Abstract
Macrophage migration inhibitory factor (MIF), originally identified as a cytokine secreted by T lymphocytes, was found recently to be both a pituitary hormone and a mediator released by immune cells in response to glucocorticoid stimulation. We report here that the insulin-secreting β cell of the islets of Langerhans expresses MIF and that its production is regulated by glucose in a time- and concentration-dependent manner. MIF and insulin colocalize by immunocytochemistry within the secretory granules of the pancreatic islet β cells, and once released, MIF appears to regulate insulin release in an autocrine fashion. In perifusion studies performed with isolated rat islets, immunoneutralization of MIF reduced the first and second phase of the glucose-induced insulin secretion response by 39% and 31%, respectively. Conversely, exogenously added recombinant MIF was found to potentiate insulin release. Constitutive expression of MIF antisense RNA in the insulin-secreting INS-1 cell line inhibited MIF protein synthesis and decreased significantly glucose-induced insulin release. MIF is therefore a glucose-dependent, islet cell product that regulates insulin secretion in a positive manner and may play an important role in carbohydrate metabolism.
Keywords: diabetes, endocrine pancreas, gene regulation, cytokine, MIF
The protein originally called macrophage migration inhibitory factor (MIF) was described 30 years ago as one of the first soluble mediators, or lymphokines, to be released by activated T lymphocytes (1, 2). A much broader role for MIF has recently emerged from studies that have identified this protein to be an abundant preformed constituent of the anterior pituitary gland and of monocytes/macrophages (3, 4). MIF is colocalized with corticotropin (ACTH) and thyroid stimulating hormone (TSH) in the secretory granules of corticotrophic and thyrotrophic cells of the pituitary gland and is released into the bloodstream in response to various proinflammatory stimuli (5). MIF plays a pivotal role in the host response to endotoxic shock, and the administration of neutralizing anti-MIF antibodies fully protects animals from lethal endotoxemia (3). A unique property of MIF is its release from immune cells after stimulation with glucocorticoid hormones (6). Once secreted, MIF acts to “override” or counter-regulate the suppressive effects of glucocorticoid on inflammatory cytokine production (6).
The identification of significant quantities of preformed MIF within the anterior pituitary gland and its release from immune cells upon stimulation with steroids suggested that MIF could function in other contexts as a protein mediator within the endocrine system. We performed immunohistochemical studies in rat tissues to examine the localization of MIF within endocrine tissues. In this report, we show that abundant quantities of MIF mRNA and protein are detected in primary rat islets of Langerhans. MIF is highly expressed in several insulin-secreting cell lines, colocalizes with insulin-containing secretory granules, and is secreted in response to glucose stimulation in a time- and concentration-dependent manner. Immunoneutralization of MIF in perifusion studies or the constitutive expression of MIF antisense RNA in an insulin-secreting cell line reduced significantly the first and second phase of glucose-induced insulin secretion. Taken together, these data suggest that pancreatic β cell MIF is an important, autocrine regulator of insulin secretion.
METHODS
Cell Lines.
The transplantable x-ray induced rat insulinoma INS-1 cell line was kindly provided by Asfari et al. and cultured as described (7). The mouse insulin-producing βTC-3 cell line, the rat insulin- and somatostatin-producing RIN-1027 B2 cell line, the hamster glucagon-producing InR1-G9 cell line, the human choriocarcinoma JEG-3 cell line, and the monkey kidney-derived COS-1 cell line were cultured in RPMI 1640 medium containing 10% fetal calf serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin (8).
RNA and Northern Blot Analysis.
Total cellular RNA was prepared by scraping cells from two 10-cm dishes in the presence of guanidinium buffer. The RNAs were pelleted at 100,000 × g for 17 hr in a Beckman Ti 50 rotor. For Northern blot analysis, 10 μg of total RNA was size-fractionated on 1× Mops/1.2% agarose gels containing formaldehyde. Gels were transferred overnight by diffusion [10× standard saline citrate (SSC)] to GeneScreen membranes (Dupont). The membranes were UV cross-linked and baked for 2 hr at 80°C. After prehybridization, the blots were hybridized overnight at 42°C with random-primed (Boehringer, Mannheim) mouse MIF, rat GLUT2, rat insulin, and actin probes (9, 10) in 5× SSC/100 mM NaHPO4, pH 6.5/5× Denhardt’s solution/50% formamide/10 mM EDTA/1% SDS/100 μg/ml yeast tRNA. The blots then were washed in 2× SSC followed by 0.2× SSC containing 0.1% SDS at 60°C. Directly after washing, the blots were quantified by electronic autoradiography with an Instant Imager 2024 (Packard). The blots then were exposed to Hyperfilm-MP (Amersham).
Islets Isolation and Northern and Western Blot Analyses.
The bile ducts of six anesthetized, Sprague–Dawley rats were cannulated with a 27-gauge needle, and the pancreas was distended with a solution containing 2 mg/ml collagenase. Each pancreas then was incubated in a tissue culture flask at 37°C and the islets isolated by the method of Gotoh et al. (11). Total RNA was extracted from 100 and 200 islets by the method of Chomczynski et al. (12). Separate experiments were performed using 400 rat pancreatic islets incubated in RPMI 1640 medium containing 2.8 or 30 mM glucose for 12 hr, after which the RNA was extracted for Northern blot analysis.
Proteins were extracted from INS-1 cells, and islets were freshly isolated or kept in culture for up to 3 days. Lysis was performed with Tris-buffered saline containing 1% Nonidet P-40 and 2 mM EDTA, after which the cellular debris was pelleted and the supernatants were collected. The protein contents were measured using the Bio-Rad protein assay kit. Cellular proteins (40 μg) were diluted with an equal volume of reducing SDS/PAGE sample buffer and electrophoresed through 18% polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell) at 50 V for 14 hr. Membranes were blocked with PBS containing 5% nonfat dry milk and incubated first with anti-MIF polyclonal rabbit serum (3) (diluted 1:800) and then with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (diluted 1:5,000). Incubations were performed for 1 hr each. MIF was visualized with the chemiluminescence ECL system (Amersham).
Immunocytochemistry.
Adult mice were anesthetized and rapidly perfused with 20 ml of PBS followed by a continuous perfusion of 4% paraformaldehyde dissolved in PBS. The pancreata were removed and paraffin-embedded, and 8-μm sections were used for immunohistochemistry. After a preincubation with 5% H2O2 for 15 min, the sections were rinsed in 0.5% Triton X-100 dissolved in PBS for 15 min and then incubated for 2 hr in PBS supplemented with 5% BSA/0.5% Triton X-100/2% goat serum. Sections then were incubated for 20 hr at 4°C with anti-recombinant MIF (anti-rMIF) or pre-immune serum, anti-insulin, anti-GLUT2 antibodies (3, 13), and anti-CD14 antibodies (PharMingen). Polyclonal anti-MIF antibody was raised to mouse rMIF and has been shown previously to react specifically with MIF in tissue sections (14). Anti-MIF IgGs were purified by protein G affinity chromatography. Sections then were incubated 4 hr at room temperature with the secondary antibody (biotinylated goat anti-rabbit IgG diluted 1:200 in PBS) and then for 2 hr at room temperature with avidin-biotinylated peroxidase complex (diluted 1:50 in PBS, ABC kit, Vector Laboratories). The peroxidase reactions were visualized with 3,3′-diaminobenzidine tetrahydrochloride dihydrate (Fluka) dissolved in PBS containing 0.3% H2O2. Photographs were taken with a Reichert polyvar microscope using transmitted light optics and photographed on Kodak Ektachrome EPJ 320 film.
For the confocal experiments, INS-1 cells were plated on glass coverslips 48 hr before the immunostaining. The cells were fixed in ice-cold methanol/acetone (50:50) and incubated for 1 hr in a solution containing PBS, 0.5% BSA, and a monoclonal anti-MIF or a guinea-pig anti-insulin antibody (each diluted 1:800). Detection was by fluorescein isothiocyanate-labeled goat anti-mouse antibodies or Texas Red-labeled goat anti-guinea pig antibodies. The specificity of the antisera was tested by preabsorption of the antiserum with the respective antigen (10 μg/ml of rMIF). The fluorescence images were obtained with a complete inverted confocal laser scan microscope LSM 410 equipped with a HeNe-laser (543 nm) and an Ar-laser (514 nm/488 nm). The HeNe-laser was used to excite the Texas Red (emission filter LP 570 nm), and the Ar-laser was used to excite the fluorescein (emission filter BP 515 nm/525 nm). The scanner and detectors were attached to an inverted microscope (Axiovert M135, Zeiss). An oil-immersion, 1.4 n.a., plan-apochromat 100 × objective (Zeiss) was used for image acquisitions.
Perifusion Protocol.
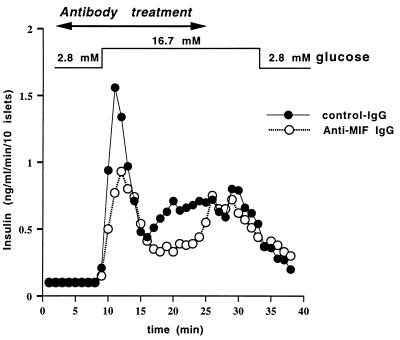
Ten isolated rat pancreatic islets were incubated in a perifusion chamber and perifused at 1 ml/min with Krebs–Ringer buffer. Three separate experiments were performed using different preparations of rat pancreatic islets. The glucose concentration was increased experimentally from 2.8 mM (−40 to +7 min) to 16.7 mM (+8 to +33 min) and then returned to 2.8 mM (+34 to +40 min). The perifusion chamber was maintained at 37°C, and the gas phase was 95:5 O2/CO2. Nonimmune IgG or anti-MIF IgG, each at a concentration of 50 μg/ml, was added to the chamber from +2 to +24 min. The anti-MIF IgG was previously shown to be able to immunoneutralize MIF in vivo (3, 15). The antibody preparations were determined to contain no significant endotoxin (<25 pg/mg) when analyzed by the chromogenic Limulus amebocyte assay (BioWhittaker). The control and immunoneutralized perifusions were repeated three times. The insulin radioimmunoassay was done according to the manufacturer’s recommendations (Linco Research Immunoassay, St. Charles, MO). No interference with the insulin assay was observed with the use of nonimmune IgG or anti-MIF IgG.
MIF-Induced Secretion of Insulin in Rat Pancreatic Islets.
Eight rat pancreatic islets per well were incubated in Krebs–Ringer buffer supplemented with 0.5% BSA for 60 min with 2.7 mM glucose followed by a 60-min incubation period in the presence of 16.7 mM glucose and 1 mM 3-isobutyl-1-methyl-xanthine. rMIF was produced exactly as described previously (3, 4), and 0.1–100 pg/ml of the cytokine was added during the high glucose concentration incubation period. The secreted insulin was measured in the 2.7 and 16.7 mM glucose incubation medium, and the percentage of insulin secretion during 60 min was normalized to the insulin content of the eight pancreatic islets determined at the end of the experiment by acid-ethanol extraction. The experiments were done twice in triplicate.
MIF Antisense RNA Experiments.
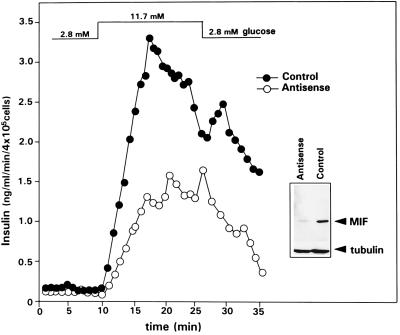
A MIF antisense expression plasmid was constructed by cloning the murine MIF cDNA (9) in 3′–5′ orientation (KpnI/PstI) into the cytomegalovirus promoter-containing expression vector pBK (Stratagene). The pBK/antisense MIF expression vector or the parental (control) pBK vector was transfected into INS-1 cells with the cationic detergent DOTAP (Boehringer Mannheim), and stable clones were selected by resistance to G418. Either antisense (4 × 105) or control INS-1 cells were incubated in a perifusion chamber, and the glucose concentration was increased from 2.8 mM (−40 to +8 min) to 11.7 mM together with 1 mM 3-isobutyl-1-methyl-xanthine and 1 μM forskolin (+9 to +26 min) and then returned to 2.8 mM (+27 min).
RESULTS
Immunohistochemical Localization of MIF in the Islets of Langerhans of the Pancreas.
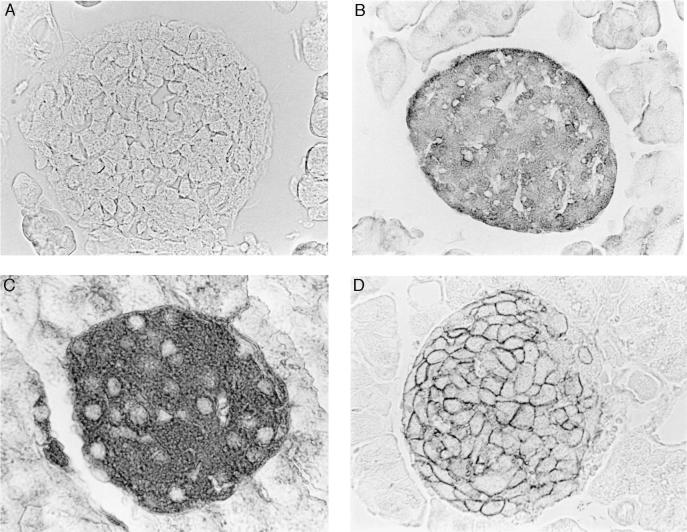
Mouse pancreata were immunostained with anti-MIF, anti-insulin, and anti-GLUT2 antibodies. MIF was visualized within the cytoplasm of islet cells that also were positive for insulin and GLUT2 (Fig. 1). The GLUT2-positive staining was predominantly localized to cell membranes (10, 13). The cells that were positive for MIF, insulin, and GLUT2 did not react with a specific anti-CD14 antibody (data not shown), indicating that the MIF staining was not due to contaminating islet macrophages, as cells of the monocyte/macrophage lineage have been shown previously to contain preformed MIF protein (4).
Figure 1.
Immunohistochemical localization of MIF in the islets of Langerhans of the pancreas. MIF is detected within the cytoplasm of islet cells (B) that also are positive for insulin (C) and GLUT2 (D). The GLUT2-positive staining is localized predominantly to cell membranes (10, 13). No MIF is detected when cells are stained with preimmune serum (negative control) (A).
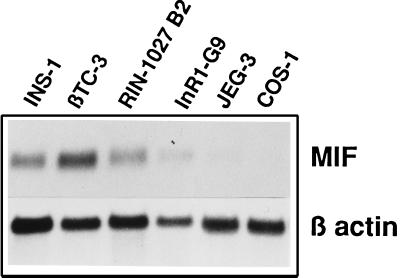
The MIF Gene Is Expressed in β Cell Lines and MIF Protein Colocalizes to the Insulin-Containing Granules.
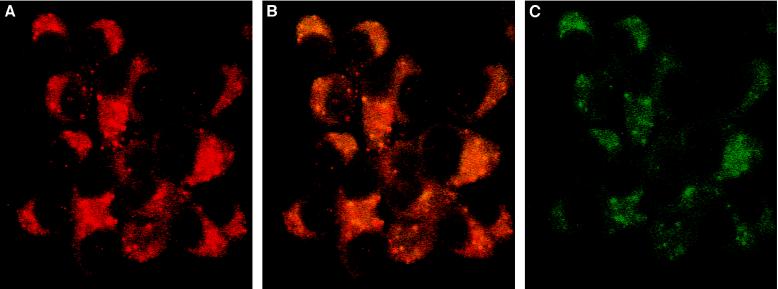
Several phenotypically distinct cell lines derived from the islets of Langerhans were analyzed for MIF expression by Northern blotting. High levels of MIF mRNA were detected at baseline in the insulin-producing cell lines INS-1 and βTC3, and in RIN-1027 B2 cells, which also secrete somatostatin and glucagon (Fig. 2). MIF mRNA also was detected in a much lower extent in the glucagon-producing cell line (InR1-G9). Double immunostaining then was performed in the insulin-secreting INS-1 cells to determine the subcellular localization of MIF protein. As shown in Fig. 3, MIF and insulin localize to the cytoplasm of INS-1 cells. The immunofluorescence intensity was greater for insulin than for MIF (Fig. 3 A and C, respectively), suggesting that INS-1 cells may contain more insulin than MIF. Some granules (stained in red in Fig. 3B) contain only insulin, whereas others contain a mixture of MIF and insulin (appearing as orange-yellow granules in Fig. 3B). No granules appear to contain MIF alone. Because both hormones colocalize within secretory granules of INS-1 cells, the secretion of MIF may be regulated in the same manner as insulin.
Figure 2.
MIF is highly expressed in pancreatic β cell lines. Northern blotting analyses of MIF mRNA (0.6 kb) and β-actin mRNA (1.8 kb) expression in two insulin-producing cell lines (INS-1 and βTC3), an insulin and somatostatin cell line (RIN1027-B2), a glucagon-producing cell line (InR1-G9), and nonpancreatic cell lines (JEG-3 and COS-1). Total RNA (10 μg) was prepared and analyzed as described in Methods. The MIF transcript is detected in high abundance in the insulin-producing cell lines.
Figure 3.
MIF is colocalized in the insulin-containing granule of β cells. INS-1 cells were stained with anti-insulin (A), anti-insulin and anti-MIF (B), or anti-MIF IgG (C) and revealed by immunofluorescence (Texas Red for the anti-insulin antibodies and fluorescein isothiocyanate for the anti-MIF). Insulin granules are red, MIF staining is green, and the orange-yellow staining corresponds to granules containing MIF and insulin. Fluorescence images were obtained with confocal laser scan microscope. (×60.)
Expression and Glucose-Dependent Regulation of MIF mRNA in Pancreatic β Cell Lines and in Isolated Rat Pancreatic Islets.
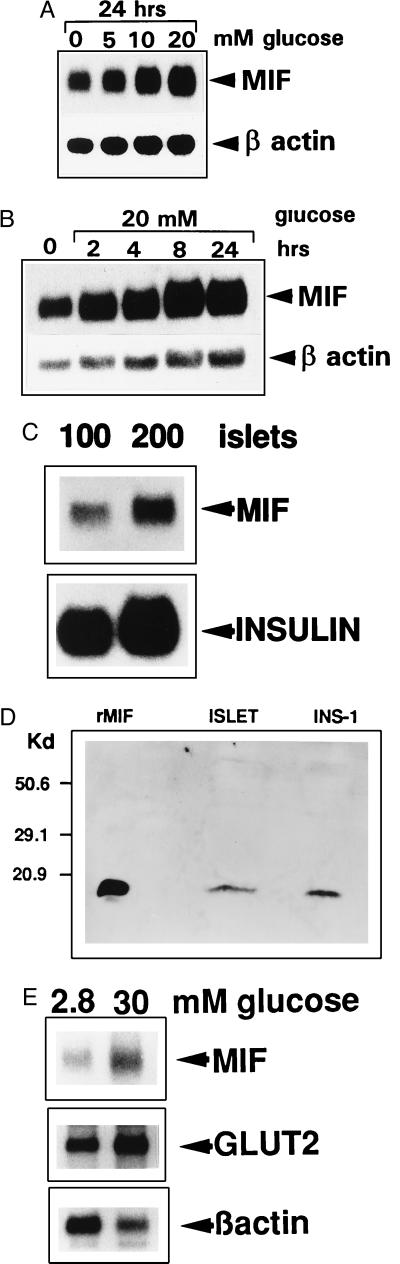
The MIF gene was found to be constitutively expressed in the highly differentiated, insulin-secreting INS-1 cells and to be up-regulated by glucose in a concentration- and time-dependent manner (Fig. 4 A and B) (7). Normalized to β-actin levels, MIF mRNA expression increased 3-fold after stimulation with 20 mM glucose for 24 hr when assessed by laser densitometric analysis (Table 1). MIF also was found to be expressed in primary cells of the endocrine pancreas. Islets of Langerhans from Sprague–Dawley rats were isolated, and the RNA was extracted from 100 and 200 islets and analyzed by Northern blotting. MIF mRNA was detected in as few as 100 pancreatic islets (Fig. 4C), and MIF protein was detected in 40 μg of crude cellular extracts obtained from these islets (Fig. 4D). Islet MIF mRNA levels also increased after stimulation in vitro with 30 mM glucose, as did mRNA for the glucose transporter GLUT2, which has been shown previously to be regulated by glucose at the transcriptional level (16) (Fig. 4E). MIF/β-actin and GLUT2/β-actin mRNA ratios increased 4-fold over 24 hr in the presence of 30 mM glucose (Table 1).
Figure 4.
Expression and glucose-dependent regulation of MIF mRNA in pancreatic β cell lines and in isolated rat pancreatic islets. (A) Northern blot analysis of MIF mRNA and β-actin mRNA expression by INS-1 cells incubated with 0, 5, 10, and 20 mM glucose in RPMI 1640 medium for 24 hr. (B) Time course of MIF mRNA expression by INS-1 cells incubated with 20 mM glucose. Total RNA was extracted at intervals, and MIF mRNA was analyzed by Northern blotting as described in the Methods. (C) Islets of Langerhans were isolated from Sprague–Dawley rats, total RNA was extracted from 100 and 200 islets and analyzed by Northern blotting using MIF and rat proinsulin II cDNA probes. (D) Western blot analysis of the MIF content of INS-1 cells or isolated islets of Langerhans. Protein extracts (40 μg) obtained from INS-1 cells or islets of Langerhans were size-fractionated on an SDS/18% polyacrylamide gel, transferred to nitrocellulose, and blotted with an anti-MIF antiserum. rMIF (50 ng) served as a positive control and size marker. (E) Northern blot analysis of MIF, GLUT-2, and β-actin mRNA expression by 400 pancreatic islets incubated for 12 hr in RPMI 1640 medium containing 2.8 or 30 mM glucose. Normalized to β-actin, MIF and GLUT2 mRNA increased 4-fold when the glucose concentration was raised from 2.8 to 30 mM.
Table 1.
Quantitative assessment of MIF/β-actin expression
| Cells | Glucose concentration, mM | Expression, %
|
||||
|---|---|---|---|---|---|---|
| Incubation time, hr
| ||||||
| 0 | 2 | 4 | 8 | 24 | ||
| INS-1 | 20 | 100 | 140 | 172 | 220 | 320 |
| INS-1 | 0 | 100 | ||||
| 5 | 132 | |||||
| 10 | 190 | |||||
| 20 | 310 | |||||
| Pancreatic islets | 2.8 | 100 | ||||
| 30 | 410 | |||||
MIF expression was assessed by laser densitometric scanning and measured by Northern blot analysis (100% = value obtained in controls at 0 mM glucose concentration in INS-1 cells or at 2.8 mM glucose in the isolated pancreatic islets).
Immunoneutralization of Islet Cell MIF Inhibits Insulin Secretion.
The observations that MIF production by the β cells of the islets is induced by glucose and that glucose is the most important physiological stimulus of insulin release (17) suggested to us a possible role for MIF in insulin secretion by pancreatic islets. To address that question, insulin release was measured in the presence of neutralizing anti-MIF antibody or control IgG in an isolated rat islet perfusion system during stepwise changes in glucose concentrations from 2.8 to 16.7 mM (Fig. 5). Anti-MIF IgG, at a concentration of 50 μg/ml, had been shown previously to neutralize MIF in vivo (3, 15). As shown in Fig. 5, anti-MIF IgG inhibited the first and second phases of glucose-induced insulin secretion by 39% and 31%, respectively. Antibody treatment had no effect on insulin release during the first 6 min at low glucose concentration. At the end of the neutralizing antibody treatment period, the glucose-induced insulin secretion returned to control levels, suggesting that the effects observed with these antibodies are directly related to the loss of endogenous MIF. We next examined whether recombinant MIF could potentiate insulin secretion from rat pancreatic islets. In a static experiment, the addition of 1 or 10 pg/ml of recombinant MIF increased by 140% the glucose-induced insulin secretion (data not shown).
Figure 5.
Immunoneutralization of islet cell MIF inhibits insulin secretion. Anti-MIF antibodies decrease the first and second phase of glucose-induced insulin secretion by isolated pancreatic islets. Ten purified rat islets were incubated in a perifusion chamber and the glucose concentration of the perifused buffer was increased experimentally from 2.8 mM (−40 to +7 min) to 16.7 mM (+8 to +33 min) and then returned to 2.8 mM (+34 min) as shown. The results are expressed as the mean ± SEM of three separate perifusion experiments. Control, nonimmune IgG or anti-MIF IgG (each at 50 μg/ml) was added to the chamber from +2 to +24 min. The quantity of insulin released during the first phase (+8 to +15 min) and the second phase (+16 to +25 min) of insulin secretion showed a decrease after immunoneutralization of MIF from 1.17 ± 0.19 ng/ml to 0.71 ± 0.06 ng/ml and 0.74 ± 0.02 ng/ml to 0.51 ± 0.01 ng/ml, respectively (P < 0.01 and P < 0.001 for the first and second phases of insulin secretion, respectively; Student’s t test).
MIF Antisense RNA Inhibits MIF Protein Expression and Glucose-Induced Insulin Release.
We next produced stably transfected INS-1 clones containing either an integrated MIF antisense expression plasmid or a parental (control) plasmid. Western blot analyses of the MIF content of cell lysates obtained from the clone transfected with the MIF antisense RNA showed a significant reduction of the MIF protein when compared with the control clone (Fig. 6). We then examined the insulin secretion of both clones during glucose administration in a perifusion system. Compared with INS-1 cells transfected with the control vector, cells transfected with the MIF antisense RNA showed a marked decrease in the glucose-induced insulin secretion (Fig. 6). Taken together with the data obtained with anti-MIF IgG, these studies indicate that islet cell MIF potentiates the insulin secretion induced by glucose.
Figure 6.
MIF antisense RNA inhibits MIF protein expression and glucose-induced insulin release by INS-1 cells. Antisense MIF or parental (control) expression vector were stably integrated into INS-1 cells. Western blotting of the MIF content of the cell lysates (20 μg total protein) obtained from an antisense transfected clone versus a control clone showed a significant decrease in the MIF protein content in cells containing the antisense construct (Inset). Control or antisense INS-1 cells then were incubated in a perifusion chamber, and the glucose concentration of the perifused buffer (supplemented with 1 mM 3-isobutyl-1-methyl-xanthine and 1 μM forskolin) increased from 2.8 mM (−40 to +8 min) to 11.7 mM (+9 to +26 min) and then returned to 2.8 mM (+27 min).
DISCUSSION
Recent studies have identified MIF to be a new pituitary peptide that colocalizes to the ACTH and TSH secretory granules of the corticotrophic and thyrotrophic cells (3, 5). MIF is released from the pituitary gland upon activation of the hypothalamic–pituitary–adrenal axis and contributes to the increase in the serum concentration of MIF during endotoxemia and stress (3, 6). MIF also was found to be a proinflammatory mediator of macrophages and T cells and to act as a physiological counter-regulator of the anti-inflammatory and immunosuppressive effects of glucocorticoid hormones (6, 18). As such, MIF fulfills a previously unrecognized but critical function in the elaboration of the inflammatory and immune responses.
The present report provides the first description of the presence of MIF within the insulin-secreting β cells of the pancreas. In the highly differentiated, insulin-secreting cell line INS-1, the MIF gene is constitutively expressed and is up-regulated by glucose in a concentration- and time-dependent manner. This effect could be transcriptionally regulated as observed for the GLUT2 gene (16) and/or secondary to stabilization of MIF mRNA. The low level of expression of the MIF transcript in the glucagon-producing cell line InR1-G9 may reflect the tumoral origin of the cell line or some features of an ancestral origin of the β cells. As the precursor cells of the endocrine pancreas coexpress insulin, glucagon and neuropeptide Y and subsequently undergo differentiation with single hormone secretion, it may be not surprising to observe the presence of MIF transcript in these InR1-G9 glucagon-producing cells (19). Moreover, MIF protein was found to be present in large quantities within primary rat islets of Langerhans, where it is localized to the insulin-containing granules of the β cells.
The role of MIF within the endocrine pancreas was assessed by immunoneutralization of endogenous rat islet MIF and by inhibition of MIF protein expression in insulin-secreting INS-1 cells transfected with an MIF antisense RNA. In both systems, inhibition of endogenous MIF resulted in a significant decrease in the first and second phases of glucose-induced insulin secretion. Furthermore, the addition of rMIF to rat pancreatic islets increased significantly the secretion of insulin induced by glucose. These data provide strong evidence that MIF is a secretory product of the β cell that functions as an autocrine regulator of insulin release.
With the possible exception of the metabolite ATP, MIF is the first mediator identified to be released from islets in response to glucose stimulation and to regulate insulin secretion in a positive fashion. ATP was described previously to be coreleased with insulin and to participate in the potentiation of the glucose-induced insulin secretion (20–22). The mediator γ-aminobutyric acid, on the other hand, is secreted from synaptic-like microvesicles of β cells and may play a paracrine, inhibitory effect on glucagon secretion (23, 24). Similarly, islet amyloid polypeptide (IAPP or amylin) is a β cell product that is cosecreted with insulin and controls negatively insulin secretion within the islet (25–27). Finally, neuropeptide Y also is secreted from β cells and exerts autocrine or paracrine inhibitory effects on insulin secretion (28–30). However, in contrast to MIF, none of these molecules has been shown to directly potentiate insulin release. Within this context, it is tempting to speculate that a progressive decrease in MIF release and/or of MIF action within the islets of Langerhans may contribute to the β cell dysfunction and diminished insulin release associated with type II diabetes (31–33).
The present data significantly expand the important regulatory role of MIF in the body. MIF is an integral component of the stress response and is released by the pituitary gland, the macrophage, and the T lymphocyte in response to inflammation, infection, and stress. Within the immune system, MIF functions as an antagonist of the anti-inflammatory and immunosuppressive effects of steroids (4, 6). High circulating steroid levels are associated with increased serum glucose and a state of peripheral insulin resistance. Severe or chronic inflammatory conditions are often accompanied by a syndrome of metabolic derangement that include hyperlipidemia, hyperglycemia, and insulin resistance (34). Under such conditions, high circulating levels of MIF derived either from immune cells or other tissue compartments may serve to potentiate insulin release, preserve the overall metabolic integrity of the host, and promote survival.
Acknowledgments
We thank Myriam Steinmann for excellent technical assistance and Gabriel Centeno for taking the pictures on the confocal microscope. G.W. is supported by a career award from the Swiss National Science Foundation (32-31915.91, 32-29317.91, and 32-49673.96) and by a grant from the Juvenile Diabetes Foundation International (194183), and part of this work was supported by the Placide Nicod Foundation. J.-A.H. is supported by a career award from the Max Cloëtta Foundation. R.B. and C.N.M. are supported by National Institutes of Health Grant AI35931 and T.C. by a career award from the Swiss National Science Foundation (32-48916.96 and 32-49129.96) and by a grant from the Fonds de Perfectionnement du Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland.
ABBREVIATIONS
- MIF
macrophage migration inhibitory factor
- rMIF
recombinant MIF
References
- 1.Bloom B R, Bennet B. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 2.David J. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhagen J, Calandra T, Mitchell R A, Martin S B, Tracey K J, Voelter W, Manogue K R, Cerami A, Bucala R. Nature (London) 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 4.Calandra T, Bernhagen J, Mitchell R A, Bucala R. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishino T, Bernhagen J, Shiki H, Calandra T, Dohi K, Bucala R. Mol Med. 1995;1:781–788. [PMC free article] [PubMed] [Google Scholar]
- 6.Calandra T, Bernhagen J, Metz C N, Spiegel L A, Bacher M, Donnelly T, Cerami A, Bucala R. Nature (London) 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 7.Asfari M, Janjic D, Meda P, Li G, Halban P A, Wollheim C B. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 8.Philippe J, Chick W L, Habener J F. J Clin Invest. 1987;79:351–358. doi: 10.1172/JCI112819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell R, Bacher M, Bernhagen J, Pushkarskaya T, Seldin M F, Bucala R. J Immunol. 1995;154:3863–3870. [PubMed] [Google Scholar]
- 10.Thorens B, Sarkar H K, Kaback H R, Lodish H F. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 11.Gotoh M, Maki T, Satomi T, Porter J, Bonner-Weir S, O’Hara C J, Monaco A P. Transplantation. 1987;43:725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Chomczynski P, Sacchi N. Anal Biochem. 1993;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 13.Thorens B, Wu Y-J, Leahy J L, Weir G C. J Clin Invest. 1992;90:77–85. doi: 10.1172/JCI115858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan H Y, Mu W, Yang N, Meinhardt A, Nikolic-Paterson D J, Ng Y Y, Bacher M, Atkins R C, Bucala R. Am J Pathol. 1996;149:1119–1127. [PMC free article] [PubMed] [Google Scholar]
- 15.Bernhagen J, Bacher M, Calandra T, Metz C N, Doty S B, Donnelly T, Bucala R. J Exp Med. 1996;183:277–282. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waeber G, Thompson N, Haefliger J-A, Nicod P. J Biol Chem. 1994;269:26912–26919. [PubMed] [Google Scholar]
- 17.Meglasson M D, Matschinsky F M. Diabetes Metab Rev. 1986;2:163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- 18.Bacher M, Metz C N, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teitelman G, Alpert S, Polak J M, Martinez A, Hanahan D. Development (Cambridge, UK) 1993;118:1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 20.Wollheim C B, Sharp G W. Physiol Rev. 1981;61:914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 21.Leitner J W, Sussman K E, Vatter A E, Schneider F H. Endocrinology. 1975;95:662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- 22.Kuromi H, Mizuno N, Seino S. Diabetes. 1995;44:1213–1217. doi: 10.2337/diab.44.10.1213. [DOI] [PubMed] [Google Scholar]
- 23.Reetz A, Solimena M, Matteoli M, Folli F, Takei K, De Camilli P. EMBO J. 1991;10:1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rorsman P, Berggren P-O, Bokvist K, Ericson H, Möhler H, Ostenson C-G, Smith P A. Nature (London) 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo A, Razzaboni B, Weir G C, Yankner B A. Nature (London) 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 26.Westermark P, Wernstedt C, Wilander E, Hayden D W, O’Brien T D, Johnson K H. Proc Natl Acad Sci USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson K H, O’Brien T D, Betsholtz C, Westermark P. N Engl J Med. 1989;321:513–518. doi: 10.1056/NEJM198908243210806. [DOI] [PubMed] [Google Scholar]
- 28.Jamal H, Jones P M, Byrne J, Suda K, Ghatei M A, Kanse S M, Bloom S R. Endocrinology. 1991;129:3372–3380. doi: 10.1210/endo-129-6-3372. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z-L, Bennet W M, Wang R, Ghatei M A, Bloom S R. Endocrinology. 1994;135:200–206. doi: 10.1210/endo.135.1.8013354. [DOI] [PubMed] [Google Scholar]
- 30.Waeber G, Thompson N, Waeber B, Brunner H-R, Nicod P, Grouzmann E. Endocrinology. 1993;133:1061–1067. doi: 10.1210/endo.133.3.8396008. [DOI] [PubMed] [Google Scholar]
- 31.Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J. N Engl J Med. 1992;326:22–29. doi: 10.1056/NEJM199201023260104. [DOI] [PubMed] [Google Scholar]
- 32.Polonsky K S, Sturis J, Bell G I. N Engl J Med. 1996;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E, Schalin C, Groop L. N Engl J Med. 1989;321:337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- 34.Milzock B A. Am J Med. 1995;98:75–84. doi: 10.1016/S0002-9343(99)80083-7. [DOI] [PubMed] [Google Scholar]