Abstract
Previously we reported that pretreatment of rats with the substance P (SP) antagonist CP-96,345 inhibits the enterotoxic responses following administration of toxin A from Clostridium difficile into ileal loops, indicating that SP participates in the intestinal responses to this toxin. We now report that injection of toxin A into rat ileum causes a rapid increase in SP content in lumbar dorsal root ganglia (DRG) and mucosal scrapings 30–60 min after toxin A administration. Toxin A-mediated fluid secretion, mannitol permeability, and ileal histologic damage is significantly increased only after 2 hr. Toxin A also causes an increase in the abundance of SP mRNA in lumbar DRG and ileal mucosa as measured by reverse transcription–PCR. Lamina propria macrophages (LPMs) obtained from toxin A-injected loops release greater amounts of tumor necrosis factor α (TNFα) and SP as compared with LPMs isolated from buffer-injected loops (P < 0.01). Pretreatment of rats with the SP antagonist CP-96,345 inhibits toxin A-mediated TNFα release from isolated LPMs, whereas an inactive enantiomer (CP-96,344) of the SP antagonist has no effect. LPMs obtained from toxin A-injected ileal loops incubated in vitro with SP (10−8 to 10−9 M) show enhanced TNFα secretion, whereas LPMs isolated from buffer-injected loops do not respond to SP. In addition, LPMs obtained from toxin A-injected ileal loops incubated in vitro with CP-96,345 showed a diminished TNFα release. Our results indicate that activated LPMs secrete SP during toxin A enteritis that can lead to secretion of cytokines, suggesting an autocrine/paracrine regulation of cytokine secretion by SP from LPMs during intestinal inflammation.
Substance P (SP), an 11-aa peptide member of the tachykinin family originally isolated by Chang and Leeman (1), is a peptide distributed throughout the central and peripheral nervous system. In the intestine, SP has been found in capsaicin-sensitive sensory neurons (2), enteric neurons (3), as well as intestinal enteroendocrine cells (4). SP is also synthesized in the cell bodies of the dorsal root ganglia (DRG) (5), and evidence indicates that release of SP from DRG to spinal cord mediates nociceptive and inflammatory stimuli (6–8). Recently, Reinshagen et al. (9) reported decreased levels of SP in the DRG of rabbits 48 hr after induction of colitis, suggesting that SP may be released from sensory neurons during the acute phase of inflammation. However, there are no studies specifically evaluating the SP content in the DRG during acute intestinal inflammation mediated by bacterial enterotoxins.
In addition to its role as a neurotransmitter, SP also participates in immune and inflammatory responses. Mast cells (10), polymorphonuclear leukocytes (11), T lymphocytes (12, 13), and macrophages (14) can respond to SP, indicating that the effects of SP during inflammation may be mediated by direct activation of these cells. SP levels are also elevated in inflamed tissues (15–17), and increased binding sites for SP have been demonstrated at sites of inflammation in conditions such as arthritis (18) and Crohn disease (19). In regard to intestinal inflammation, studies from our laboratory indicate that SP-containing sensory neurons are involved in the enterotoxic effects of Clostridium difficile toxin A, the causative agent of antibiotic-associated enterocolitis in animals and humans (20). Pretreatment of rats with capsaicin, an agent that leads to depletion of neurotransmitters from primary sensory neurons, inhibits fluid secretion and intestinal inflammation mediated by injection of toxin A into ileal loops (21). Furthermore, pretreatment of rats with the specific SP nonpeptide antagonist CP-96,345, but not with its inactive enantiomer, almost completely prevents toxin A-induced fluid secretion, increased mucosal permeability, mast cell degranulation, and mucosal neutrophil infiltration (22). Mantyh et al. (23), using another SP antagonist, confirmed that SP released from sensory neurons mediates toxin A-induced ileal histologic damage. Interestingly, injection of the SP antagonist 10 min after intraluminal injection of toxin A did not inhibit the enterotoxic effects of toxin A (22), suggesting that SP is involved in the early stages of toxin A-induced enteritis. These results strongly support participation of SP in enterotoxin-mediated intestinal secretion and inflammation. However, the cellular sources of SP in this animal model have not been investigated.
Macrophages are implicated in the pathophysiology of intestinal inflammation. Lamina propria macrophages (LPMs) in the intestinal mucosa of patients with Crohn disease and ulcerative colitis are activated (24, 25). Activation of LPMs results in the release of tumor necrosis factor α (TNFα) and other mediators that can directly stimulate intestinal ion transport (26, 27), suggesting a role for these cells in the pathophysiology of inflammatory diarrhea. A number of recent studies have indicated that SP and its receptor may be important in the modulation and amplification of macrophage function. For example, SP stimulates production of IL-1 from human blood monocytes (28) and there is an enhanced response of activated monocytes to SP (29). Bost et al. (30) reported that rat peritoneal macrophages express mRNAs encoding both SP and its receptor and that the abundance of these mRNAs is increased after stimulation with lipopolysaccharide (LPS). These results provide a pathway for autocrine/paracrine effects of SP in the activation of peritoneal macrophages. However, no reports directly demonstrate a SP–macrophage interaction during acute intestinal inflammation. We report here that administration of toxin A into rat ileal loops causes an increase in SP concentration in the intestinal mucosa and lumbar DRG and an increased responsiveness of LPMs to SP during toxin A enteritis. We also present evidence of an autocrine–paracrine interaction in activated LPMs that is mediated by SP.
MATERIALS AND METHODS
Materials.
[3H]mannitol (30 Ci/mmol; 1 Ci = 37 GBq) was obtained from New England Nuclear. Toxin A was purified to homogeneity from broth culture supernatants of C. difficile strain 10,463, as described (31). Enterotoxicity and cytotoxicity of toxin A were assessed as described (32, 33). A dose of 5 μg of purified toxin A was used in all experiments since previous studies showed that this dose induces fluid secretion, increases mannitol permeability, and causes an acute inflammatory infiltrate in rat ileal loops in vivo (21, 22, 31–33). SP was purchased from Phoenix Laboratories (Belmont, CA), whereas the SP antagonist CP-96,345 and its 2R,3R inactive enantiomer, CP-96,344, were generously provided by Pfizer Diagnostics. The SP antagonist was dissolved in 0.9% saline immediately before use and injected i.p. Sodium pentobarbital was purchased from Abbott. Protein concentrations were determined by the bicinchoninic acid protein assay reagent (Pierce).
Methods. Preparation of ileal loops and measurement of fluid secretion, mannitol permeability, and histologic damage.
Fasted male Wistar rats (200–250 g) (Charles River Breeding Laboratories) were anesthetized by an i.p. injection of pentobarbital (35 mg/kg). Laparotomy was then performed and 2–3 closed, 5-cm-long ileal loops for each animal were formed and injected with either toxin A (5 μg) in 0.4 ml of 50 mM Tris buffer or buffer alone as previously described (22). Before toxin A injection, the renal pedicles were tied and 10 μCi of [3H]mannitol was injected into the inferior vena cava. The abdomen was then closed and body temperature maintained at 37°C by a heating pad. At the indicated time points, animals were killed by a bolus i.p. of pentobarbital (120 mg/kg), the ileal loops were removed, their weights and lengths were recorded, and fluid contents were collected. Mucosal permeability to mannitol and fluid secretion were estimated as described (21, 22). Full thickness section of loops were fixed in formalin, paraffin-embedded, and stained with hematoxylin and eosin, and histologic severity of enteritis was graded in a “blinded fashion” by a gastrointestinal pathologist (S.N.), as described (22). (This study was approved by the Beth Israel Deaconess Medical Center and Boston University Medical Center Institutional Animal Care and Use Committee).
Measurements of SP content in lumbar dorsal root ganglia and intestinal mucosa scrapings.
Toxin A- or buffer-injected loops were surgically removed, cut longitudinally, and washed in ice- cold Hanks’ balanced salt solution (HBSS, Sigma). The mucosa was then scraped from the underlying muscularis and homogenized in 2 ml of ice-cold 0.1 M of HCl for 20 sec. The homogenate was centrifuged at 15,000 × g (15 min at 4°C) and the supernatant collected. To collect the lumbar DRG, the lumbar column was incised bilaterally, and, using ultra fine forceps, the lumbar DRG from each side of the animal were removed (6–8 per animal), pooled, and processed as described above for intestinal mucosal scrapings for determination of SP content. To determine SP levels, samples were absorbed on C18 cartridge columns (Waters, Cambridge, MA) that had been washed with 10 ml of methanol and then equilibrated with 0.1% trifluoroacetic acid (TFA). Samples diluted 1:1 with 0.2% TFA were slowly loaded onto the column, and the column was then washed with 10 ml of 0.1% TFA. Adsorbed peptides were then eluted with 1.5 ml of 75% acetonitrile. Samples were freeze-dried and reconstituted in 0.5 ml of sample buffer. SP content was determined using a commercially available EIA kit (Peninsula Laboratories) following the instructions of the manufacturer, and results were expressed as pmol/mg protein.
Total RNA extraction and reverse transcription–PCR (RT-PCR) amplification for SP mRNA.
Rat ileal loops were formed and injected with buffer or toxin A as described above. Animals were killed at different time points, the loops were removed and washed in ice-cold HBSS, and both the mucosa scrapings and lumbar DRG were collected as described above. Total RNA from these samples was isolated by the acid guanidinium thiocyanate/phenol/chloroform extraction method as described by Chomczynski and Sacchi (34) and RNA integrity was tested by 1% agarose formaldehyde gel electrophoresis. cDNA was prepared by reverse transcribing 1 μg of total RNA as described (35). All RT-PCRs were performed in a 50-μl reaction volume containing 1 μl of the cDNA mix as described (35). [32P]dCTP (0.25 μl) (3,000 Ci/mmol, New England Nuclear) was added to the reaction mixture to radiolabel the products of the amplification reaction. The temperature profile for the RT-amplification reaction and the sequence for SP primers were as previously reported by Crofford et al. (36). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers, used as an internal standard, were purchased from CLONTECH. The experimental conditions for amplification were as described by Tso et al. (37) with 25 amplification cycles. The amplification reactions (10 μl) were analyzed directly on a 3.5% or 5% polyacrylamide gel in a Tris/EDTA buffer. The gels were then dried and exposed to Kodak X-Omat AR film (Eastman Kodak) to visualize amplification products.
Effect of SP-antagonist on toxin A-induced TNFα release from intestinal macrophages.
Rats were injected with CP-96,345 or CP-96,344 (2.5 mg/kg), or saline (i.p.). Ten minutes later ileal loops were prepared and injected with either buffer or toxin A. LPMs were purified 1 hr after toxin A injection by a modification of the method previously described by Sperber et al. (38). Briefly, ileal loops were removed, washed in sterile HBSS, cut into small pieces (2 × 2 mm) and incubated (≈90 min at 37°C) in Ca2+- and Mg2+-free HBSS supplemented with 5% heat inactivated fetal calf serum (FCS; HyClone), penicillin (100 units per ml), streptomycin sulfate (100 μg/ml), Hepes buffer (1 mM), and EDTA (1 mM) with continuous gentle stirring to remove epithelial cells. The medium was changed until no epithelial cells were seen in the medium under light microscopy. The tissue was then washed extensively with HBSS and incubated in R-10 medium [RPMI medium 1640 (Fisher) without phenol red, supplemented with 10% heat-inactivated FCS, penicillin (100 units per ml), streptomycin sulfate (100 μg/ml), and glutamine (2 mM)] containing Dispase (Boehringer Mannheim; 3 mg/ml) and DNase I (0.1 mg/ml). After 1.5 hr of incubation at 37°C with gentle, constant stirring, cells released in the supernatant were collected by centrifugation (600 × g for 10 min). Lamina propria mononuclear cells were purified by centrifugation through a discontinuous density gradient made up with Percoll (Pharmacia). Percoll solutions (44% and 67%) were prepared by diluting the stock solution (45 ml Percoll and 5 ml of 10 × PBS) in R-10 medium. Cells were first resuspended in the 44% Percoll solution, and 6 ml of this cell suspension was gently overlayered on 3 ml of the 67% Percoll solution. This mixture was then centrifuged (600 × g for 20 min), and lamina propria mononuclear cells migrating at the interface were collected and washed twice in HBSS. The cells were then resuspended in R-10 medium and seeded at a density of 5 × 105 per well onto 24-well tissue culture plates (Primaria, Becton Dickinson). After 2 hr, wells were washed extensively to remove nonadherent cells, and adherent macrophages were incubated in fresh medium. Cells were determined as >95% pure macrophages/monocytes by cytostaining with α-naphtyl acetate esterase (Sigma) and immunostaining with a mouse monoclonal antibody (BioSource International, Camarillo, CA) directed against a 97-kDa protein specific for rat macrophages (39). Cell viability in the end of the experiments was >90% as determined by Trypan blue exclusion.
Measurement of TNFα.
TNFα release from macrophage culture supernatant was determined by measuring its cytotoxic effect on WEHI 164 cells, as reported by Chung and Benveniste (40), by using recombinant mouse TNFα (Genzyme) as the internal standard. WEHI 164 cells were harvested and resuspended at a concentration of 4 × 105 cells per ml in R-10 medium containing 0.9 μg/ml of actinomycin d-mannitol (Sigma). One hundred microliters of this cell suspension was seeded onto 96-well tissue culture plates (4 × 104 cells per well), and 100 μl of either sample or standard in triplicate was added to the wells and incubated at 37°C for 16 hr. After incubation was completed, cell viability was assessed using the MTT tetrazolium (Sigma) cytotoxicity assay. Briefly, 10 μl of MTT solution (5 mg/ml in 0.9% saline) was added to each well and incubated at 37°C for 4 hr. One hundred microliters of the supernatant was removed and replaced with 200 μl of isopropanol containing 0.04 N HCl to solubilize the dark blue formazan crystals precipitated into the mitochondria of metabolically active cells. The plates were analyzed on a microplate reader (Dynatech) at a wavelength of 570 nm. Results were expressed as pg of TNFα released per 5 × 105 cells.
Measurement of SP release and identification of SP mRNA in lamina propria macrophages.
Intestinal macrophages were obtained from buffer- or toxin A-injected ileal loops as described above. LPMs (2 × 106) were seeded in 60-mm dishes and, after 2 hr of incubation, extensively washed with sterile HBSS to remove nonadherent cells. Adhered macrophages were incubated in fresh medium for an additional 4 hr; the conditioned medium was then collected to measure SP content as described above, whereas total cellular RNA was extracted from adhering macrophages. cDNA was prepared as described above and the expression of SP mRNA was detected by RT-PCR as described above.
Effect of SP on TNFα release from lamina propria macrophages.
LPMs obtained from buffer- or toxin A-injected loops were incubated (4 hr at 37°C) in fresh medium alone or medium containing 10−7 to 10−10 M of SP. In some experiments the SP antagonist CP-96345 (3 nM) or the same concentration of its inactive enantiomer CP-96,344 was added to the medium 10 min prior to the addition of SP (10−9 M). After 4 hr, the medium was collected and TNFα release was measured as described above. In separate experiments, LPMs obtained from toxin A-injected loops were incubated in the presence of 3 nM of the SP antagonist CP-96345 or its inactive enantiomer CP-96344, and after 4 hr the medium was collected to determine TNFα as described above.
Statistical analyses.
Statistical analyses were performed using sigmastat (version 1.0, Jandel, San Rafael, CA). ANOVA was used for intergroup comparisons.
RESULTS
Time-Dependent Increases in the Abundance of SP mRNA and SP Content in the Ileal Mucosa after Ileal Toxin A Administration.
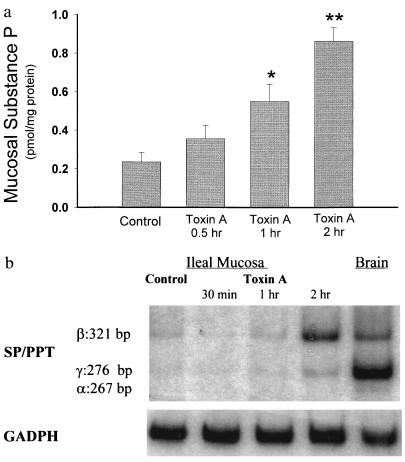
The enterotoxic effects of toxin A in rat ileal loops were assessed by measuring fluid secretion and blood-to-lumen mucosal permeability to mannitol, and by histologic examination. Injection of toxin A into ileal loops had no significant effect in any of the intestinal responses 30 min following toxin administration (Table 1), whereas after 1 hr only tissue neutrophil infiltration was significantly increased (Table 1). However, after 2 hr toxin A caused significant increases in secretion, mucosal permeability, and histologic damage, and after 4 hr further increases in all these parameters were measurable (Table 1). Toxin A also caused a time-dependent increase in SP content in the ileal mucosa (Fig. 1a); mucosal SP levels were increased approximately twofold (P < 0.05) 1 hr after toxin A injection and further increased approximately three- to fourfold (P < 0.01) after 2 hr. When we examined the time-course effect of toxin A on the abundance of mucosal SP mRNA, our results showed that SP mRNA mucosal levels were elevated 2 hr after toxin A administration (Fig. 1b).
Table 1.
Effect of toxin A on rat ileal fluid secretion, mucosa permeability to mannitol, and histologic severity of enteritis
| Intestinal secretion, cm/mg | [3H]Mannitol permeability, dpm/cm | Epithelial damage | Histologic severity, congestion, and edema | Neutrophil infiltration | |
|---|---|---|---|---|---|
| Control | 105 ± 17 | 1472 ± 270 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 |
| 0.5 hr toxin A | 146 ± 4 | 1640 ± 106 | 0.2 ± 0.1 | 0.5 ± 0.3 | 0.6 ± 0.3 |
| 1 hr toxin A | 155 ± 5 | 1260 ± 174 | 0.2 ± 0.2 | 1.0 ± 0.2 | 1.5 ± 0.2* |
| 2 hr toxin A | 213 ± 14* | 4126 ± 290* | 0.8 ± 0.2* | 2.0 ± 0.2* | 2.0 ± 0.1* |
| 4 hr toxin A | 369 ± 15** | 41695 ± 3823** | 2.4 ± 0.2** | 2.5 ± 0.2** | 2.5 ± 0.2** |
Rat ileal loops were injected with either 5 μg of toxin A or buffer (control). At the indicated time intervals, loops were excised and fluid secretion was assessed by loop weight to length ratio (mg:cm). Intestinal permeability to mannitol was estimated by scintillation counting of aliquots of loop samples, and results were expressed as dpm per cm of loop. Ileal tissues were also processed for histologic examination as described. The histologic severity of enteritis was graded by a score of 0–3 for epithelial cell damage, congestion and edema of the mucosa, and neutrophil infiltration. Data are expressed as mean ± SE. Number of loops tested (n) were 6–12 per group. ∗, P < 0.05; ∗∗, P < 0.01 vs. control.
Figure 1.
Toxin A increases SP content and SP mRNA levels in rat ileal mucosa. (a) Rat ileal loops were injected with either 5 μg of toxin A or buffer (control). At the indicated time intervals (2 hr after buffer injection) animals were killed, ileal loops were removed, and ileal mucosa was scraped and processed for measurements of SP content using an immunoenzymatic assay. Six to eight loops were tested for each time point. Results are expressed as pmol of SP per mg protein (mean ± SEM). ∗, P < 0.05; ∗∗, P < 0.01 vs. control. (b) Total RNA was purified from ileal mucosa scrapings obtained at the indicated time points. PCR of reverse transcribed cDNA was performed as described using specific primers for SP/PPT (40 cycles) or GAPDH (25 cycles). The PCR primers for SP/preprotachykinin were designed to detect the α, β, and γ forms of the PPT mRNA (36). [32P]-labeled PCR products were electrophoresed on 3.5 or 5% native polyacrylamide gels (depending on the size of the product), and autoradiograms were obtained. Arrows indicate specific SP mRNA fragments and their sizes in base pairs (bp). Negative controls performed with no RNA added to the reverse transcription, or no reverse transcriptase yielded no detectable fragments with any primer pairs.
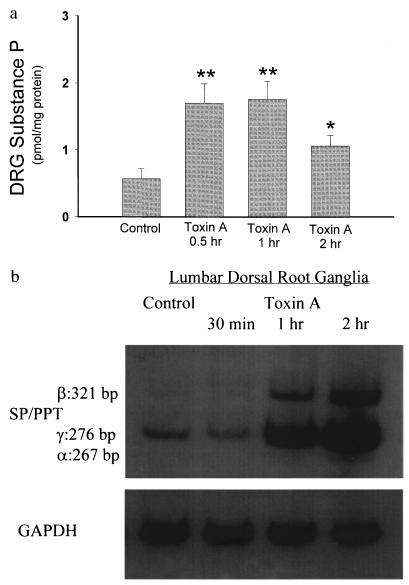
Time-Dependent Increase in the Abundance of SP mRNA and SP Content in the Lumbar DRG after Ileal Toxin A Administration.
A possible source of the increase in SP in the intestinal mucosa following toxin A exposure are sensory afferent fibers (2, 3). We (21) and others (23) previously reported that sensory afferent neurons are involved in toxin A-induced enteritis. Since the bodies of ileal sensory neurons are located in the lumbar DRG (2, 3), we determined whether administration of toxin A into ileal loops would have an effect on SP content in lumbar DRG. The results show that the abundance of SP mRNA in the lumbar DRG was increased 1 hr after toxin A administration and further increased after 2 hr (Fig. 2b). SP levels in the lumbar DRG were also significantly increased 30 min after toxin A injection and further increased after 1 hr (Fig. 2a). Two hours after toxin A administration SP levels in the DRG declined, but still remained higher as compared with controls (Fig. 2a).
Figure 2.
Toxin A increases SP content and SP mRNA in rat lumbar dorsal root ganglia. (a) Rat ileal loops were prepared and injected with either 5 μg toxin A or buffer (control) and animals were killed at the indicated time points (2 hr after buffer injection). Lumbar DRG from each side (n = 6–8) were removed, and SP content was measured by immunoenzymatic assay. Each bar represents the mean ± SEM of six to eight different determinations for each time point. ∗, P < 0.05; ∗∗, P < 0.01 vs. control. (b) Total RNA extracted from lumbar DRG was reverse transcribed as described. RT-PCR was performed using primers specific for SP/PPT (40 cycles) or GAPDH (25 cycles). [32P]-labeled PCR products were electrophoresed on 3.5 or 5% native polyacrylamide gels (depending on the size of the product) and autoradiograms were obtained. Arrows indicate the specific SP mRNA fragments and their sizes in bp. Negative controls were performed as described in Fig. 1b.
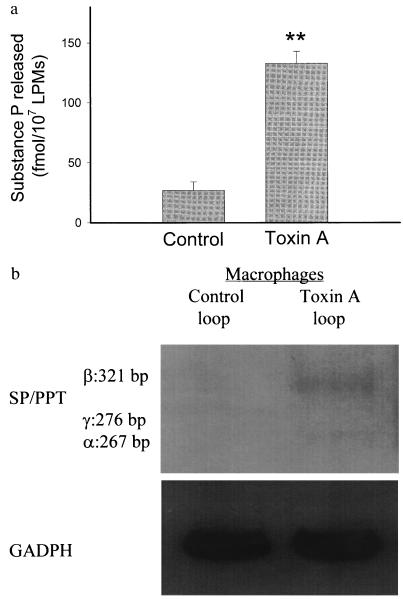
Lamina Propria Macrophages Release Increased SP.
LPMs obtained from toxin A-injected ileal loops released in vitro significantly higher amounts of SP as compared with the amount of SP released from LPMs obtained from buffer-exposed loops (Fig. 3a). LPMs isolated from toxin A-injected loops also showed increased levels of SP mRNA as compared with controls (Fig. 3b).
Figure 3.
Toxin A increases release of SP and stimulates SP mRNA expression from LPMs. (a) Rat ileal loops were injected with either toxin A or buffer. After 1 hr, LPMs were purified and placed in tissue culture (6 hr at 37°C) as described. SP released into the culture medium was measured by an immunoenzymatic assay. Each bar represents the mean ± SEM of values derived from six to eight animals, each with quadruplicate determinations for each experimental condition. ∗, P < 0.01 vs. control. (b) Total RNA was extracted from LPMs after 2 hr of culture in vitro and reverse transcribed to obtain cDNA as described. SP/preprotachykinin mRNA was measured by RT-PCR as described in legend to Fig. 2b.
Inhibition of Toxin A-Induced Lamina Propria Macrophage Activation by Administration of the SP Antagonist CP-96,345 in Vivo.
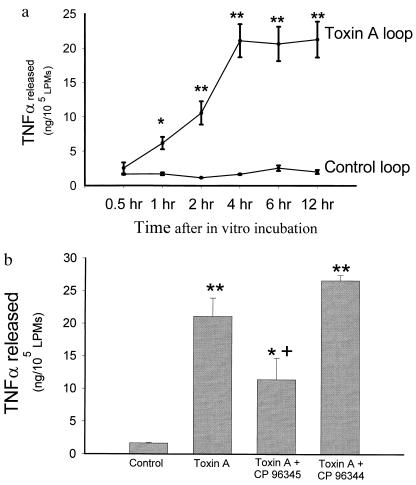
To determine whether intestinal macrophages are activated during toxin A-induced enteritis we measured TNFα release from LPMs obtained from toxin A-injected ileal loops. Fig. 4a shows that macrophages obtained from toxin A-injected loops released significantly higher amounts of TNFα as compared with macrophages obtained from control loops (Fig. 4). Injection of the SP antagonist CP-96,345 to animals 10 min prior to intraluminal administration of toxin A, but not its inactive enantiomer, significantly inhibited TNFα release from LPMs measured at 4 hr (Fig. 4b).
Figure 4.
(a) Intestinal lamina propria macrophages isolated from toxin A-injected loops show enhanced release of TNFα. Ileal loops were injected with either toxin A or buffer. After 1 hr, LPMs were purified and cultured and TNFα release was determined as described. Each data point = mean ± SEM of values derived from six to eight rats, each with quadruplicate determinations for each experimental condition. ∗, P < 0.05; ∗∗, P < 0.01 vs. control. (b) Pretreatment of rats with the SP antagonist CP-96,345 reduces toxin A-induced TNFα release from LPMs. Rats were injected i.p. with either saline or saline containing CP-96,345 (2.5 mg/kg) or CP-96,344 (2.5 mg/kg) 10 min before administration of toxin A or buffer into ileal loops. After 1 hr LPMs were isolated and cultured and TNFα release after 4 hr was determined as described. Bars = mean ± SEM of values derived from six to eight rats, each with quadruplicate determinations for each experimental condition. ∗, P < 0.05; ∗∗, P < 0.01 vs. control; +, P < 0.05 vs. toxin A.
TNFα Release from Activated Lamina Propria Macrophages in Vitro Is Mediated by SP.
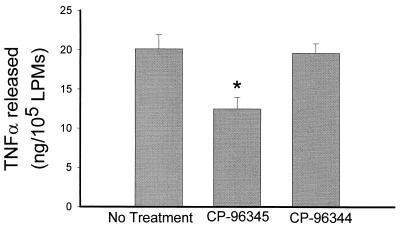
To examine whether cytokine release from activated LPMs in vitro is mediated by SP we measured TNFα release from LPMs obtained from toxin A-injected loops in the presence of the SP antagonist in the cell medium. Our results (Fig. 5) show that incubation of activated LPMs with the SP antagonist CP-96,345 significantly inhibited the release of TNFα, whereas the inactive enantiomer of the SP antagonist had no significant inhibitory effect (Fig. 5).
Figure 5.
Release of TNFα from activated LPMs in vitro is inhibited by the SP antagonist CP-96,345. Rat ileal loops were injected with toxin A, and after 1 hr animals were killed, loops were excised, and intestinal LPMs were purified and plated as described. LPMs were cultured in vitro in the presence or absence of the SP receptor antagonist CP-96,345 (3 nM) or same dose of its inactive enantiomer CP-96,344. TNFα release was determined as described. Bar = mean ± SEM six different experiments, each with quadruplicate determinations per experimental condition. ∗, P < 0.05 vs. no treatment.
Increased Responsiveness of Activated Lamina Propria Macrophages to SP in Vitro.
Because previous studies indicated that activated peritoneal macrophages are able to respond to SP (14), we next examined the effect of SP on lamina propria macrophages obtained from buffer or toxin A-injected loops. Macrophages isolated from buffer-injected loops exposed to various doses of SP (10−7–10−10 M, n = 6–8 per group) did not show increased TNFα release. In contrast, macrophages isolated from toxin A-injected loops showed increased TNFα release in response to 10−8 and 10−9 M of SP (by 27% and 25%, respectively, n = 6–7 per group, P < 0.05). This effect of SP can be partially inhibited by incubating LPMs with the SP antagonist CP-96,345 30 min prior to addition of 10−8 M SP (by 57%, P < 001, n = 6), whereas its inactive enantiomer CP-96,344 (n = 6) had no inhibitory effect.
DISCUSSION
We demonstrate here that injection of C. difficile toxin A into ileal loops causes an early (30 min) increase in SP mRNA and SP peptide concentration in lumbar DRG after intraluminal administration of C. difficile toxin A (Fig. 1), indicating increased synthesis of SP in lumbar DRG in response to the toxin. Thus, following application of toxin A to the intestinal lumen, there is a rapid communication to the cell bodies in the DRG via intestinal sensory neurons. Interestingly, these responses occur prior to changes in fluid secretion, mucosal permeability to mannitol, and histologic evidence of enteritis in response to toxin (Table 1). These results are in agreement with previous observations that SP-containing sensory neurons are involved in the intestinal effects of this toxin (21–23).
There have been conflicting reports of changes in the content of SP in tissues of the intestinal tract in response to inflammation (17, 41–43). These differences may be explained by differences in the experimental conditions, particularly the time after the initiation of the inflammatory response. However, the results presented here clearly show increased mucosal mRNA and SP peptide following administration of toxin A into ileal loops (Fig. 2). SP-containing sensory neurons may serve as a source of increased SP measurable in the intestinal mucosa after toxin A administration.
We demonstrate here most likely for the first time that the abundance of the mRNA encoding for SP also increases in intestinal macrophages in response to toxin A administration, and that these macrophages incubated in vitro release increased amounts of SP as determined by radioimmunoassay (Fig. 3). These results provide evidence that LPMs must be considered as a source of SP in C. difficile toxin A-mediated enteritis. Several other studies show that immune and inflammatory cells synthesize and release SP, including rat peritoneal macrophages (30), the murine macrophage cell line P388D1 (44), mouse granuloma eosinophils (16), and human eosinophils (45). Moreover, Bost et al. (30) demonstrated that stimulation of rat peritoneal macrophages with LPS increased the abundance of SP mRNA. Interestingly, preliminary studies in this laboratory (I.C. and C.P., unpublished data) indicate that exposure of rat lamina propria macrophages to LPS also stimulates release of SP and causes increased expression of SP mRNA.
The in vivo application of toxin A leads to activation of LPMs to release TNFα, and this activation is inhibited by prior exposure of rats to the SP antagonist CP-96345 in vivo (Fig. 4). Thus, activation of LPMs in response to toxin A in vivo is mediated, at least in part, by SP. In support of this conclusion we show here that SP can stimulate the secretion of TNFα from intestinal LPMs, but that prior activation of LPMs caused by administration of toxin A leads to a marked enhancement of TNFα release. The importance of activation in the response to SP has been previously reported. Luber-Narod et al. (46) reported that SP enhances the secretion of TNFα from activated neuroglial cells. Lotz et al. (29) showed that physiologic concentrations of SP in vitro stimulate release of interleukin 1 (IL-1) and TNF from human peripheral monocytes, Laurentzi et al. (28) reported that LPS-activated human blood monocytes have a greater response to SP, and Calvo et al. (12) showed that SP enhances IL-2 release from activated human T cells. Prior activation of LPMs in our experiments is also important to the response to SP, because the enhanced TNFα secretion was evident only in LPMs obtained from toxin A-injected, not control, loops. This enhanced response of LPMs to SP is inhibited by the SP antagonist, CP-96,345, applied in vitro, indicating that stimulation by SP is mediated via the presence of specific SP receptors on LPMs. The presence of functional, high-affinity binding sites for SP on guinea pig macrophages has been previously shown (14).
A question of interest is whether prior exposure of LPMs to SP, which is a priming effect of SP, leads to changes in the responsiveness of these cells. A priming effect of SP has been demonstrated by Berman et al. (47), who showed that exposure of rat peritoneal macrophages to SP, prior to incubation with LPS, results in enhanced release of proinflammatory cytokines.
Another important observation reported here is that the increase in TNFα release from LPMs obtained from toxin A-exposed loops can be inhibited by the in vitro addition of the SP antagonist CP-96,345 to the medium, whereas the inactive enantiomer of the SP antagonist is ineffective (Fig. 5). These results indicate that SP released in vitro from intestinal macrophages can mediate an increase in TNFα secretion in these cells in an autocrine or paracrine fashion.
Our evidence that administration of toxin A into rat ileum stimulates an increase in the synthesis of SP in lumbar DRG suggests that it is likely that there is also an increase in release of SP from the terminals of these sensory neurons. The pathway(s) by which signals from the intestinal epithelium are communicated to the sensory nerves is still under investigation. An interesting possibility is that inflammatory mediators, like macrophage inflammatory protein-2 (48), released from intestinal epithelial cells in response to toxin A may directly activate sensory neuronal nerve endings in the intestinal lamina propria. Another source of SP are the intestinal macrophages from the lamina propria (Fig. 3), but release of SP from these cells involves an activation process that may require participation of sensory neurons or direct activation of LPMs by inflammatory mediators such as macrophage inflammatory protein-2. SP released into the mucosa is likely to interact with receptors on multiple local cells, including intestinal nerves (23); immune cells, including macrophages; mast cells (10); neutrophils (11); as well as intestinal epithelial cells (49). We postulate that such interactions lead to the initiation and perpetuation of inflammatory diarrhea that characterizes C. difficile toxin A-mediated enteritis.
Acknowledgments
We thank Pfizer for generously providing the SP antagonist CP-96,345 and its inactive enantiomer CP-96,344. This work was supported by Research Grants DK-47343 (C.P. and S.E.L.) and DK-02128 (C.P.K.) from the National Institutes of Health. I.C. is a recipient of a Research Fellowship from the Crohn’s and Colitis Foundation of America.
ABBREVIATIONS
- SP
substance P
- DRG
dorsal root ganglia
- LPMs
lamina propria macrophages
- LPS
lipopolysaccharide
- TNFα
tumor necrosis factor α
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HBSS
Hanks’ balanced salt solution
References
- 1.Chang M M, Leeman S E. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 2.Maggi C A. Arch Int Pharmacol. 1990;303:157–166. [PubMed] [Google Scholar]
- 3.Costa M, Furness J B, Llewellyn-Smith I J. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. Vol. 1. New York: Raven; 1987. pp. 1–40. [Google Scholar]
- 4.Keast J R, Furness J B, Costa M. J Comp Neurol. 1985;236:403–422. doi: 10.1002/cne.902360308. [DOI] [PubMed] [Google Scholar]
- 5.Marchand J E, Wurm W H, Kato T, Kream R M. Pain. 1994;58:219–231. doi: 10.1016/0304-3959(94)90202-X. [DOI] [PubMed] [Google Scholar]
- 6.Badalamente M A, Dee R, Ghillani R, Chien P, Daniels K. Spine. 1987;12:552–555. doi: 10.1097/00007632-198707000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Smith G D, Harmar A J, McQueen D S, Seckl J R. Neurosci Lett. 1992;137:257–260. doi: 10.1016/0304-3940(92)90417-6. [DOI] [PubMed] [Google Scholar]
- 8.Donnerer J, Schuligor R, Stein C. Neuroscience. 1992;49:693–698. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- 9.Reinshagen M, Adler G, Eysselein V E. Regul Pept. 1995;59:53–58. doi: 10.1016/0167-0115(95)00073-k. [DOI] [PubMed] [Google Scholar]
- 10.Shanahan F, Denburg J A, Fox J, Bienenstock J, Befus D. J Immunol. 1985;135:1331–1337. [PubMed] [Google Scholar]
- 11.Perianin A, Snyderman R, Malfroy B. Biochem Biophys Res Comm. 1989;161:520–524. doi: 10.1016/0006-291x(89)92630-2. [DOI] [PubMed] [Google Scholar]
- 12.Calvo C F, Chavanel G, Senik A. J Immunol. 1992;148:3498–3504. [PubMed] [Google Scholar]
- 13.Wozniak A, McLennan G, Betts W H, Murphy G A, Scicchitano R. Immunology. 1989;68:359–364. [PMC free article] [PubMed] [Google Scholar]
- 14.Hartung H P, Wolters K, Toyka K V. J Immunol. 1986;136:3856–3863. [PubMed] [Google Scholar]
- 15.Stanisz A M, Befus D, Bienenstock J. J Immunol. 1986;136:152–160. [PubMed] [Google Scholar]
- 16.Tissot M, Pradelles P, Giroud J P. Inflammation. 1988;12:25–35. doi: 10.1007/BF00915889. [DOI] [PubMed] [Google Scholar]
- 17.Swain M G, Agro A, Blennerhassett P, Stanisz A, Collins S M. Gastroenterology. 1992;102:1913–1919. doi: 10.1016/0016-5085(92)90313-n. [DOI] [PubMed] [Google Scholar]
- 18.Lotz M, Carson D A, Vaughan J H. Science. 1987;235:893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- 19.Mantyh CR, Gates TS, Zimmerman ML, Welton ML, Passaro EPJ, Vigna SR, Maggio JE, Kruger L, Mantyh PW. Proc Natl Acad Sci USA. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly P C, Pothoulakis C, LaMont J T. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 21.Castagliuolo I, LaMont J T, Letourneau R, Kelly C, O’Keane J C, Jaffer A, Theoharides T C, Pothoulakis C. Gastroenterology. 1994;107:657–665. doi: 10.1016/0016-5085(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 22.Pothoulakis C, Castagliuolo I, LaMont J T, Jaffer A, O’Keane J C, Snider R M, Leeman S E. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantyh C R, Pappas T N, Lapp J A, Washington M K, Neville L M, Chilardi J R, Rogers S D, Mantyh P W, Vigna S R. Gastroenterology. 1996;111:1272–1280. doi: 10.1053/gast.1996.v111.pm8898641. [DOI] [PubMed] [Google Scholar]
- 24.Johnston R B., Jr N Engl J Med. 1988;318:747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 25.Ligumsky M, Simon P L, Karmeli F, Rachmilewitz D. Gut. 1990;31:686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandil H M, Berschneider H M, Argenzio R A. Gut. 1994;35:934–940. doi: 10.1136/gut.35.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang E B, Musch M W, Mayer L. Gastroenterology. 1990;98:1518–1524. doi: 10.1016/0016-5085(90)91084-j. [DOI] [PubMed] [Google Scholar]
- 28.Laurentzi M A, Person M A A, Dalsgaard C, Hoegerstrand A. Scand J Immunol. 1990;31:529–536. doi: 10.1111/j.1365-3083.1990.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 29.Lotz M, Vaughan J H, Carson D A. Science. 1988;243:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 30.Bost K L, Breeding S A L, Pascual D W. Reg Immunol. 1992;4:105–113. [PubMed] [Google Scholar]
- 31.Pothoulakis C, LaMont J T, Eglow R L, Gao N, Rubins J B, Theoharides T C, Dickey B R. J Clin Invest. 1991;88:1461–1465. doi: 10.1172/JCI115267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pothoulakis C, Kelly C P, Joshi M A, Gao N, O’Keane C, LaMont J T. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 33.Pothoulakis C, Karmeli F, Kelly C P, Eliakim R, Joshi M A, O’Keane C, Castagliuolo I, LaMont J T, Rachmilewitz D. Gastroenterology. 1993;105:701–707. doi: 10.1016/0016-5085(93)90886-h. [DOI] [PubMed] [Google Scholar]
- 34.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 35.Castagliuolo I, LaMont J T, Qiu B, Fleming S M, Bhaskar K R, Nikulasson S T, Kornetsky C, Pothoulakis C. Am J Physiol. 1996;271:G884–G892. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- 36.Crofford L J, Sano H, Karalis K, Webster E L, Goldmuntz E A, Chrousos G P, Wilder R L. J Clin Invest. 1992;90:2555–2564. doi: 10.1172/JCI116150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tso J T, Sun X H, Kao T H, Reece K S, Wu R. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperber K, Ogata S, Sylvester C, Aisenberg J, Chen A, Mayer L, Itzkowitz S. Gastroenterology. 1993;104:1302–1309. doi: 10.1016/0016-5085(93)90338-d. [DOI] [PubMed] [Google Scholar]
- 39.Yam L T, Li C Y, Crosby W H. Am J Clin Pathol. 1971;55:283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- 40.Chung I Y, Benveniste E N. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 41.Goldin E, Karmeli F, Selinger Z, Rachmilewitz D. Dig Dis Sci. 1989;34:754–757. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- 42.Eysselein V E, Reinshagen M, Cominelli F, Sternini C, Davis W, Patel A, Nast C, Bernstein D, Anderson K, Khan H, Snape W J., Jr Gastroenterology. 1991;101:1211–1219. doi: 10.1016/0016-5085(91)90069-w. [DOI] [PubMed] [Google Scholar]
- 43.Sharkey K A. Ann NY Acad Sci. 1992;664:425–442. doi: 10.1111/j.1749-6632.1992.tb39781.x. [DOI] [PubMed] [Google Scholar]
- 44.Pascual D W, Bost K L. Immunology. 1990;71:52–56. [PMC free article] [PubMed] [Google Scholar]
- 45.Aliakbari J, Sredharan S P, Turck C W, Goetzl E J. Biochem Biophys Res Commun. 1987;148:1440–1445. doi: 10.1016/s0006-291x(87)80293-0. [DOI] [PubMed] [Google Scholar]
- 46.Luber-Narod J, Kage R, Leeman S E. J Immunol. 1994;152:819–824. [PubMed] [Google Scholar]
- 47.Berman A S, Chancellor-Freeland C, Zhu G, Black P H. Neuroimmunomodulation. 1996;3:141–149. doi: 10.1159/000097239. [DOI] [PubMed] [Google Scholar]
- 48.Castagliuolo I, Qiu B S, Shuck K, Keates A, Kelly C P, LaMont J T, Pothoulakis C. Gastroenterology. 1996;110:A878. (abstr.). [Google Scholar]
- 49.Pothoulakis C, Castagliuolo I, Mezey E, Li H, Hoffman B J, LaMont J T, Leeman S. Gastroenterology. 1995;108:A896. (abstr.). [Google Scholar]