Abstract
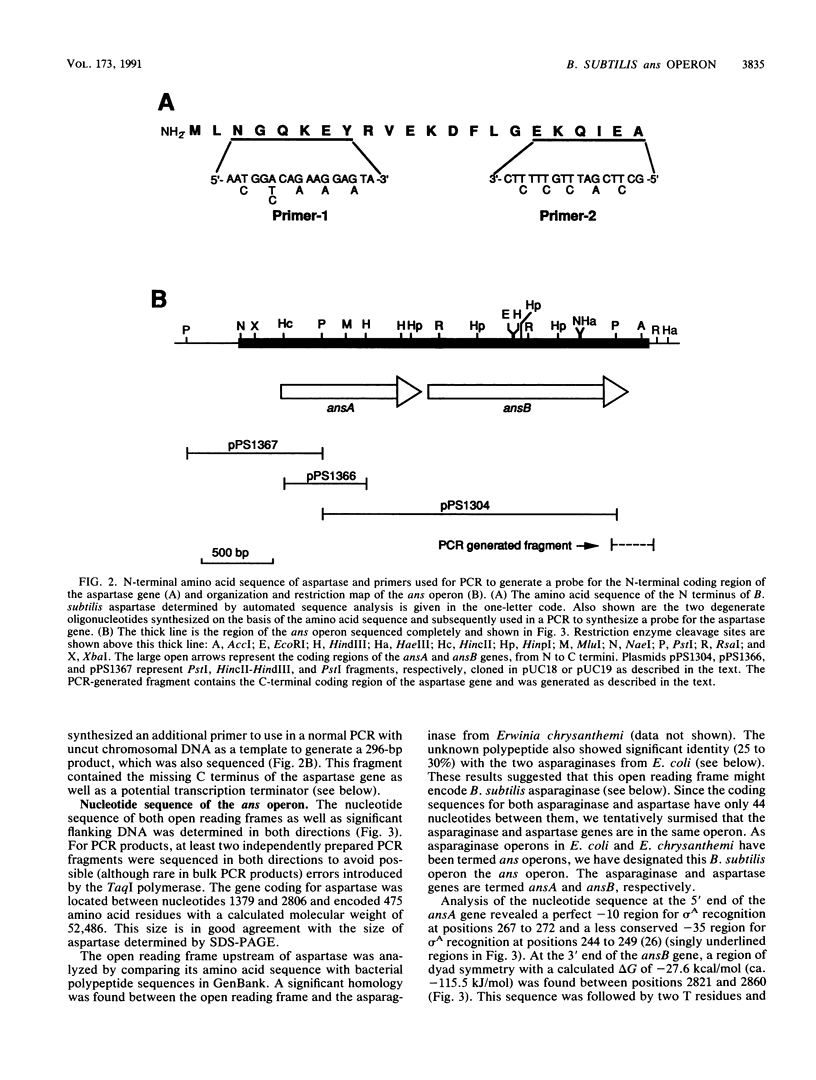
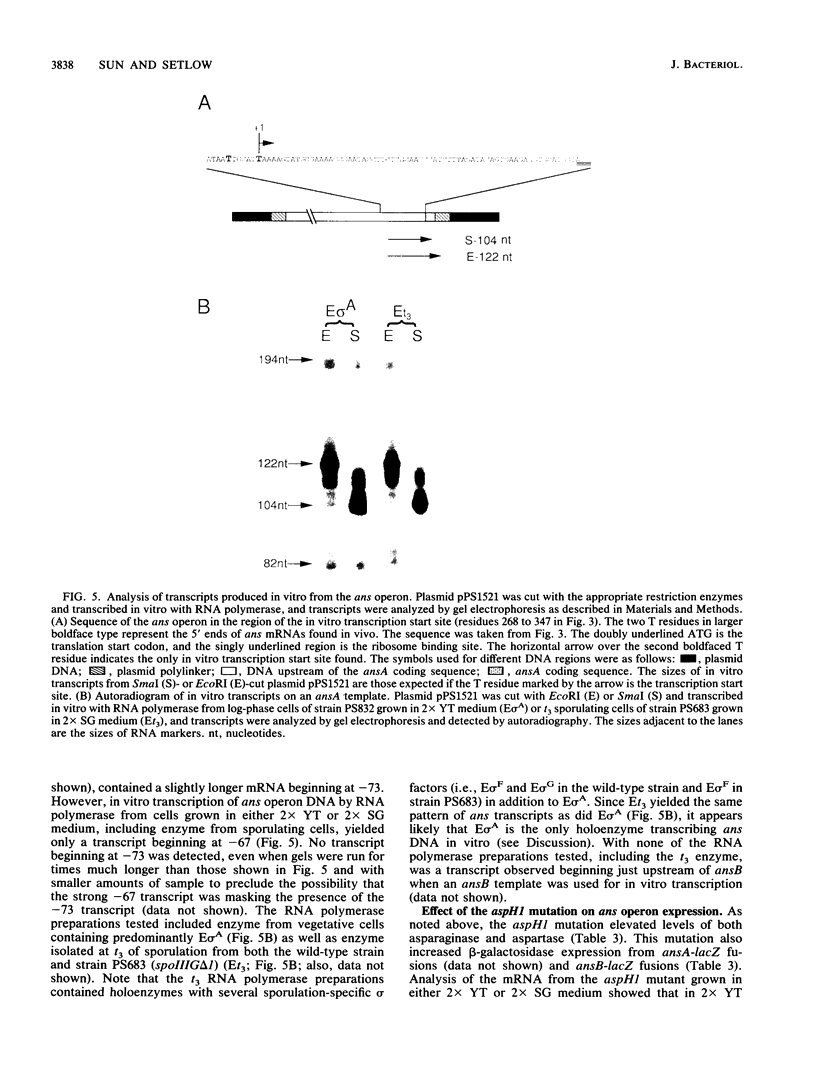
L-Aspartase was purified from Bacillus subtilis, its N-terminal amino acid sequence was determined to construct a probe for the aspartase gene, and the gene (termed ansB) was cloned and sequenced. A second gene (termed ansA) was found upstream of the ansB gene and coded for L-asparaginase. These two genes were in an operon designated the ans operon, which is 80% cotransformed with the previously mapped aspH1 mutation at 215 degrees. Primer extension analysis of in vivo ans mRNA revealed two transcription start sites, depending on the growth medium. In wild-type cells in log-phase growth in 2x YT medium (tryptone-yeast extract rich medium), the ans transcript began at -67 relative to the translation start site, while cells in log-phase growth or sporulating (t1 to t4) in 2x SG medium (glucose nutrient broth-based moderately rich medium) had an ans transcript which began at -73. The level of the -67 transcript was greatly increased in an aspH mutant grown in 2x YT medium; the -67 transcript also predominated when this mutant was grown in 2x SG medium, although the -73 transcript was also present. In vitro transcription of the ans operon by RNA polymerase from log-phase cells grown in 2x YT medium and log-phase or sporulating cells grown in 2x SG medium yielded only the -67 transcript. Depending on the growth medium, the levels of asparaginase and aspartase were from 2- to 40-fold higher in an aspH mutant than in wild-type cells, and evidence was obtained indicating that the gene defined by the aspH1 mutation codes for a trans-acting transcriptional regulatory factor. In wild-type cells grown in 2x SG medium, the levels of both aspartase and asparaginase decreased significantly by t0 of sporulation but then showed a small increase, which was mirrored by changes in the level of beta-galactosidase from an ansB-lacZ fusion. The increase in the activities of ans operon enzymes between t2 and t5 of sporulation was found primarily in the forespore, and the great majority of the increased was found in the mature spore. However, throughout sporulation the only ans transcript detected was the -73 form, and no sporulation-specific RNA polymerase tested yielded a -73 transcript in vitro.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli A. J., Suehiro S., Sakiyama D., Takemoto J., Vivanco E., Lara J. C., Klute M. C. Release and recovery of forespores from Bacillus cereus. J Bacteriol. 1973 Sep;115(3):1159–1166. doi: 10.1128/jb.115.3.1159-1166.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. R., Fisher S. H. Identification of genes and gene products whose expression is activated during nitrogen-limited growth in Bacillus subtilis. J Bacteriol. 1991 Jan;173(1):23–27. doi: 10.1128/jb.173.1.23-27.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. R., Wray L. V., Jr, Fisher S. H. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J Bacteriol. 1990 Sep;172(9):4758–4765. doi: 10.1128/jb.172.9.4758-4765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther D. L-asparaginase and human malignant disease. Nature. 1971 Jan 15;229(5281):168–171. doi: 10.1038/229168a0. [DOI] [PubMed] [Google Scholar]
- Feavers I. M., Price V., Moir A. The regulation of the fumarase (citG) gene of Bacillus subtilis 168. Mol Gen Genet. 1988 Mar;211(3):465–471. doi: 10.1007/BF00425702. [DOI] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulger D., Errington J. The role of the sporulation gene spoIIIE in the regulation of prespore-specific gene expression in Bacillus subtilis. Mol Microbiol. 1989 Sep;3(9):1247–1255. doi: 10.1111/j.1365-2958.1989.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. P., Milikin E. B., Bobbitt J. L., Grinnan E. L., Burck P. J., Frank B. H., Boeck L. D., Squires R. W. Crystalline L-asparaginase from Escherichia coli B. I. Purification and chemical characterization. J Biol Chem. 1970 Jul 25;245(14):3708–3715. [PubMed] [Google Scholar]
- Iijima T., Diesterhaft M. D., Freese E. Sodium effect of growth on aspartate and genetic analysis of a Bacillus subtilis mutant with high aspartase activity. J Bacteriol. 1977 Mar;129(3):1440–1447. doi: 10.1128/jb.129.3.1440-1447.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. P., Beacham I. R. Analysis of the Escherichia coli gene encoding L-asparaginase II, ansB, and its regulation by cyclic AMP receptor and FNR proteins. J Bacteriol. 1990 Mar;172(3):1491–1498. doi: 10.1128/jb.172.3.1491-1498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlström P. G., Bezjak D. A., Jennings M. P., Beacham I. R. Structure and expression in Escherichia coli K-12 of the L-asparaginase I-encoding ansA gene and its flanking regions. Gene. 1989 May 15;78(1):37–46. doi: 10.1016/0378-1119(89)90312-0. [DOI] [PubMed] [Google Scholar]
- Karsten W. E., Hunsley J. R., Viola R. E. Purification of aspartase and aspartokinase-homoserine dehydrogenase I from Escherichia coli by dye-ligand chromatography. Anal Biochem. 1985 Jun;147(2):336–341. doi: 10.1016/0003-2697(85)90280-5. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kunkel B., Losick R. Switch protein alters specificity of RNA polymerase containing a compartment-specific sigma factor. Science. 1989 Jan 27;243(4890):526–529. doi: 10.1126/science.2492118. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Loshon C. A., Fliss E. R., Setlow B., Foerster H. F., Setlow P. Cloning and nucleotide sequencing of genes for small, acid-soluble spore proteins of Bacillus cereus, Bacillus stearothermophilus, and "Thermoactinomyces thalpophilus". J Bacteriol. 1986 Jul;167(1):168–173. doi: 10.1128/jb.167.1.168-173.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Fajardo-Cavazos P., Setlow P. Levels of mRNAs which code for small, acid-soluble spore proteins and their LacZ gene fusions in sporulating cells of Bacillus subtilis. Nucleic Acids Res. 1988 Jul 25;16(14A):6567–6583. doi: 10.1093/nar/16.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Moir A., Feavers I. M., Guest J. R. Characterization of the fumarase gene of Bacillus subtilis 168 cloned and expressed in Escherichia coli K12. J Gen Microbiol. 1984 Nov;130(11):3009–3017. doi: 10.1099/00221287-130-11-3009. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Nicholson W. L., Sun D. X., Setlow B., Setlow P. Promoter specificity of sigma G-containing RNA polymerase from sporulating cells of Bacillus subtilis: identification of a group of forespore-specific promoters. J Bacteriol. 1989 May;171(5):2708–2718. doi: 10.1128/jb.171.5.2708-2718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. G., Richards F. F., Handschumacher R. E. Structure of peptide from active site region of Escherichia coli L-asparaginase. J Biol Chem. 1977 Mar 25;252(6):2072–2076. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorenstein R. G., Losick R. Comparative size and properties of the sigma subunits of ribonucleic acid polymerase from Bacillus subtilis and Escherichia coli. J Biol Chem. 1973 Sep 10;248(17):6170–6173. [PubMed] [Google Scholar]
- Sun D. X., Cabrera-Martinez R. M., Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor sigma G. J Bacteriol. 1991 May;173(9):2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Stragier P., Setlow P. Identification of a new sigma-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989 Feb;3(2):141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- Svobodová O., Strbánová-Necinová S. Induction of L-asparaginase synthesis in Escherichia coli. Biochim Biophys Acta. 1973 Oct 10;321(2):643–652. doi: 10.1016/0005-2744(73)90208-8. [DOI] [PubMed] [Google Scholar]
- Switzer R. L., Bond R. W., Ruppen M. E., Rosenzweig S. Involvement of the stringent response in regulation of protein degradation in Bacillus subtilis. Curr Top Cell Regul. 1985;27:373–386. doi: 10.1016/b978-0-12-152827-0.50039-6. [DOI] [PubMed] [Google Scholar]
- Takagi J. S., Ida N., Tokushige M., Sakamoto H., Shimura Y. Cloning and nucleotide sequence of the aspartase gene of Escherichia coli W. Nucleic Acids Res. 1985 Mar 25;13(6):2063–2074. doi: 10.1093/nar/13.6.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J. S., Tokushige M., Shimura Y. Cloning and nucleotide sequence of the aspartase gene of Pseudomonas fluorescens. J Biochem. 1986 Sep;100(3):697–705. doi: 10.1093/oxfordjournals.jbchem.a121762. [DOI] [PubMed] [Google Scholar]
- Takagi T., Kisumi M. Isolation of a versatile Serratia marcescens mutant as a host and molecular cloning of the aspartase gene. J Bacteriol. 1985 Jan;161(1):1–6. doi: 10.1128/jb.161.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis R. C., Woolfolk C. A. Asparagine utilization in Escherichia coli. J Bacteriol. 1974 Apr;118(1):231–241. doi: 10.1128/jb.118.1.231-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. A., Miles J. S., Roberts R. E., Guest J. R. Structural and functional relationships between fumarase and aspartase. Nucleotide sequences of the fumarase (fumC) and aspartase (aspA) genes of Escherichia coli K12. Biochem J. 1986 Jul 15;237(2):547–557. doi: 10.1042/bj2370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]






