Abstract
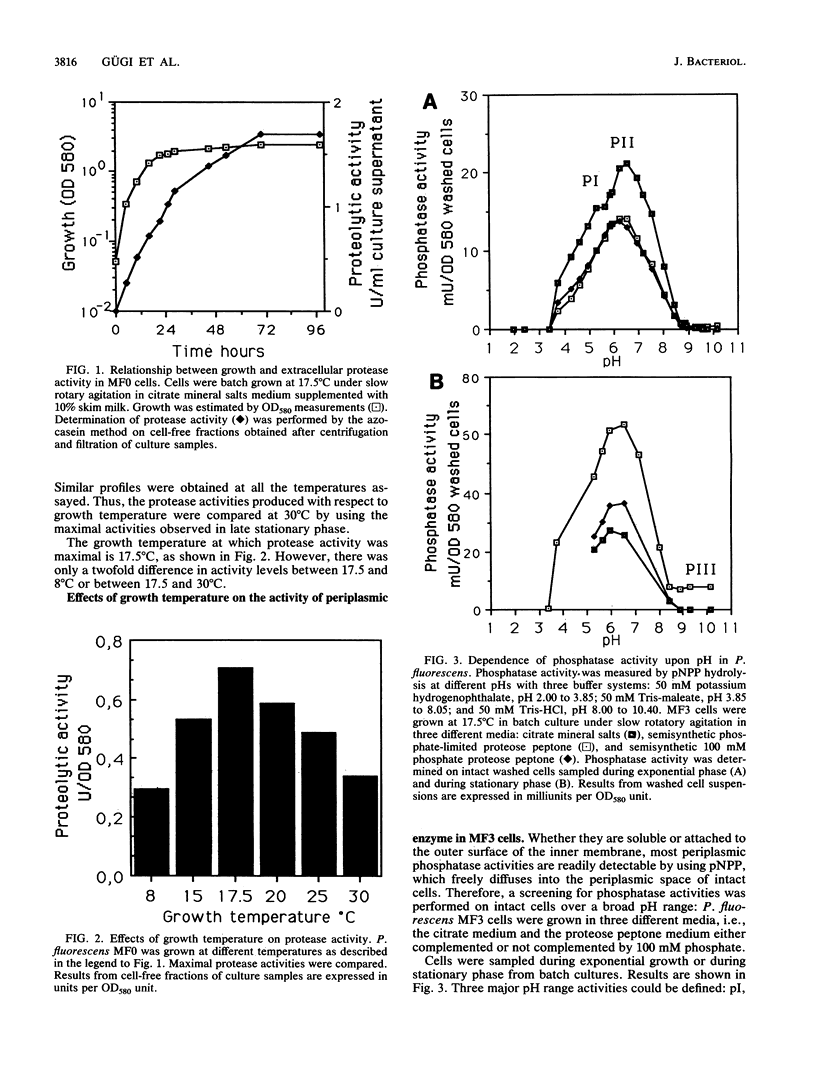
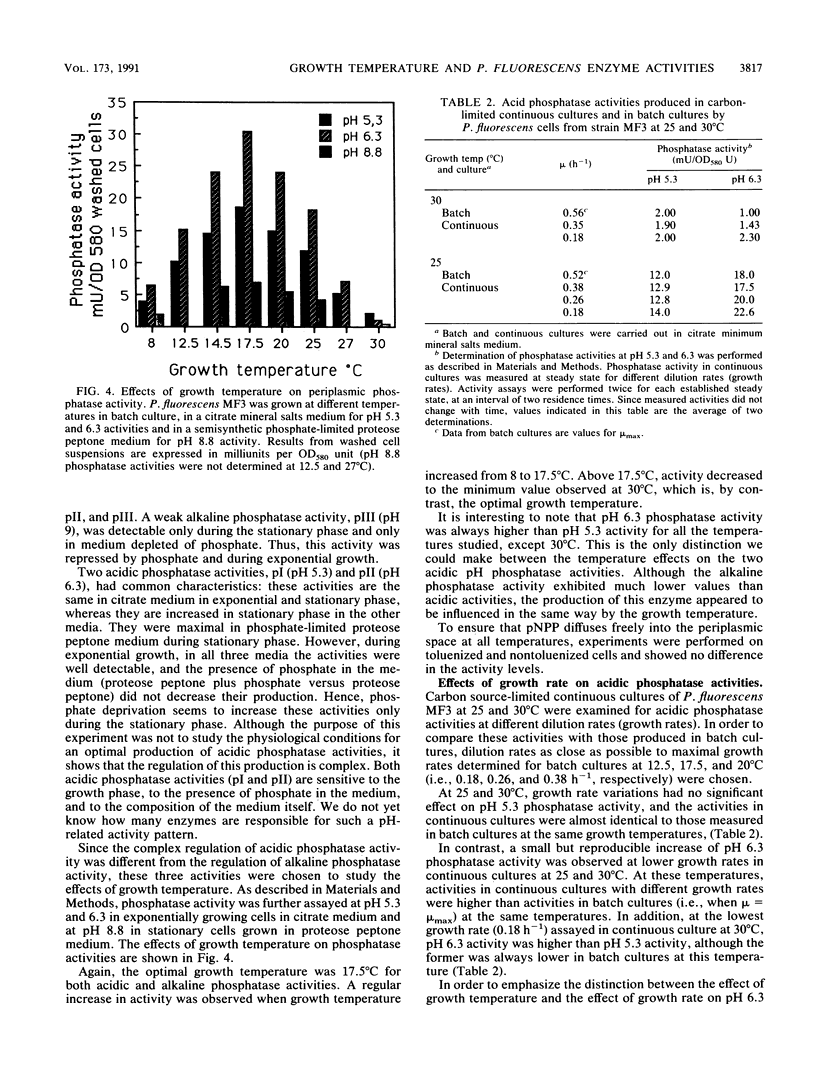
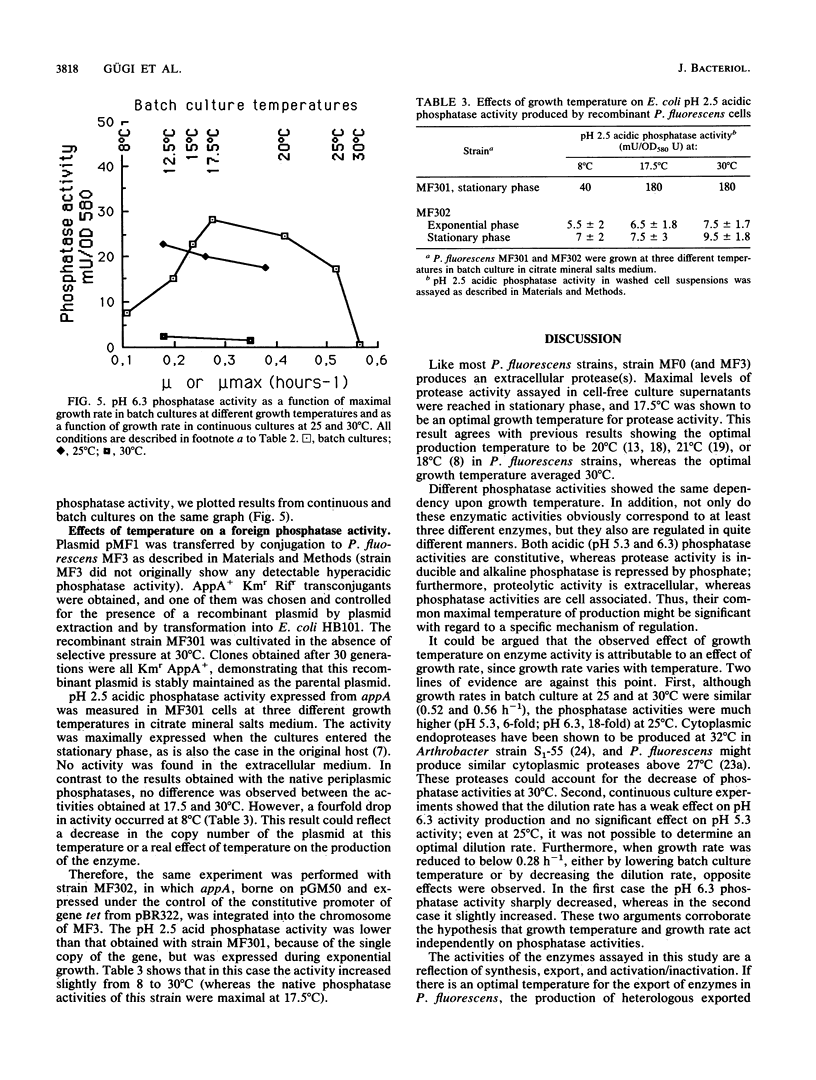
In accordance with previous results, the activity of extracellular proteases from Pseudomonas fluorescens MF0 is maximal at a growth temperature of 17.5 degrees C, well below the optimal growth temperature. In addition, the activities of three periplasmic phosphatases display the same growth temperature optimum. Chemostat experiments have shown that it is the growth temperature itself and not the value of the growth rate that regulates these activities. In contrast, a foreign periplasmic phosphatase, expressed under the control of its own promoter, displays a different sensitivity toward temperature. We conclude that in the psychrotrophic strain P. fluorescens MF0, growth temperature exerts a specific control upon the activity of certain enzymes. The critical temperature (17.5 degrees C) is within the range of normal growth, suggesting that this control is probably different from a cold shock or heat shock response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P. L., Manoil C., Beckwith J. Use of TnphoA to detect genes for exported proteins in Escherichia coli: identification of the plasmid-encoded gene for a periplasmic acid phosphatase. J Bacteriol. 1987 Apr;169(4):1663–1669. doi: 10.1128/jb.169.4.1663-1669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa E., Boquet P. L. Identification of the gene appA for the acid phosphatase (pH optimum 2.5) of Escherichia coli. Mol Gen Genet. 1985;200(1):68–73. doi: 10.1007/BF00383314. [DOI] [PubMed] [Google Scholar]
- Fairbairn D. J., Law B. A. Proteinases of psychrotrophic bacteria: their production, properties, effects and control. J Dairy Res. 1986 Feb;53(1):139–177. doi: 10.1017/s0022029900024742. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., VanBogelen R. A., Neidhardt F. C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987 May;169(5):2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffs H. S. Effects of temperature and nutrients on proteinase production by Pseudomonas fluorescens and Ps. aeruginosa in broth and milk. J Appl Bacteriol. 1976 Feb;40(1):23–32. doi: 10.1111/j.1365-2672.1976.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Mayerhofer H. J., Marshall R. T., White C. H., Lu M. Characterization of a heat-stable protease of Pseudomonas fluorescens P26. Appl Microbiol. 1973 Jan;25(1):44–48. doi: 10.1128/am.25.1.44-48.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar R. C. Factors influencing the production of extracellular proteinase by Pseudomonas fluorescens. J Appl Bacteriol. 1982 Dec;53(3):305–316. doi: 10.1111/j.1365-2672.1982.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Stepaniak L., Fox P. F., Daly C. Isolation and general characterization of a heat-stable proteinase from Pseudomonas fluorescens aft 36. Biochim Biophys Acta. 1982 Aug 6;717(2):376–383. doi: 10.1016/0304-4165(82)90192-1. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]


