Abstract
Aim
To compare the efficacy of two altered fractionation radiotherapy treatment protocols (hyperfractionation and accelerated fractionation with concomitant boost) with conventional fractionation in improvement of locoregional disease control and survival of patients with squamous cell carcinoma of the larynx, oropharynx, or hypopharynx.
Methods
From March 1999 to December 2000, 51 patients with previously untreated squamous cell carcinoma of the larynx, oropharynx or hypopharynx underwent conventionally fractionated radiotherapy and received 66-70 Gy in 6˝-7 weeks (2 Gy per fraction a day, 5 fractions a week). From January 2001 to June 2004, 101 patients with the same diagnoses underwent either hyperfractionated radiotherapy, with 74.4-79.2 Gy delivered in 6.2-7 weeks (1.2 Gy per fraction twice a day), or accelerated fractionation with concomitant boost, which delivered 68.7-72 Gy in 6 weeks (1.8 Gy per fraction a day and 1.5 Gy per fraction a day to a boost filed as a second daily treatment for the last 11-12 treatment days). Locoregional relapse and overall survival were recorded.
Results
Complete response to treatment was obtained in 31 of 51 patients treated with conventional fractionation, 33 of 50 patients treated with hyperfractionation, and 36 of 51 patients treated with accelerated fractionation. No significant differences were observed among the patients treated with conventional, hyperfractionated, or accelerated radiotherapy modalities either in locoregional control rate (41% vs 35% vs 49%, respectively; P = 0.690) or overall survival rate (50% vs 40% vs 51%, respectively; P = 0.760). The grade of acute reactions of the larynx significantly differed among the treatment groups (Fisher exact test; P = 0.039). The difference in the grade of acute side effects in the skin among the treatment groups was of borderline significance (χ22 test; P = 0.054). There was also a borderline difference among the groups in the grade of late side effects in the mucous membrane (χ22 test; P = 0.055).
Conclusion
Altered fractionation regimens were not more efficacious than conventional fractionation in the treatment of previously untreated head and neck carcinoma.
Clinical Trial Registration
ClinicalTrials.gov Identifier: NCT00291434
Irrespectively of the modality of primary treatment for head and neck squamous cell carcinoma, local or locoregional residual or recurrent tumors represent the major cause of treatment failure, emphasizing the role of locoregional control for the patients’ long-term survival (1). Primary definitive radiotherapy used as a single treatment modality in patients with head and neck cancer is expected to allow preservation of the form and function of organs in this region (2). Primary definitive radiotherapy in the treatment of early stages of squamous cell carcinoma of the larynx, oropharynx or hypopharynx achieves equal probability of tumor control as surgery does. Large primary tumors and/or advanced neck disease treated with primary definitive radiotherapy demand delivery of large total doses of irradiation to enhance the tumor control. Considering the results of conventionally fractionated radiotherapy, rational modification of radiation fractionation regimens has been intensively investigated for more than three decades, with an aim to improve the outcome of patients with locally advanced head and neck carcinomas (3).
The two prototypes of altered radiation fractionation regimens are hyperfractionation and accelerated fractionation. Hyperfractionation increases locoregional control of the diseases by increasing total tumor dose delivered, whereas accelerated fractionation should increase the control by counteracting the accelerated tumor clonogen proliferation during irradiation and uses a shortened overall treatment time (4-6). Large randomized trials showed that a number of altered fractionation schedules improved the locoregional control rates, but had only a modest impact on survival (7,8).
The aim of our study was to evaluate two different altered fractionation regimens – hyperfractionation and accelerated fractionation with concomitant boost as a late accelerating component – in comparison with conventional fractionation in primary definitive radiotherapy of patients with squamous cell carcinomas of the larynx, oropharynx or hypopharynx.
Patients and methods
Patients
The study conducted at the Institute of Radiotherapy and Oncology in Skopje from March 1999 to June 2004 involved 152 consecutive, previously untreated patients with histologically proved squamous cell carcinoma of the larynx, oropharynx or hypopharynx. There were 137 men and 15 women aged between 36 and 74 years (median age, 58.3 years). The inclusion criteria were age 18-75 years, Karnofsky performance score ≥60% (9), and all stages of the disease except stage IVC. Disease staging was done according to TNM classification of International Union Against Cancer (UICC) and American Joint Committee of Cancer (AJCC) from 1997 (10). Patients with previous or concurrent malignancy other then basal cell carcinoma were excluded.
Pretreatment diagnostic work-up other than medical history and physical examination included panendoscopy with tumor biopsies for histological proof, neck ultrasound and fine-needle biopsy for cytological proof of cervical metastases, blood chemistries, chest x-ray, and liver ultrasound. The extent of the disease was also defined by computed tomography scanning and/or magnetic resonance imaging.
Treatment assignment
Eligible patients admitted from March 1999 to December 2000 were treated with conventionally fractionated radiotherapy. Eligible patients admitted between January 2001 and June 2004 were first stratified by site of cancer origin (larynx vs oropharynx vs hypopharynx), Karnofsky performance score (60-70% vs 80-100%), and stage of disease (I and II vs III and IV) and then randomly assigned to either hyperfractionation regimen or accelerated fractionation with concomitant boost regimen and followed up. Exceptions occurred when patients either refused treatment with two daily fractions or were not offered twice-a-day irradiation because of lack of machine time.
Radiotherapy regimens
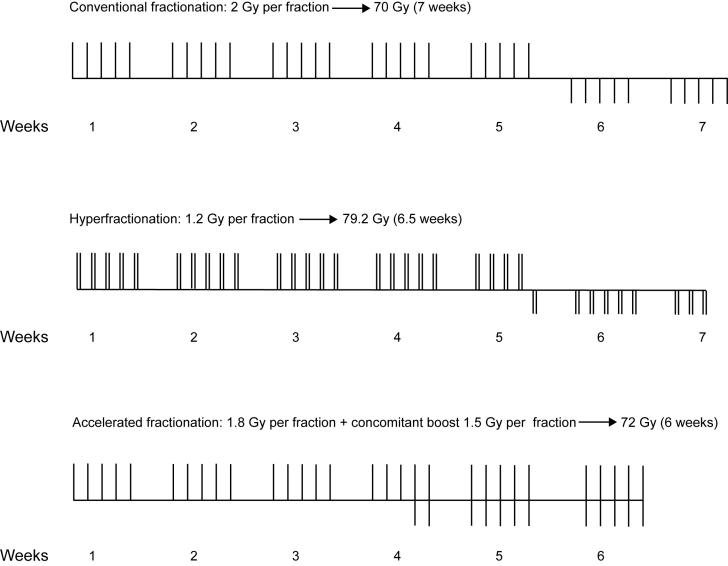
Cobalt-60 machine was used for irradiation therapy. The conventionally fractionated radiotherapy schedule was 66-70 Gy in 6˝-7 weeks (one fraction of 2 Gy a day, 5 fractions per week), whereas the hyperfractionation treatment schedule was 74.4-79.2 Gy in 6.2-7 weeks (two fractions of 1.2 Gy a day, 10 fractions per week, with an interfraction interval of at least 6 hours; Figure 1). The treatment schedule in accelerated fractionation with concomitant boost consisted of a fraction of 1.8 Gy a day, 5 days a week, up to 32.4 Gy, including all sites of disease and electively irradiated areas of the neck, followed by two daily fractions for the last 11-12 days. The first daily fraction delivering dose of 1.8 Gy encompassed all sites of the disease and electively irradiated neck nodes. The second daily fraction was the concomitant boost delivered through reduced fields to encompass the gross disease only, delivering a fraction of 1.5 Gy up to a total of 68.7-72 Gy in 6 weeks (Figure 1). The interval between the two daily fractions was at least 6 hours.
Figure 1.
Schematic illustration of the fractionation regimens used. Each bar represents one radiation fraction. Bars above the lines represent large field irradiation and those bellow the lines stand for coned-down boost irradiation.
The use of therapeutic ionizing radiation leads to cell killing. In radiotherapy the most important biologic endpoint is loss of cellular reproductive ability or clonogenicity. When a population of cells is irradiated, a proportion of cells sustain injury lethal to their reproductive integrity, while the remaining proportion retains the ability to reproduce indefinitely, which is the endpoint for survival most commonly used in radiobiology (11). The linear-quadratic model allows for analysis of the relationship between cell survival and radiation dose. In this widely accepted model in radiobiology, the cell survival is approximated mathematically by the following formula: SFd = exp (-αd -βd2), where SFd is surviving fraction of target cells after a dose per fraction d is given (12,13). The equation for cell survival describes logarithmic cell killing by two coefficients, α for single hit killing (a linear function of dose) and β for multihit killing (a function of the square of the dose) (13). In fractionated irradiation, each successive fraction in a series is equally effective, ie, the same proportion of cells is killed for a given dose increment (11,14). According to the linear-quadratic model, the survival curve continuously bends and its shape is determined by the α/β ratio. The dimensions of α/β ratio are Gy. This is the dose at which the linear contribution to damage (αd on the logarithmic scale) equals the quadratic contribution (βd2) (12).
The therapeutic use of ionizing radiations is predicated on sparing normal tissue while attempting to achieve lethal effects on tumor cells (15). When fractionated irradiation is used, responses to fractionation are different in different types of tissues. The three generic tissue types are (a) early-responding normal tissues, characterized by rapid cell turnover, such as skin, mucosa, intestinal epithelia, and hemopoetic system; (b) late-responding normal tissues, characterized by slow rate of renewal, such as spinal cord, kidney, and dermis; and (c) tumors, as a general class (13,16). In the linear-quadratic model, the values for α/β ratio for early-responding tissues are high, 10 Gy, and for late-responding tissues are lower, 2-4 Gy. This implies that the cell killing that underlies acute radiation responses is a result of irreparable single-hit mechanisms. In terms of cell survival curve shapes, the target cells in early-responding tissues have a relatively long initial linear region, whereas the curve for slowly responding tissues is “curvier.” Thus, the fractionation effect is relatively smaller in early-responding tissues than in late-responding tissues (13). This is reflected in isoeffect curves, which show the relationships between total doses and dose per fraction for early- and late-responding tissues. The isoeffective total doses increase more rapidly with decrease in dose per fraction for late effects than for early effects. In terms of fractionation response, tumors tend to behave like early-responding tissues, providing that late reactions are dose-limiting (4).
Hyperfractionation is based on the difference between the fractionation response of early- and late-responding tissues. A therapeutic benefit of hyperfractionation can be achieved if tumors have small fractionation sensitivity characterized by α/β values greater than the α/β values that characterize the fractionation sensitivity of late-responding normal tissues (17). Hyperfractionation is generally expected to allow for an escalation of total dose, thereby increasing tumor control rate without increasing the risk of late complications (5).
The biologic equivalence sought in the conventional and hyperfractionated regimens is in their late effects. The value for α/β ratio for late-responding tissues used in our calculations was 3.5 Gy (α/β for head and neck various late effects) (14).
The calculation of biologically effective dose (BED) (18) as a measure of the effect of a course of irradiation was done by using the following formula: BED = D [1 + d/ (α/β)], where D is the total dose in n fractions of size d.
The calculated BED for late effects in the conventionally fractionated regimen was 112 Gy. The calculated BED for the late effects in the hyperfractionation regimen was 103 Gy. The calculated BED for the tumor using α/β ratio of 10 Gy was 84 Gy for the conventional fractionation and 88.7 Gy for the hyperfractionation. According to these calculations, the conventional fractionation seemed to be less effective for tumor effects than hyperfractionation protocol and, in addition, it appeared around 8% more damaging for late-responding tissues.
The accelerated fractionation, which uses a reduced overall treatment time below the conventional 7 weeks, should increase tumor cure rates by restricting the time available for tumor cell proliferation. Using the accelerated fractionation with concomitant boost as a late accelerating component we shortened the overall treatment time by one week. Considering the effect of proliferation being equivalent to the loss of radiation dose of about 0.6 Gy per day (6), the dose in this 6-week schedule would be effectively larger than that in a 7-week schedule by factor 0.6 × (7-6) × 7 = 4.2 Gy, or 6% of a conventional treatment of 70 Gy. The use of the concomitant boost in the last 12 days of the course of radiotherapy seems to be a biologically reasonable strategy. In normal tissues with earliest response, a repopulation response probably begins within about 2 weeks from the start of radiotherapy (6). Withers et al (19) concluded that there was no repopulation by clonogenic mucosal epithelial cells during the first 12-14 days of a 2.0 Gy per fraction regimen, 5 times per week, but thereafter, mucosal isoeffect dose increases by about 7.0 Gy/week between the third and seventh week of irradiation. Since the accelerated repopulation as a regenerative response of the mucous membranes is already in full swing by the time of the concomitant boost, the tolerance should be improved. Hence, delivering the boost during the last part of the basic treatment course has the greatest probability of insuring that the full dose will be given without treatment interruption (13,20).
Radiotherapy was delivered by cobalt-60 machine with a source-to-surface or source-to-isocenter distance of 80 cm. Lateral opposing fields were used to treat the primary tumor and the lymph nodes in the upper neck. Elective low-neck radiation therapy was realized through a single anterior field. There was no elective nodal irradiation in patients with early glottic cancer (T1 and T2 primary tumors). All fields were treated on each treatment day. A shrinking-field technique was adopted for all patients. The first field reduction off the spinal cord occurred at 46 Gy for conventional fractionation, 45.6 for hyperfractionation, and 45 Gy for accelerated fractionation. The second field reduction occurred at 56 Gy for conventional fractionation and 55-57.6 Gy for hyperfractionation. Electrons or partial semi field technique were used for boosting the dose to involved nodes in spinal chains, while protecting the cord. These fields and the low neck field were treated once a day.
Follow-up and treatment response
According to our follow-up policy, all patients were followed by both ear, nose and throat (ENT) surgeons and radiation oncologists. During the radiological treatment, patients were examined weekly. The patients were seen for clinical examination monthly during the first year after they completed the treatment, every other month in the second year, every 4 months in the third year, every 6 months in the fourth and fifth year, and annually thereafter.
Tumor response was documented by direct measurement at every examination. When feasible, biopsy was performed of clinically suspected persistence or regrowth of the tumor, but systematic biopsies were not performed. Patients were considered to have achieved an initial complete primary response if the primary disease totally disappeared after radiotherapy. A complete nodal response was similarly defined for patients with initial involvement of neck lymph nodes. A patient was considered a “complete responder” when both complete primary and nodal response was achieved. For estimating the locoregional control rates, the following definitions for failures were used: patients who initially achieved only partial response to the planned radiotherapy were considered failures on study day 1; patients who did achieve an initial complete response were considered as failures on the study day when a recurrence either at the primary site of tumor or the lymph node was first reported. Locoregional control was defined as persisting tumor clearance above clavicles after complete response at the end of radiation therapy.
Assessment of treatment toxicity
Acute reactions of skin, mucous membrane, salivary glands, pharynx and larynx were evaluated according to the scales of the European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group (EORTC/RTOG) (15) and scored weekly during the course of radiotherapy and monthly during the first 2 months after irradiation. Late radiation changes in the skin, subcutaneous tissue, and mucous membrane were also graded according to the same criteria (15), beginning 6 months after the end of radiotherapy.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Association between categorical data was analyzed with contingency tables and χ2 test (21). Fisher exact test was used when contingency tables had expected frequencies of <5 (21-24). Differences of means between the three treatment groups were tested with ANOVA (25). Actuarial locoregional control and survival curves were plotted according to Kaplan-Meier method (26) and compared by the log-rank test (25). The rates of locoregional control and overall survival are reported as 95% confidence intervals (95% CI). The statistical analyses were done by the BioMeDical (BMDP) statistical software package (27) and specially designed software for Kaplan-Meier estimation and graphic presentation (28), including the log-rank test for comparison of locoregional control and survival (29). Post hoc power calculation was performed for all comparisons with G*Power (http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/), PS Power (http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/PowerSampleSize), and NCSS/PASS Power Analysis Statistical Software 2005 (NCSS, Kaysville, UT, USA).
Results
The three treatment groups did not significantly differ in sex, age, and performance status (P = 0.802, P = 0.630, and P = 0.851, respectively; Table 1). The distribution of tumor-related variables such as site, T stage, N stage, and UICC/AJCC stage were not significantly different in the three treatment groups (P>0.95, P>0.95, P = 0.932, and P = 0.921, respectively; Table 2).
Table 1.
Pretreatment characteristics of the patients undergoing radiotherapy for cancer of the larynx, oropharynx or hypopharynx according to fractionation regimen
| Fractionation regimen (No. of patients) |
|||
|---|---|---|---|
| Characteristics | conventional (n = 51) | hyper-fractionation (n = 50) | accelerated (n = 51) |
| Sex: | |||
| male | 45 | 45 | 47 |
| female | 6 | 5 | 4 |
| Age (y): | |||
| ≤40 | 2 | 1 | 3 |
| 41-70 | 45 | 43 | 40 |
| >70 | 4 | 6 | 8 |
| Karnofsky performance status (%):* | |||
| 60-70 | 8 | 10 | 9 |
| 80-100 | 43 | 40 | 42 |
*Karnofsky performance status according to ref. 9.
Table 2.
Cancer characteristics according to fractionation regimen
| Fractionation regimen (No. of patients) |
|||
|---|---|---|---|
| Characteristics | conventional (n = 51) | hyper-fractionation (n = 50) | accelerated (n = 51) |
| Primary site: | |||
| larynx | 28 | 27 | 29 |
| oropharynx | 16 | 17 | 16 |
| hypopharynx | 7 | 6 | 6 |
| T stage: | |||
| T1 | 2 | 2 | 1 |
| T2 | 9 | 11 | 10 |
| T3 | 26 | 24 | 28 |
| T4 | 14 | 13 | 12 |
| N stage: | |||
| N0 | 24 | 21 | 26 |
| N1 | 7 | 8 | 6 |
| N2 | 15 | 13 | 12 |
| N3 | 5 | 8 | 7 |
| UICC/AJCC* stage: | |||
| I | 2 | 2 | 1 |
| II | 8 | 10 | 7 |
| III | 18 | 15 | 21 |
| IV | 23 | 23 | 22 |
*International Union Against Cancer (UICC) and American Joint Committee of Cancer (AJCC) classification of TNM stages.
All patients in each treatment group completed the assigned treatment in accordance with the protocol or with minor variations. The mean total dose delivered at mid-depth of the central axis of the parallel-opposed fields was 69.4 ± 1.34 Gy for patients irradiated with conventional fractionation, 78.4 ± 1.40 Gy for patients receiving hyperfractionation, and 70.4 ± 9.08 Gy for patients treated with accelerated fractionation. The average overall treatment time was 52.5 ± 4.10 days for patients treated with conventionally fractionated radiotherapy, hyperfractionation was accomplished in an average time of 48.0 ± 3.81 days, and accelerated fractionated radiotherapy was completed in 43.3 ± 3.41 days. There was a significant difference between the overall treatment time and fractionation regimen accomplished (ANOVA; P<0.001).
Outcome
Complete primary response was achieved in 31 of 51 patients treated with conventional fractionation, 33 of 50 patients assigned to hyperfractionation, and 36 of 51 patients assigned to accelerated fractionation. The complete nodal response rates were obtained in 16 of 27 patients treated with conventional fractionation, 19 of 29 patients treated with hyperfractionation, and 18 of 25 patients treated with accelerated fractionation. Complete nodal response with persistent primary lesion was present in 4 of 16 patients treated conventionally, 2 of 19 patients treated with hyperfractionation, and 6 of 18 patients treated with accelerated fractionation. The “complete responders” rate within 2 months from the end of radiotherapy was 31 of 51 in the group of patients treated with conventional fractionation, 33 of 50 in the group irradiated with hyperfractionation, and 36 of 51 in the accelerated fractionation group. The median follow-up was 23 months (range, 6-64).
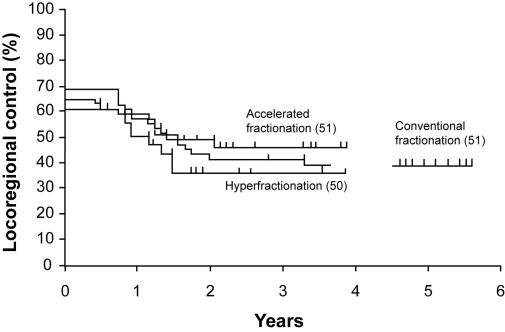
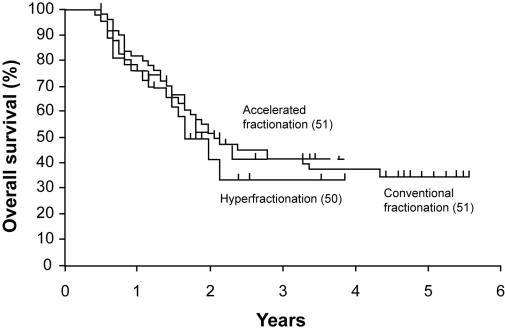
No significant differences in locoregional control and overall survival rates were found between the three treatment modalities (log-rank test; P = 0.690 and P = 0.760, respectively). The 2-year locoregional control rate was 41.0% (95% CI, 27.5-54.5) for the patients treated with conventionally fractionated radiotherapy. The 2-year locoregional control rate was 35.2% (95% CI, 22.0-48.4) for the patients irradiated with hyperfractionation and 48.7% (95% CI, 35.0-62.4) for the patients irradiated with accelerated fractionation (Figure 2). The overall survival rate at two years was 50.2% (95% CI, 36.5-63.9) for the patients irradiated with conventional fractionation, 40.1% (95% CI, 26.6-53.6) for the patients treated with hyperfractionation, and 50.5% (95% CI, 36.8-64.2) for the patients treated with accelerated radiotherapy (Figure 3). Subgroup analysis according to UICC/AJCC stage also did not show significant differences between the three treatment groups in locoregional control (log-rank test; P = 0.310 for stages I and II; log-rank test; P = 0.330 for stages III and IV) and survival (log-rank test; P = 0.290 for stages I and II; log-rank test; P = 0.150 for stages III and IV).
Figure 2.
Locoregional control according to fractionation regimen (Kaplan-Meier estimates). Log-rank test; χ22 = 0.760; P = 0.690.
Figure 3.
Overall survival according to fractionation regimen (Kaplan-Meier estimates). Log-rank test; χ22 = 0.640; P = 0.760002E
Patterns of failure
The recurrence at the primary site and the simultaneous manifestation of local and nodal recurrence were the most common patterns of failure (Table 3). One patient in the group treated with hyperfractionation developed recurrent tumor in the neck nodes only. Two patients in the conventionally treated group, one patient in the group treated with hyperfractionation, and two patients in the group irradiated with accelerated fractionation had developed distant metastases without locoregional failure (Table 3).
Table 3.
Recurrence and metastases of cancer of the larynx, oropharynx or hypopharynx in patients with complete primary and nodal response according to fractionation regimen
| Fractionation regimen (No. of patients) |
|||
|---|---|---|---|
| Tumor recurrence | conventional (n = 31) | hyper-fractionation (n = 33) | accelerated (n = 36) |
| Local recurrence | 5 | 3 | 5 |
| Regional recurrence | 0 | 1 | 0 |
| Distant metastases | 2 | 1 | 2 |
| Locoregional recurrence | 4 | 4 | 5 |
| Local recurrence and distant metastases | 1 | 1 | 1 |
| Locoregional recurrence and distant metastases | 1 | 1 | 0 |
Side effects
Acute side effects. The worst grades of acute side effects (Grade 3) during the treatment and up to 2 months after irradiation were most commonly found in the mucous membrane and the pharynx (Table 4). We did not find any significant difference in the distribution of acute reactions of the mucous membrane, salivary glands, and pharynx among the three fractionation regimens (Fisher exact test; P = 0.510 and χ22 test; P = 0.325, P = 0.560, respectively). There was a significant difference in the grade of acute reactions in the larynx among the three treatment groups (Fisher exact test; P = 0.039), whereas the difference in the grade of acute reactions of the skin was of borderline significance (χ22 test; P = 0.054). In comparison with the conventional fractionation group, the altered fractionation groups had slightly worse Grade 2 acute side effects.
Table 4.
Acute reactions to radiotherapy according to fractionation regimen
| Fractionation regimen (No. of patients) |
|||
|---|---|---|---|
| Acute reaction grade in organ/tissue | conventional (n = 51) | hyper-fractionation (n = 50) | accelerated (n = 51) |
| Skin: | |||
| 1 | 29 | 20 | 26 |
| 2 | 16 | 19 | 23 |
| 3 | 6 | 11 | 2 |
| Mucous membrane: | |||
| 0 | 9 | 7 | 7 |
| 1 | 3 | 4 | 3 |
| 2 | 19 | 12 | 11 |
| 3 | 20 | 27 | 30 |
| Salivary gland: | |||
| 0 | 16 | 11 | 12 |
| 1 | 23 | 18 | 24 |
| 2 | 12 | 21 | 15 |
| Pharynx: | |||
| 1 | 9 | 6 | 8 |
| 2 | 26 | 20 | 23 |
| 3 | 16 | 24 | 20 |
| Larynx: | |||
| 0 | 3 | 3 | 1 |
| 1 | 33 | 18 | 21 |
| 2 | 11 | 22 | 24 |
| 3 | 4 | 7 | 5 |
Late side effects. The worst late side effects (Grade 3) were most commonly found in the mucous membrane (Table 5). No significant difference existed among the three treatment groups in the late reactions in the skin and in the subcutaneous tissue (Fisher exact test; P = 0.520 and P = 0.071, respectively). There was a borderline significant difference in the grade of late effects in the mucous membrane among the three fractionation groups (χ22 test; P = 0.055). The Grade 3 mucosal reactions were slightly worse in altered fractionation groups as compared with conventional fractionation group.
Table 5.
Late reactions to radiotherapy according to fractionation regimen
| Fractionation regimen (No. of patients) |
|||
|---|---|---|---|
| Late reaction grade in organ/tissue | conventional (n = 51) | hyper-fractionation (n = 50) | accelerated (n = 51) |
| Skin: | |||
| 0 | 16 | 9 | 10 |
| 1 | 24 | 26 | 28 |
| 2 | 10 | 11 | 12 |
| 3 | 1 | 4 | 1 |
| Subcutaneous tissue: | |||
| 0 | 26 | 21 | 17 |
| 1 | 21 | 21 | 21 |
| 2 | 3 | 4 | 12 |
| 3 | 1 | 4 | 1 |
| Mucous membrane: | |||
| 0 | 11 | 10 | 9 |
| 1 | 12 | 5 | 4 |
| 2 | 23 | 24 | 21 |
| 3 | 5 | 11 | 17 |
Discussion
Our results did not show any significant difference between conventional fractionation and hyperfractionation or accelerated fractionation in locoregional control and overall survival rates of patients with squamous cell carcinoma of the larynx, oropharynx or hypopharynx. Similar findings were obtained in the Toronto randomized trial (30). However, our results were opposite to those of four other randomized trials (31-34), which showed that hyperfractionation improved either locoregional control or survival rates in patients with head and neck squamous cell carcinoma. The EORTC 22791 trial with the longest follow-up showed a 5-year local control rate of 59% in patients treated with hyperfractionation, compared with 40% rate in patients treated with conventional treatment (32). An improvement in 5-year overall survival was also reported for patients treated with hyperfractionation (32). In the Radiation Therapy Oncology Group trial (RTOG 9003) of 1073 patients, the locoregional control significantly increased with the increase in the total dose without changing the overall time using hyperfractionation, but no difference was found in the overall survival between hyperfractionation and conventional fractionation treatment groups (35). The role of hyperfractionation in the control of head and neck cancer has been controversial (36). Two different meta-analyses came to different conclusions regarding the role of hyperfractionation (5,17), whereas EORTC 22791 and RTOG 9003 trials together provided evidence of the efficacy of hyperfractionation compared to standard fractionation.
Results of randomized trials of accelerated fractionation are less consistent since there are numerous ways to accelerate treatment (37-39). We adopted the concept of acceleration using concomitant boost and administering it during the last 2˝ weeks of treatment, because it has been proved most efficacious (40). The results of our study did not show significant difference in the outcome of patients treated with accelerated fractionation compared with conventional fractionation, which is in accordance with previous findings (41). In contrast, Ang et al (40) and Johnson et al (38) obtained significantly better locoregional control and survival rates in patients irradiated with accelerated fractionation with concomitant boost than in those treated with conventional fractionation. The results of RTOG 9003 trial also confirmed that accelerated fractionation with concomitant boost could improve the therapeutic gains achievable with irradiation. Locoregional control significantly increased in patients treated with accelerated fractionation with concomitant boost compared with standard fractionation, but there was no significant difference in the overall survival (35).
In our study, the most common sites of Grade 3 acute reactions were the mucous membrane and the pharynx, which is in accordance with other studies (34,35,37,41). The most common site of Grade 3 late side effects was the mucous membrane. This is opposite to the results of RTOG 9003 trial (35) where the most common sites of Grade 3 late effects were the pharynx and the salivary gland. In our study, moderately enhanced Grade 2 acute reactions were observed in the skin and the larynx of patients in the altered fractionation groups compared with those in the conventional fractionation group. In contrast, Horiot et al (37) and Johnson et al (38) reported significant enhancement of acute reactions of the mucous membrane in the altered fractionation groups. In our study, Grade 3 late mucosal reactions were modestly enhanced in the altered fractionation groups compared to the conventional fractionation group. Significant difference in late mucosal reactions between altered fractionation group and conventional fractionation group has been reported by Fu et al (35) and Horiot et al (37). Antognoni et al (41) reported greater proportion of mild complications of skin and salivary glands in patients treated with accelerated fractionation, but found no differences in other normal tissues.
Chemotherapy applied concurrently with irradiation improves survival compared with radiation alone (42). The use of more toxic simultaneous radiochemotherapy protocols and altered fractionated irradiation increases the risk of severe acute normal tissue reactions, which represent the major limitation of these treatment modalities (43). On the other hand, modern conformal and intensity-modulated radiotherapy techniques diminish the incidence of severe acute side effects of irratiation in normal tissues. Conformal radiotherapy has the capability of improving tumor coverage while minimizing the dose to and volume of the surrounding normal tissues irradiated (44). Intensity-modulated radiotherapy can achieve higher total doses in tumors while sparing more normal tissue by delivering larger doses per fraction to the tumor only, while maintaining lower doses per fraction to subclinical disease and normal tissues (45).
One of the possible limitations of our study was that treatment was delivered by Cobalt-60 machine, but we expected that fractionated irradiation delivered by new, more sophisticated machines would produce similar results. Another limitation could be a relatively small sample size of our patient groups. However, the fact that we found no differences among the groups could not be attributed to the low power of the study. Although it was performed post hoc, power calculation in our study was sufficiently high, ranging between 70% and 90%. Furthermore, our findings were in accordance with results of some of larger trials.
In conclusion, our findings did not indicate that the therapeutic effects of evaluated altered fractionation schedules were different from those of conventional treatment schedule. However, we support the implementation of conformal radiotherapy for patients with previously untreated head and neck squamous cell carcinoma as it leaves open the possibility of improvement of their outcome.
References
- 1.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Shah JP, Lydiatt W. Treatment of cancer of the head and neck. CA Cancer J Clin. 1995;45:352–68. doi: 10.3322/canjclin.45.6.352. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK. Concurrent radiation chemotherapy for locally advanced head and neck carcinoma: are we addressing burning subjects? J Clin Oncol. 2004;22:4657–9. doi: 10.1200/JCO.2004.07.962. [DOI] [PubMed] [Google Scholar]
- 4.Thames HD, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–26. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 5.Beck-Bornholdt HP, Dubben HH, Liertz-Petersen C, Willers H. Hyperfractionation: where do we stand? Radiother Oncol. 1997;43:1–21. doi: 10.1016/s0167-8140(97)01911-7. [DOI] [PubMed] [Google Scholar]
- 6.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–46. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LN, Ang KK. Radiotherapy for cancer of the head and neck: altered fractionation regimens. Lancet Oncol. 2002;3:693–701. doi: 10.1016/s1470-2045(02)00906-3. [DOI] [PubMed] [Google Scholar]
- 8.Bernier J, Bentzen SM. Altered fractionation and combined radio-chemotherapy approaches: pioneering new opportunities in head and neck oncology. Eur J Cancer. 2003;39:560–71. doi: 10.1016/s0959-8049(02)00838-9. [DOI] [PubMed] [Google Scholar]
- 9.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–7. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Wittekind Ch, editors. TNM classification of malignant tumors. International Union Against Cancer (UICC). 5th ed. New York (NY): John Wiley and Sons; 1997. [Google Scholar]
- 11.Withers HR, Peters LJ. Biologic aspects of radiation therapy. In: Fletcher GH, editor, Textbook of radiotherapy. Philadelphia (PA): Lea & Febiger; 1980. p. 103-80. [Google Scholar]
- 12.Joiner MC. Models of radiation cell killing. In: Steel GG, editor. Basic clinical radiobiology. 2nd ed. London: Arnold; 1997. p. 52-7. [Google Scholar]
- 13.Withers HR. Biologic basis for altered fractionation schemes. Cancer. 1985;55(9 Suppl):2086–95. doi: 10.1002/1097-0142(19850501)55:9+<2086::aid-cncr2820551409>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Joiner MC, Kogel AJ. The linear-quadratic approach to fractionation and calculation of isoeffect relationships. In: Steel GG, editor. Basic Clinical Radiobiology. London: Arnold; 1997. p. 106-21. [Google Scholar]
- 15.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 16.Stewart F, van der Kogel AJ. Proliferative and cellular organization of normal tissue. In: Steel GG, editor. Basic clinical radiobiology. 2nd ed. London: Arnold; 1997. p. 24-9. [Google Scholar]
- 17.Stuschke M, Thames HD. Fractionation sensitivities and dose-control relations of head and neck carcinomas: analysis of the randomized hyperfractionation trials. Radiother Oncol. 1999;51:113–21. doi: 10.1016/s0167-8140(99)00042-0. [DOI] [PubMed] [Google Scholar]
- 18.Fowler JF. The linear quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 19.Withers HR, Maciejewski B, Taylor JM. Biology of options in dose fraction. In: McNally NJ, editor. The scientific basis of modern radiotherapy (British Institute Radiology Report ser.: No. 19). London: Medical Physics Pub Corp; 1989. p. 27-36. [Google Scholar]
- 20.Peters LJ, Ang KK, Thames HD. Accelerated fractionation in the radiation treatment of head and neck cancer. A critical comparison of different strategies. Acta Oncol. 1988;27:185–94. doi: 10.3109/02841868809090339. [DOI] [PubMed] [Google Scholar]
- 21.Everitt BS. The analysis of contingency tables. London: Chapman and Hall; 1977. [Google Scholar]
- 22.Mehta CR, Patel NR. A network algorithm for performing Fisher's exact test in r × c contingency tables. J Am Stat Assoc. 1983;78:427–34. [Google Scholar]
- 23.Mehta CR, Patel NR. Algorithm 643: FEXACT: A FORTRAN subroutine for Fisher's exact test on unordered r x c contingency tables. ACM Transactions on Mathematical Software. 1986;12:154–61. [Google Scholar]
- 24.Mehta CR, Patel NR. A hybrid algorithm for Fisher's exact test in unordered r x c contingency tables. Communications in Statistics. Series A. 1986;15:387–404. [Google Scholar]
- 25.Mould RE. Cancer statistics. Bristol: Adam Hilger Ltd; 1983. [Google Scholar]
- 26.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 27.Dixon WJ, editor. BMDP statistical software manual. Berkeley (CA): University of California Press; 1993. [Google Scholar]
- 28.Cabukovski V. Statistical analysis of surviving: 1. Surviving, non-parametric estimation and graphic presentation [in Macedonian]. God Zb Med Fak Skopje. 1985;31:113–9. [Google Scholar]
- 29.Cabukovski V. Statistical analysis of surviving: 2. A non-parametric test for comparison of surviving probabilities [in Macedonian]. God Zb Med Fak Skopje. 1985;32:159–61. [Google Scholar]
- 30.Cummings BJ, Keane TJ, Pintilie M, O'Sullivan B, Payne D, Warde P, et al. A prospective randomized trial of hyperfractionated versus conventional once daily radiation for advanced squamous cell carcinomas of the larynx and pharynx. Int J Radiat Oncol Biol Phys. 1996;36(1) Suppl 1:S235. [Google Scholar]
- 31.Datta NR, Choudhry AD, Gupta S, Bose AK. Twice a day versus once a day radiation therapy in head and neck cancer. Int J Radiat Oncol Biol Phys. 1989;17(Suppl 1):S132–3. [Google Scholar]
- 32.Horiot JC, Le Fur R, N'Guyen T, Chenal C, Schraub S, Alfonsi S, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25:231–41. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 33.Sanchiz F, Milla A, Torner J, Bonet F, Artola N, Carreno L, et al. Single fraction per day versus two fractions per day versus radiochemotherapy in the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 1990;19:1347–50. doi: 10.1016/0360-3016(90)90342-h. [DOI] [PubMed] [Google Scholar]
- 34.Pinto LH, Canary PC, Araujo CM, Bacelar SC, Souhami L. Prospective randomized trial comparing hyperfractionation versus conventional radiotherapy in stages III and IV oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:557–62. doi: 10.1016/0360-3016(91)90670-y. [DOI] [PubMed] [Google Scholar]
- 35.Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinoma: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 36.Garden AS. Altered fractionation for head and neck cancer. Oncology (Williston Park) 2001;15:1326–41. [PubMed] [Google Scholar]
- 37.Horiot J-C, Bontemps P, van den Bogaert W, Le Fur R, van den Weijngaert D, Bolla M, et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol. 1997;44:111–21. doi: 10.1016/s0167-8140(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 38.Johnson CR, Schmidt-Ullrich RK, Wazer DE. Concomitant boost technique using accelerated superfractionated radiation therapy for advanced squamous cell carcinoma of the head and neck. Cancer. 1992;69:2749–54. doi: 10.1002/1097-0142(19920601)69:11<2749::aid-cncr2820691120>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–40. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 40.Ang KK, Peters LJ, Weber RS, Maor MH, Morrison WH, Wendt CD, et al. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 1990;19:1339–45. doi: 10.1016/0360-3016(90)90341-g. [DOI] [PubMed] [Google Scholar]
- 41.Antognoni P, Bignardi M, Cazzaniga LF, Poli AM, Richetti A, Bossi A, et al. Accelerated radiation therapy for locally advanced squamous cell carcinomas of the oral cavity and oropharynx selected according to tumor cell kinetics-a phase II multicenter study. Int J Radiat Oncol Biol Phys. 1996;36:1137–45. doi: 10.1016/s0360-3016(96)00403-8. [DOI] [PubMed] [Google Scholar]
- 42.Bourhis J, Pignon J. Meta-analyses in head and neck squamous cell carcinoma. What is the role of chemotherapy? Hematol Oncol Clin North Am. 1999;13:769–75. doi: 10.1016/s0889-8588(05)70091-5. [DOI] [PubMed] [Google Scholar]
- 43.Brizel DM. Radiotherapy and concurrent chemotherapy for the treatment of locally advanced head and neck squamous cell carcinoma. Semin Radiat Oncol. 1998;8:237–46. doi: 10.1016/s1053-4296(98)80021-0. [DOI] [PubMed] [Google Scholar]
- 44.Eisbruch A, Marsh LH, Martel MK, Ship JA, Haken RT, Pu AT, et al. Comprehensive irradiation of head and neck cancer using conformal multisegmental fields: assessment of target coverage and noninvolved tissue sparing. Int J Radiat Oncol Biol Phys. 1998;41:559–68. doi: 10.1016/s0360-3016(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 45.Butler EB, Teh BS, Grant WH, 3rd, Uhl BM, Kuppersmith RB, Chiu JK, et al. SMART (Simultaneous Modulated Accelerated Radiation Therapy) boost: a new accelerated fractionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:21–32. doi: 10.1016/s0360-3016(99)00101-7. [DOI] [PubMed] [Google Scholar]