Abstract
Insulin-like growth factors (IGF-I and IGF-II) are well known trophic factors and their specific receptors are uniquely distributed throughout the brain, being especially concentrated in the hippocampal formation. IGFs possess neurotrophic activities in the hippocampus, an area severely affected in Alzheimer disease. These data, together with the evidence that β-amyloid (Aβ)-derived peptides likely play an important role in the neurodegenerative process observed in Alzheimer disease, led us to investigate if IGFs could be neuroprotective to hippocampal neurons against toxicity induced by amyloidogenic derivatives. Exposure of rat primary hippocampal neurons to different concentrations of Aβ25–35, Aβ1–40, Aβ1–42, and human amylin produced marked toxicity, while similar concentrations of two control Aβ peptides—reverse (Aβ40–1) and scrambled sequence (Aβ25–35)—and rat amylin failed to exhibit any significant effect on neuronal survival. IGF-I (10–100 nM) significantly protected hippocampal neurons against neurotoxicity induced by Aβ derivatives and human amylin. The homolog IGF-II was also effective although less potent than IGF-I suggesting the involvement of a typical IGF-I receptor in the observed neuroprotective effect. Most interestingly, IGF-I (10–100 nM) was even able to rescue neurons pre-exposed (up to 4 days) to amyloidogenic peptides. Other neurotrophic factors are reported to lack such rescuing abilities. These results suggest that IGF-I may have unique properties as a potent neuroprotective and neurorescuing agent against amyloid-related neurotoxicity.
Keywords: neurorescuing effect, Alzheimer disease
β-Amyloid (Aβ1–40, Aβ1–42) is believed to play a role in the neurodegenerative process occurring in Alzheimer disease (AD) (1, 2). This protein is found deposited in extracellular neuritic plaques, one of the hallmarks of the AD brain along with the presence of neurofibrillary tangles and cell losses in various regions, including the basal forebrain (3). Aβ is derived proteolytically from a larger transmembrane protein, the amyloid protein precursor, which is expressed widely throughout the brain (4, 5). The direct, toxic properties of Aβ-related fragments (Aβ1–40, Aβ1–42, Aβ25–35) in cultured rat and human neurons and in in vivo are well established (6–8), although the mechanism of action involved remains to be established.
Insulin-like growth factors (IGF-I and IGF-II) play an important role in the normal development and maintenance of the cellular integrity of the organism, including the central nervous system (9). Both trophic factors are selectively localized in the brain and their specific receptors are uniquely distributed in various neuroanatomical regions, being especially concentrated in the hippocampal formation (10). The IGF-I receptor is composed of two α chains where the ligand binds and two β chains possessing a tyrosine kinase domain (11). In contrast, the IGF-II receptor is made of a single transmembrane segment containing a binding site for IGF-II and another for mannose-6-phosphate residues. Both receptors bind specifically to their cognate ligands but can also recognize the other with lower affinity. We have recently shown that cultured hippocampal neurons are highly enriched with IGF-I and IGF-II receptors each being differentially internalized (12), and serving distinct functions (13). Earlier studies have shown that IGFs possess neurotrophic activities in the hippocampus (14–16), an area severely affected in AD. Interestingly, it was also observed that the levels of IGF-I binding sites are significantly increased in cortical areas of AD brains (17). It is unclear if these increases in IGF-I receptors represent a protective and/or compensatory mechanism against neuronal losses.
Considering the broad actions of IGFs on the maintenance of normal cellular functions and the presence of high levels of IGF receptors in the hippocampus, we investigated the potential neuroprotective effects of IGFs against Aβ-induced toxicity in rat hippocampal neurons. Human amylin, which also has amyloidogenic properties (18), was also studied for comparison. Our results show that IGF-I is able not only to protect neurons against Aβ-induced toxicity but even to rescue them up to a few days following exposure to Aβ derivatives and human amylin. Preliminary results were presented in abstract form (58).
MATERIALS AND METHODS
Materials.
Different fragments of Aβ peptides including Aβ25–35 lot no. ZM501; Aβ1–28 lot no. ZK792; Aβ1–40 lot no. ZM365; Aβ40–1 lot no. ZL511; Aβ1–42 lot no. ZN052 (Figs. 1, 2, 3) and Aβ1–42 lot no. ZM823 (Figs. 4, 5, 6) were purchased from Bachem. The scrambled sequence of Aβ25–35 was generously provided by P. Gaudreau (Notre-Dame Hospital Research Center, Montreal). Rat and human amylins were bought from Peninsula Laboratories. Recombinant human (rh)IGF-I was obtained from Genentech while hIGF-II was bought from Lilly Research Laboratories (Indianapolis). Materials used for cell culture were obtained from GIBCO/BRL. Unless stated otherwise, all other chemicals were purchased from Sigma.
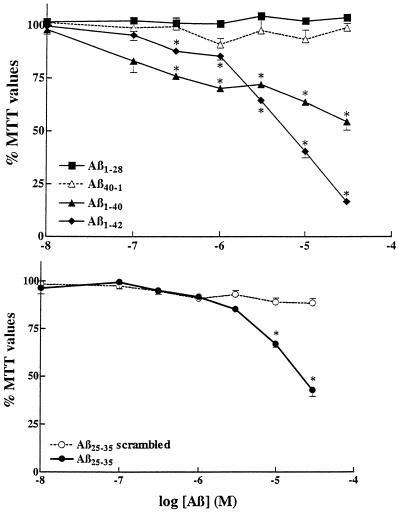
Figure 1.
Neuronal toxicity induced by different Aβ-related peptides. Rat primary hippocampal neurons were treated for 6 days with different concentrations of Aβ1–28, Aβ1–40, the control reversed sequence Aβ40–1, Aβ1–42 (Upper) and Aβ25–35, and its control scrambled sequence (Aβ25–35 scrambled) (Lower). ∗, P < 0.01 as control treatments.
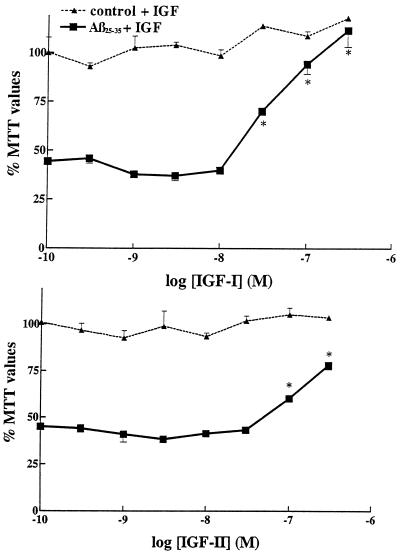
Figure 2.
Neuroprotective effect of IGF-I (Upper) and IGF-II (Lower) against Aβ25–35-induced toxicity in rat primary hippocampal neurons. Aβ25–35 alone (30 μM), induced a marked loss in MTT values down to 44% of controls. Concentration-dependent neuroprotective effects of IGF-I and IGF-II are observed against Aβ25–35 toxicity when the trophic factors are added simultaneously with Aβ25–35. Increasing concentrations of IGFs, by themselves, failed to have significant effects in control cultures (dashed lines). IGF-I was clearly more effective than IGF-II. ∗, P < 0.01 as Aβ-treated neurons.
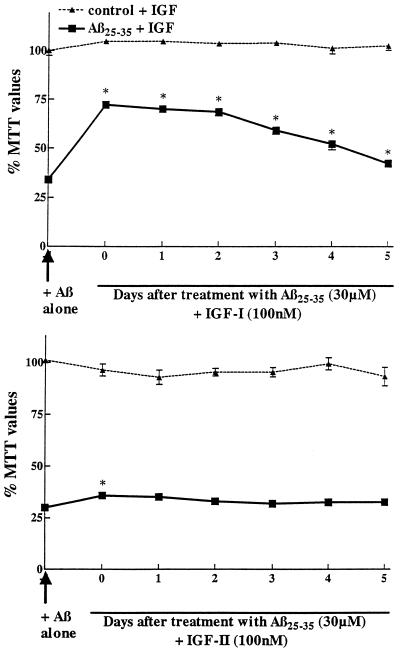
Figure 3.
Effect of IGF-I (Upper) and IGF-II (Lower) on the survival of primary hippocampal neurons previously exposed to Aβ25–35. Neurons were first incubated with 30 μM Aβ25–35 and at different days posttreatment, 100 nM of IGF-I or IGF-II was added. Neurons incubated with Aβ alone showed marked loss in MTT values down to 40% of controls. Most interestingly, IGF-I can rescue neurons from Aβ toxicity even up to 3 days posttreatment (Upper). At later stages, the rescuing effect of IGF-I is not as evident (although still significant) likely because neurons are too affected to benefit from the action of IGF-I. At the same concentration, IGF-II showed protective action when incubated simultaneously with Aβ but did not show any significant rescuing properties (Lower). ∗, P < 0.01 Aβ-treated neurons.
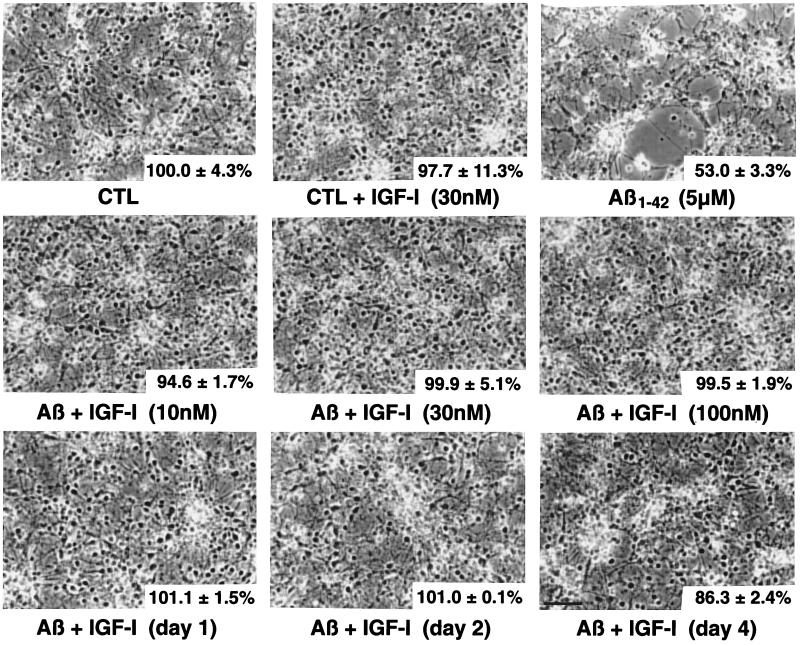
Figure 4.
Photomicrographs showing Aβ1–42 (5 μM)-induced cytotoxicity and neuroprotective effects of IGF-I. Phase contrast micrographs of primary hippocampal neurons exposed for 6 days with different treatments. MTT values are provided (lower right corner) to better assess the correlation with morphological changes. The upper left corner is control (CTL) representing neurons incubated with vehicle only, and MTT value is being fixed at 100%. The subsequent panel is the control cells (CTL) + IGF-I (30 nM) and then Aβ1–42 (5 μM) by itself. The second row pictured neurons incubated with Aβ1–42 and different concentrations of IGF-I (10, 30 and 100 nM). The bottom row shows neurons treated with Aβ1–42 and with IGF-I (30 nM) added subsequently and at different days (days 1, 2, and 4). The numbers indicated in the bottom right corner are the MTT values collected from these cells. (Bar = 50 μM.)
Figure 5.
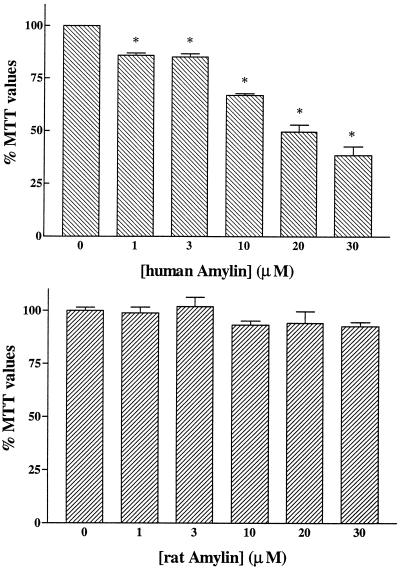
Neuronal toxicity induced by human (Upper) and rat (Lower) amylin. Rat primary hippocampal neurons were treated for 6 days with different concentrations of amylins. Human amylin induced concentration-dependent marked losses in MTT values down to 38% of controls. Rat amylin did not induce significant changes in MTT values at the concentrations used. ∗, P < 0.01 as control treatments.
Figure 6.
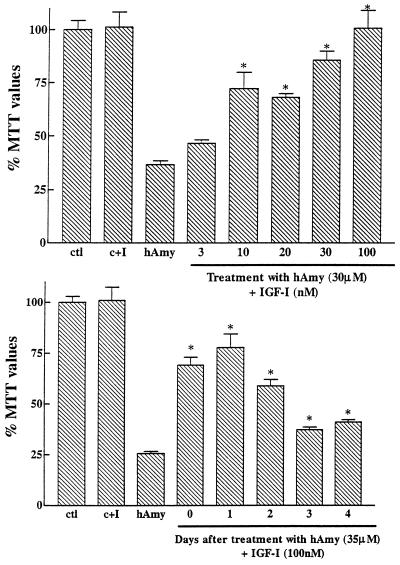
Neuroprotective effects of IGF-I against human amylin-induced toxicity in rat primary hippocampal neurons. (Upper) Human amylin (hAmy) (30 μM) by itself, induced a marked loss in MTT values down to 36% of controls. Concentration-dependent neuroprotective effects of IGF-I are observed when added simultaneously with human amylin. IGF-I (100 nM), by itself, failed to have a significant effect in control cultures (C+I). (Lower) The effect of IGF-I on neuronal survival previously exposed to human amylin. Neurons were first incubated with 35 μM human amylin and at different days posttreatment, 100 nM of IGF-I was added. IGF-I can significantly rescue neurons from human amylin toxicity even up to 4 days posttreatment. ∗, P < 0.01 as hAmy treated neurones.
Hippocampal Neuron Cultures and Experimental Treatments.
Hippocampal neuronal cells, as described earlier (12), were prepared from fetuses (embryonic day 19) obtained from time-pregnant Sprague–Dawley rats (Charles River Breeding Laboratories). Animal care was according to protocols and guidelines approved by the McGill University Animal Care Committee and the Canadian Council for Animal Care.
Following dissection, hippocampal cells were plated at high density (7.5 × 105 cells per well) in 16-mm tissue culture dishes coated with poly-d-lysine (10 μg/ml) under serum-free conditions with high glucose DMEM supplemented with B27 (GIBCO). High-density cultures offer the opportunity to score cell viability by a nonsubjective, easily quantifiable enzymatic assay (see below). On the day following plating, the medium was replaced with fresh culture medium, one-third of the medium being changed again after 3 days. Experiments were performed after 6 days in culture, at which time pyramidal neurons are fully differentiated (19).
Experimental treatments that include exposure to various Aβ-related peptides, human amylin, rat amylin, IGF-I, and IGF-II, and were conducted in the N-2 supplement Hepes-buffered high glucose neurobasal medium (GIBCO). Aβ peptides were dissolved in dimethyl sulfoxide and used immediately by directly diluting to the indicated concentrations in the chemically defined experimental culture medium. Sister cultures were treated with appropriate vehicle controls and the final concentration of dimethyl sulfoxide did not exceed 0.01%. Following treatments, neurons were maintained for an additional period of 6 days and their survival was assessed by phase-contrast microscopy and quantified using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay. The neuroprotective effect of IGF was evaluated by treating the cells simultaneously with IGF and different Aβ- or amylin-related peptides, while the neurorescuing action of IGF was determined by treating the cells at different days following initial exposure to Aβ- or amylin-related peptides.
MTT Colorimetric Assay.
MTT is a purported indicator of the mitochondrial activity in living cells (20). Evidence suggests that Aβ-induced toxicity measured as a percentage of MTT reduction correlates with percentage release of lactate dehydrogenase (21) and it represents an excellent marker of metabolic compromise that ultimately leads to neuronal degeneration and cell death (20–22). In the present study, after 6 days of experimental treatments, the culture medium was replaced with DMEM and freshly dissolved MTT (stock: 5 mg/ml in 0.1 M PBS) was aseptically added to a final concentration of 10%. The plates were then returned into the incubator for a 3-hr period. Cells and MTT formazan crystals were solubilized by trituration in a solution of anhydrous isopropanol⋅HCl 0.1 N and spectrophotometrically measured at 570 nm. Survival of control vehicle-treated neuronal cells not exposed to Aβ- or amylin-related peptides was set at 100% and treated groups were represented as percentage of control values. All experiments were repeated at least with three separate batches of cultures and the means were analyzed using GraphPad (San Diego) prism 2.01. Results were represented as the mean ± SEM with P < 0.01 was considered significant. Student’s t test was used to establish significance.
RESULTS
Neuronal Toxicity Induced by Different Aβ-Related Peptides.
Various Aβ peptides (i.e., Aβ1–40, Aβ1–42, and Aβ25–35) are found to be highly toxic to cultured rat hippocampal neurons as evidenced by concentration-dependent reduction in MTT values (Fig. 1). Of these three fragments, Aβ1–42 was the most potent to affect cell survival. In contrast, Aβ1–28 and the two control peptides, Aβ40–1 (reverse sequence) and Aβ25–35 (scrambled sequence), were not toxic and did not affect the survival of neurons thus confirming the specificity to the observed toxic effects.
Evidence suggests that pre-aggregated Aβ peptide is highly toxic to a variety of cultured neurons. To compare the relative potency of pre-aggregated Aβ peptide, we aged Aβ25–35 (1 mM) in the incubator for 4 days at 37°C prior to the experiment (23). The aged Aβ25–35 was also neurotoxic (data not shown), although not more effective than the freshly solubilized peptide. This is likely due to the fact that our experimental procedures include exposure of cultured neurons to Aβ fragments for a six-day period at 37°C, aggregation likely occurring during this period, hence artificial aging not being required.
Neuroprotective Effects of IGF-I and IGF-II Against Aβ-Induced Toxicity.
As shown in Fig. 2, increasing concentrations of IGF-I and IGF-II are able to protect hippocampal neurons against Aβ25–35-induced toxicity (30 μM Aβ25–35; 44% of control values). IGF-I is found to be more potent than IGF-II (Fig. 2). When treated alone with either IGF-I or IGF-II, the survival of neurons is not found to be significantly affected (Fig. 2, top lines).
Neurorescuing Effects of IGF-I Against Aβ-Induced Toxicity.
Fig. 3 shows that IGF-I at 100 nM is able to significantly protect neurons against toxicity induced by 30 μM Aβ25–35 even if added up to 3–5 days post-Aβ treatments. Neurons incubated with Aβ alone were highly affected with MTT values down to 34% of control. When the cells were incubated with IGF-I immediately or up to 2 days after Aβ exposure, IGF-I rescued neurons with MTT values being significantly increased between 68–72% of controls. At subsequent days (3–5), the rescuing effect was still significant but less evident indicating that neurons are too affected to fully benefit from IGF-I rescuing properties. At the same concentration, IGF-II had a slight protective effect when incubated simultaneously with Aβ but failed to demonstrate any rescuing abilities (Fig. 3).
Morphological Features of Neurons Exposed to Aβ1–42 and/or IGF-I.
Fig. 4 summarizes the morphological features of exposure to IGF-I, Aβ, and their combination (simultaneously or post-Aβ treatment). Aβ1–42, at a concentration of 5 μM, induced degeneration with shrinkage of cell soma, neuronal clustering, and debris. IGF-I added simultaneously at 10, 30, and 100 nM was clearly neuroprotective (Fig. 4, second row). Moreover, IGF-I (30 nM) was able to rescue neurons against Aβ1–42 toxicity even when added 1, 2, or 4 days later (Fig. 4, bottom row).
Neuroprotective/Neurorescuing Effects of IGF-I Against Amylin-Induced Toxicity.
Human amylin was highly toxic to cultured hippocampal neurons as shown by decreased concentration-dependent MTT values (Fig. 5). In contrast, rat amylin was unable to induce toxicity, in accordance with an earlier report (18). Fig. 6 Upper shows that increasing concentrations of IGF-I were able to protect neurons against toxicity induced by 30 μM human amylin. Neurons incubated with human amylin alone (35 μM) were highly affected with MTT values down to 26% of control (Fig. 6 Lower). IGF-I (100 nM) was able to significantly rescue neurons for up to 4 days after treatment with human amylin, with important rescuing abilities observed after 2 days. At subsequent days, the rescuing ability of IGF-I was still significant but less evident (Fig. 6).
DISCUSSION
The major finding of the present study relates to the neuroprotective and neurorescuing properties of IGF-I against Aβ-induced toxicity. While various neurotrophins and neurotrophic factors (24) have been shown to block the toxic effect of Aβ derivatives in vitro and in vivo, the rescuing action of IGF-I is, to our knowledge, unique. Indeed, we observed that the incubation of IGF-I was able to rescue primary rat hippocampal neurons pre-exposed to Aβ for up to 4–5 days. This is particularly interesting in the clinical context and suggests that the mechanism of action of Aβ-induced toxicity involved a rather long process allowing for various pharmacological interventions such as the use of IGF-I, its mimetics, or molecules activating the IGF-I receptor signaling pathway.
It is now rather well established that various Aβ derivatives, especially in their aggregated forms, are toxic to many cell types, including neurons both in vitro and in vivo (8, 25, 26). We confirmed and extended these findings in the present study. The main amyloidogenic components of the neuritic plaques are the Aβ1–42 and Aβ1–40 fragments (1, 27). Aβ1–40, Aβ1–42 fragments and the Aβ25–35 peptide (which contains the active toxic domain) are highly toxic to rat primary hippocampal neurons as exemplified by the reduction in MTT values and the altered morphological features of the culture. In contrast, the nonamyloidogenic fragment Aβ1–28 and the controls (including the random sequence of the Aβ25–35 and the reverse sequence peptide, Aβ40–1) did not affect neuronal survival. These results confirm that the neurotoxic properties of Aβ derivatives are highly sequence dependent and possibly related to their amphiphilic nature. This hypothesis is supported further by the fact human, but not rat, amylin was found to be neurotoxic in various models (18, 28), including ours. Human amylin, which is present in the brain (28), is a highly amphiphilic peptide that has a high tendency to aggregate in contrast to rat amylin that is nontoxic (29).
The initial event leading to Aβ-induced toxicity is unknown at present but may involve plasma membrane receptors for advanced glycation end products (RAGE; ref. 30), class A scavenger receptor-related proteins (31), certain G-proteins (32), heparan sulfate (33, 34), and α2-macroglobulin extracellular domains (35). Whether IGF-I can directly interact with these various receptor sites to block Aβ-induced toxicity remains to be established. Moreover, we cannot rule out the possibility that IGF-I could interfere with the aggregation of Aβ or human amylin. Preliminary experiments, however failed to provide evidence for differential fibril formation in the presence (Aβ25–35; 30 μM plus IGF-I, 100 nM) or absence (Aβ alone) of IGF-I (unpublished results).
We observed that both IGF-I and IGF-II were able to protect rat hippocampal neurons against Aβ-induced neurotoxicity. However, IGF-I was clearly more potent than IGF-II. Additionally, IGF-II, at the concentration tested (100 nM), was unable to rescue neurons previously exposed to Aβ25–35. Taken together, these results suggest the involvement of the IGF-I receptor subtype known to be activated by both IGF-I and IGF-II, the latter with markedly lower potency.
Other trophic factors have been reported to protect neurons against various types of insults. For example, nerve growth factor (NGF) (36), basic fibroblast growth factor (bFGF) (37), and transforming growth factors (TNF) α and β (38) have been shown to protect cultured hippocampal neurons against Aβ-induced toxicity. However, none of these trophic factors were effective post-Aβ-treatment hence lacking neurorescuing properties. Interestingly, TGFβ1 and TGFβ2, but not TGFβ3, were apparently able to have a slight protective effect in neurons pre-exposed to Aβ25–35 for 24 hr while such effect was not observed with TNF, NGF, aFGF, or bFGF (39). The rescuing ability of TGFβ1 and TGFβ2 was not as pronounced as the one observed with IGF-I and was not as effective against longer exposures to Aβ25–35. Moreover, preliminary results with TGFβ (100 nM) and NGF (10 to 1,000 nM) failed to reveal any rescuing abilities of these two factors against toxicity induced by Aβ25–35 in our model (unpublished results). Taken together, it appears that the neuroprotective/neurorescuing abilities of IGF-I against Aβ- and human amylin-induced toxicities observed in the present study are rather unique.
The mechanism of action involves in neuroprotective and especially neurorescuing properties of IGF-I against Aβ-induced toxicity remains to be clarified. The activation of the IGF-I tyrosine kinase receptor induces protein phosphorylation followed by a cascade of intracellular events that include the activation of insulin receptor substrates 1 and 2, and phosphoinositide 3-kinase, phosphotyrosine phosphatases, S6 kinase, Ras-mitogen-activating protein kinase and transcription factors leading to alterations in Ca2+ storage and mobilization, and mitochondrial respiration (40, 41). It has been proposed that Aβ-induced toxicity is due to free radicals production/oxidative stress (2, 42) and/or increased free intracellular Ca2+ levels (43) leading to necrosis and cell death (21). The toxic properties of Aβ-derivatives could also relate to their abilities to stimulate apoptotic genes/cellular events (7, 44, 45). Accordingly, IGF-I could interfere at different stages of the necrotic or apoptotic pathway to block and even rescue neurons against Aβ-induced cell death. Interestingly, IGF-I has already been shown to block programmed cell death in various models. For example, IGF-I and the IGF-I receptor prevent topoisomerase I inhibitor etopiside-induced apoptosis in 3T3 cells (46), inhibit interleukin-3-dependent cell death in various cell lines (47), inhibit apoptosis induced by either TNFα, radiation and dysregulated c-myc expression (48) and protect differentiated PC12 cells against cell death following NGF withdrawal (49). IGF-I has also been shown to prevent apoptosis associated with K+-deprivation in cerebellar granule neurons, other factors tested including aFGF, bFGF, platelet-derived growth factor, and neurotrophin 3 being inactive (49). Similar protective effects were observed in a hybrid dopaminergic cell line against oxidation and hypoglycemia-induced cell death, IGF-I being more potent than bFGF, epidermal growth factor, and NGF (50). More recently, it was demonstrated that IGF-I (and supraphysiologic concentrations of insulin) activates a phosphoinositide 3-kinase and the serine-threonine protein kinase Akt to promote the survival of rat primary cerebellar neurons after induction of apoptosis by serum deprivation (51). It would be of interest to investigate if this pathway is also involved in the neuroprotective/neurorescuing properties of IGF-I observed in the present study. In vivo, IGF-I has been shown to reduce neuronal cell losses observed following hypoxic–ischemic insults (15, 52) and is beneficial in the treatment of amyotrophic lateral sclerosis patients (53). Hence, IGF-I by acting on necrotic and/or apoptotic cellular events could protect and more importantly rescue neurons against Aβ- and human amylin-induced toxicity.
It is well established that insulin-like family members are critically involved in maintenance of body homeostasis. Aging is associated with changes in basic functions among which glucose metabolism is central to the nervous system. In aged rats and humans, poor cognitive performances have been correlated with impaired glucose regulation (54). In sporadic AD cases, significant reductions in glucose utilization have been reported (55) and neuroglucopenia reduced the formation rate of ATP and acetylcholine from 54% to 47% of control values, respectively (55). Moreover, IGF-I receptor binding levels are apparently increased in affected areas of the AD brain (17), may be as an attempt to counteract energy metabolism deficits and cell losses. Interestingly, IGF-I is also known to be neuroprotective against toxicity induced by glucose deprivation (14, 50, 56) and it has been shown that exposure to subthreshold doses of Aβ derivatives render neurons more susceptible to glucose deprivation (57). The unique capacity of IGF-I to protect and rescue neurons against Aβ-induced toxicity, in parallel to its homeostatic potential to insure adequate glucose/energy metabolism exemplified even further the critical relevance of this trophic factor in normal and pathological brain functioning.
In summary, we observed that IGF-I is able to protect, and more importantly, to rescue rat hippocampal primary neurons against Aβ- and human amylin-induced toxicity. IGF-II is less potent suggesting the activation of the IGF-I receptor and the related signaling pathway. These unique properties of IGF-I, in parallel to its well known involvement in various metabolic pathways, suggest that the development of IGF-I-related mimetics could be a promising strategy toward the treatment of various neurodegenerative diseases including AD.
Acknowledgments
This work was supported by the Medical Research Council of Canada. S.D. is a Post-Doctoral Fellow of the Alzheimer Society of Canada. R.Q. and S.K are “Chercheurs Boursiers” from the Fonds de la Recherche en Santé du Québec.
ABBREVIATIONS
- Aβ
β-amyloid
- AD
Alzheimer disease
- FGF
fibroblast growth factor
- IGF
insulin-like growth factor
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NGF
nerve growth factor
- TGF
transforming growth factor
References
- 1.Roher A E, Lowenson J D, Clarke S, Woods A S, Cotter R J, Gowing E, Ball M J. Proc Natl Acad Sci USA. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris M E, Hensley K, Butterfield D A, Leedle A, Caney J M. Exp Neurol. 1995;131:193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 3.Price D L, Sisodia S S, Gandy S E. Curr Opin Neurol. 1995;8:268–274. doi: 10.1097/00019052-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Buxbaum J D, Greengard P. Ann NY Acad Sci. 1996;777:327–331. doi: 10.1111/j.1749-6632.1996.tb34441.x. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe D J, Yamazaki T, Citron M, Podlisny M B, Koo E H, Teplow D B, Haass C. Ann NY Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 6.Busciglio J, Yeh J, Yankner B A. J Neurochem. 1993;61:1565–1568. doi: 10.1111/j.1471-4159.1993.tb13658.x. [DOI] [PubMed] [Google Scholar]
- 7.Loo D T, Copani A, Pike C J, Hittemore E R, Walencewicz A J, Cotman C W. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurice T, Lockhart B P, Privat A. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 9.de Pablo F, de la Rosa E J. Trends Neurosci. 1995;18:143–150. doi: 10.1016/0166-2236(95)93892-2. [DOI] [PubMed] [Google Scholar]
- 10.Kar S, Baccichet A, Quirion R, Poirier J. Neuroscience. 1993;55:69–80. doi: 10.1016/0306-4522(93)90455-o. [DOI] [PubMed] [Google Scholar]
- 11.LeRoith D, Werner H, Beitner-Johnson D, Roberts C T. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 12.Doré, S., Kar, S. & Quirion, R. (1997) Neuroscience, in press.
- 13.Kar S, Seto D, Hanisch U K, Quirion R. Soc Neurosci Abstr. 1994;20:81. [Google Scholar]
- 14.Cheng B, Mattson M P. J Neurosci. 1994;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu C Z, Auer R N. Neurol Res. 1994;16:116–120. doi: 10.1080/01616412.1994.11740207. [DOI] [PubMed] [Google Scholar]
- 16.Lindholm D, Carroll P, Tzimagiorgis G, Thoenen H. Eur J Neurosci. 1996;8:1452–1460. doi: 10.1111/j.1460-9568.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 17.Crews F T, McElhaney R, Freund G, Ballinger W E, Jr, Raizada M K. J Neurochem. 1992;58:1205–1210. doi: 10.1111/j.1471-4159.1992.tb11330.x. [DOI] [PubMed] [Google Scholar]
- 18.May P C, Boggs L N, Fuson K S. J Neurochem. 1993;61:2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- 19.Mattson M P, Wang H, Michaelis E K. Brain Res. 1991;565:94–108. doi: 10.1016/0006-8993(91)91740-r. [DOI] [PubMed] [Google Scholar]
- 20.Shearman M S, Hawtin S R, Tailor V J. J Neurochem. 1995;65:218–227. doi: 10.1046/j.1471-4159.1995.65010218.x. [DOI] [PubMed] [Google Scholar]
- 21.Behl C, Davis J B, Klier F G, Schubert D. Brain Res. 1994;645:253–264. doi: 10.1016/0006-8993(94)91659-4. [DOI] [PubMed] [Google Scholar]
- 22.Connolly D T, Knight M B, Harakas N K, Wittwer A J, Feder J. Anal Biochem. 1986;152:136–140. doi: 10.1016/0003-2697(86)90131-4. [DOI] [PubMed] [Google Scholar]
- 23.Lockhart B P, Benicourt C, Junien J-L, Privat A. J Neurosci Res. 1994;39:494–505. doi: 10.1002/jnr.490390416. [DOI] [PubMed] [Google Scholar]
- 24.Yanker B A, Caceres A, Duffy L K. Proc Natl Acad Sci USA. 1990;87:9020–9023. doi: 10.1073/pnas.87.22.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haass C. Eur Arch Psychiatry Clin Neurosci. 1996;246:118–123. doi: 10.1007/BF02189111. [DOI] [PubMed] [Google Scholar]
- 26.Iversen L L, Mortishire-Smith R J, Pollack S J, Shearman M S. Biochem J. 1996;311:1–16. doi: 10.1042/bj3110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selkoe D J. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- 28.Asai J, Nakazato M, Miyazato M, Kangawa K, Matsuo H, Matsukura S. Biochem Biophys Res Commun. 1990;169:788–795. doi: 10.1016/0006-291x(90)90400-h. [DOI] [PubMed] [Google Scholar]
- 28a.Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, Kimura H. Proc Natl Acad Sci USA. 1995;92:1989–1993. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean L R, Balasubramaniam A. Biochim Biophys Acta. 1992;112:317–320. doi: 10.1016/0167-4838(92)90411-6. [DOI] [PubMed] [Google Scholar]
- 30.Yan S D, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt A M. Nature (London) 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 31.El Khoury J, Hickman S E, Thomas C A, Cao L, Silverstein S C, Loike J D. Nature (London) 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto T, Takeda S, Murayama Y, Ogata E, Nishimoto I. J Biol Chem. 1995;270:4205–4208. doi: 10.1074/jbc.270.9.4205. [DOI] [PubMed] [Google Scholar]
- 33.Narindrasorasak S, Lowery D, Gonzalez-DeWhitt P, Poorman R A, Greenberg B, Kisilevsky R. J Biol Chem. 1991;266:12878–12883. [PubMed] [Google Scholar]
- 34.Gupta-Bansal R, Frederickson R C, Brunden K R. J Biol Chem. 1995;270:18666–18671. doi: 10.1074/jbc.270.31.18666. [DOI] [PubMed] [Google Scholar]
- 35.Qiu W Q, Borth W, Ye Z, Haass C, Teplow D B, Selkoe D J. J Biol Chem. 1996;271:8443–8451. doi: 10.1074/jbc.271.14.8443. [DOI] [PubMed] [Google Scholar]
- 36.Rabizadeh S, Bittle C M, Butcher L L, Bredsen D E. Proc Natl Acad Sci USA. 1994;91:10703–10706. doi: 10.1073/pnas.91.22.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattson M P, Barger S W, Cheng B, Lieberburg I, Smith-Swintosky V L, Rydel R E. Trends Neurosci. 1993;16:409–414. doi: 10.1016/0166-2236(93)90009-b. [DOI] [PubMed] [Google Scholar]
- 38.Barger S W, Horster D, Furukawa K, Goodman Y, Kieglestein J, Mattson M P. Proc Natl Acad Sci USA. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren R F, Flanders K C. Brain Res. 1996;732:16–24. doi: 10.1016/0006-8993(96)00458-1. [DOI] [PubMed] [Google Scholar]
- 40.Lienhard G E. Nature (London) 1994;372:128–129. doi: 10.1038/372128a0. [DOI] [PubMed] [Google Scholar]
- 41.Waters S B, Pessin J E. Trends Cell Biol. 1996;6:1–3. doi: 10.1016/0962-8924(96)81024-5. [DOI] [PubMed] [Google Scholar]
- 42.Behl C, Davis J B, Lesly R, Schubert D. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 43.Goodman Y, Mattson M P. Brain Res. 1994;650:170–174. doi: 10.1016/0006-8993(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 44.Forloni G, Chiesa R, Smiroldo S, Verga L, Salmona M, Tagliavini F, Angeretti N. NeuroReport. 1993;4:523–526. doi: 10.1097/00001756-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Gschwind M, Huber G. J Neurochem. 1995;65:292–300. doi: 10.1046/j.1471-4159.1995.65010292.x. [DOI] [PubMed] [Google Scholar]
- 46.Sell C, Baserga R, Rubin R. Cancer Res. 1995;55:303–306. [PubMed] [Google Scholar]
- 47.Rodriguez-Tarduchy G, Collins M K L, Garcia I, Lopez-Rivas A. J Immunol. 1992;149:535–540. [PubMed] [Google Scholar]
- 48.Harrington E A, Bennet M R, Fanadi A, Evan G I. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Mello S R, Galli C, Ciotti T, Calissano P. Proc Natl Acad Sci USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le W D, Xie W -J, Smith R G, Appel S H. Neurodegeneration. 1995;2:227–236. [Google Scholar]
- 51.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 52.Beilharz E J, Bassett N S, Sirimanne E S, Williams C E, Gluckman P D. Mol Brain Res. 1995;29:81–91. doi: 10.1016/0169-328x(94)00232-4. [DOI] [PubMed] [Google Scholar]
- 53.Murphy M F, Felice K, Gawel M, Gelinas D, Kratz R, Lai F, Lange D, Natter H, Rudnicki S ALS/North America Myotrophin group. Am Neurol Assn Abstr. 1995;38:335. [Google Scholar]
- 54.Long J M, Davis B J, Garofalo P, Spangler E L, Ingram D K. Physiol Behav. 1992;51:411–418. doi: 10.1016/0031-9384(92)90159-y. [DOI] [PubMed] [Google Scholar]
- 55.Hoyer S. Life Sci. 1994;55:1977–1983. doi: 10.1016/0024-3205(94)00377-7. [DOI] [PubMed] [Google Scholar]
- 56.Harper S J, Macaulay A J, Hill R G, Priestley T. Brain Res. 1996;709:303–310. doi: 10.1016/0006-8993(95)01355-5. [DOI] [PubMed] [Google Scholar]
- 57.Copani A, Koh J-Y, Cotman C W. NeuroReport. 1991;2:763–765. doi: 10.1097/00001756-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Doré S, Quirion R, Kar S. Soc Neurosci Abstr. 1995;21:475. [Google Scholar]