Kaiser R., Holmquist B., Hempel J., Vallee B. L., Jörnvall H. Class III human liver alcohol dehydrogenase: a novel structural type equidistantly related to the class I and class II enzymes. Biochemistry. 1988 Feb 23;27(4):1132–1140. doi: 10.1021/bi00404a009. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Michaliszyn G. A., Wing S. S., Meighen E. A. Purification and properties of a NAD(P)H:flavin oxidoreductase from the luminous bacterium, Beneckea harveyi. J Biol Chem. 1977 Nov 10;252(21):7495–7499. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Wilson G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9070–9074. doi: 10.1073/pnas.83.23.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainthorpe A. C., Lees V., Salmond G. P., Dalton H., Murrell J. C. The methane monooxygenase gene cluster of Methylococcus capsulatus (Bath). Gene. 1990 Jul 2;91(1):27–34. doi: 10.1016/0378-1119(90)90158-n. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yubisui T., Matsuki T., Takeshita M., Yoneyama Y. Characterization of the purified NADPH-flavin reductase of human erythrocytes. J Biochem. 1979 Mar;85(3):719–728. [PubMed] [Google Scholar]