Abstract
Aim
To evaluate long-term cognitive consequences of subarachnoid hemorrhage with good outcome and the opinion of patients and their relatives about these consequences.
Methods
The study included 10 patients surgically treated for subarachnoid hemorrhage due to the rupture of aneurysm of the anterior communicating artery 2 or more years earlier, and 10 age- and sex-matched healthy controls. The preoperative and postoperative course in the patients was uneventful. Clinical and psychosocial factors and cognitive status of the patients were assessed by use of checklists and neuropsychological tests for executive functions, attention, and memory, and event-related potential recordings (waves P3a and P3b) with tree-stimulus auditory oddball paradigm, which was also performed in healthy controls.
Results
The number of reported cognitive problems negatively correlated with the patients’ level of community integration (ρ range, -0.22 to -0.75). The average neuropsychological results ranged between the 12th and 46th percentile. Impaired results were found in 7 patients across different tests and were most frequent for visual memory, followed by verbal memory and executive functions. A clear decline in cognitive functioning was observed in 3 patients. Neither P3a nor P3b wave could be found in 3 patients. In comparison with controls, patients had significantly longer P3b wave latencies (364 vs 334 ms; Mann-Whitney U test, P = 0.025). We found statistically non-significant, but still prominent negative correlations between the sustained attention results and latencies of P3a (ρ = -0.58; P = 0.172) and P3b (ρ = -0.58; P = 0.172) waves.
Conclusion
Despite good outcome after subarachnoid hemorrhage, persistent cognitive consequences were still manifest, limiting the patients' psychosocial functioning. The correlation between neuropsychological and neurophysiological measures indicated frontal lobe damage, which in some patients persisted for years after the hemorrhage.
Aneurysmal subarachnoid hemorrhage leaves around 50% of the patients permanently disabled (1), with the same percentage unable ever to return to the same level of work they had had before the event (2). Cognitive dysfunction, a common consequence of aneurysmal subarachnoid hemorrhage (3), is present to various degrees even in patients with good outcomes and without neurological deficits (4), causing considerable distress not only to the patients themselves, but also to their families. The persistence of cognitive dysfunction can have a profound influence on the rehabilitation process and social and occupational reintegration of the patients.
Patients may experience deficits in executive functions, memory, psychomotor speed, attention, visuospatial abilities, and other cognitive domains (3,5-7). The typical pattern is thought to be mild-to-moderate dysfunction across multiple cognitive domains (8,9), although some studies have confirmed only severe impairment in a subset of patients rather than mild-to-moderate impairment in most of them (3).
Reports on different patterns of cognitive deficit, depending on the site of the aneurysm, are inconsistent (3,10). Different patterns may be present in patients with aneurysmal subarachnoid hemorrhage in identical locations (11). Earlier studies that explored cognitive deficits after subarachnoid hemorrhage from the anterior communicating artery (ACoA) described a special “ACoA syndrome”, characterized by amnesia, personality changes, and confabulation as the main symptoms (12-16), where damage to particular anterior cerebral structures was a suspected cause (14,15,17). However, other studies did not find specific deficits in ACoA patients, and a more diffuse pattern of brain damage was thought to be responsible for the deficits (9,18,19).
Accurate cognitive assessment of patients after aneurysmal subarachnoid hemorrhage could improve treatment measures in the preoperative and postoperative periods and help in accurate guidance of rehabilitation. Previous studies have not simultaneously addressed patients’ and relatives’ views of cognitive deficits, their level of community integration, and the results of neuropsychological and neurophysiological tests. In this study, we explored long-term cognitive deficits in a small, selected group of patients with good outcome after subarachnoid hemorrhage due to the rupture of the anterior communicating artery aneurysm. Our aim was to determine whether long-term changes in brain functioning could be detected with a simple and objective method that could be of potential use at different time points after subarachnoid hemorrhage, and to determine if diminished cognitive capacities seen on neuropsychological test results were also reflected in neurophysiological measurements, since this could also provide some information regarding localization and the degree of brain damage.
Patients and methods
Patients
Ten right-handed patients (6 men and 4 women) were chosen from a pool of 66 consecutive patients with solitary aneurysms of the anterior communicating artery that had been operated over a 3-year period at the Department of Neurosurgery, University Medical Center, Ljubljana, Slovenia. The patients had to meet the following inclusion criteria: experience of spontaneous subarachnoid hemorrhage from the rupture of the solitary non-giant aneurysm of the anterior communicating artery at least 2 years previously; good preoperative condition (grade 1, according to the World Federation of Neurological Surgeons) (20); aneurysm totally excluded by surgically placing a clip on the aneurysm’s neck as confirmed on the control angiography; no major postoperative complications; no clinical vasospasm after operation; good outcome one year after the surgery (grade 5 on Glasgow Outcome Scale) (21); and no neurological or psychiatric disease before or after the surgery. The purpose and course of the study was explained to all patients. Special care was taken not to cause any psychological stress or harm during the study. The study was approved by the National Ethical Committee.
The median age of patients at the time of operation was 55 years (range, 45-65), and the median time interval between the operation and the assessment was 41 months (range, 28-60) (Table 1). All patients were operated via the right pterional transsylvian approach, except for 2 patients (No. 2 and 8) in whom the left pterional transsylvian approach was used. All patients were operated in the acute period, ie, within 14 days after subarachnoid hemorrhage, except for patient No. 6 in whom the aneurysm was clearly visible only on control angiography performed a few months after subarachnoid hemorrhage. Subarachnoid hemorrhage was not visible on initial computer tomography (CT) head scans in patients No. 3, 6, and 8, but was detected only by lumbar puncture. The degree of subarachnoid hemorrhage was mostly mild (grade 2 on the Fisher scale) (22) in all patients, except in patient No. 1 who had extensive subarachnoid hemorrhage and patient No. 5 who had extensive subarachnoid hemorrhage, interhemispheric frontal hematoma, and hematocephalus. Neither intracerebral hematoma nor hematocephalus was visible in other patients.
Table 1.
Clinical characteristics of the patients operated for subarachnoid hemorrhage*
| Patient No. | Age at operation | Time to operation (d) | Side of surgical approach | Preoperative head CT scan | SAH grade |
|---|---|---|---|---|---|
| 1 | 45 | 0 | right | extensive SAH in basal cisterns and cortical sulci | 3 |
| 2 | 56 | 2 | left | SAH in basal cisterns and cortical sulci | 2 |
| 3 | 54 | 8 | right | no SAH, blood in CSF (lumbar puncture) | 1 |
| 4 | 53 | 2 | right | minimal SAH in basal cisterns | 2 |
| 5 | 64 | 3 | right | extensive SAH, interhemispheric frontal hematoma, hematocephalus | 4 |
| 6 | 61 | 115 | right | no SAH, blood in CSF (lumbar puncture) | 1 |
| 7 | 60 | 5 | right | SAH in basal cisterns and cortical sulci | 2 |
| 8 | 51 | 13 | left | no SAH, blood in CSF (lumbar puncture) | 1 |
| 9 | 65 | 1 | right | interhemispheric frontal SAH | 2 |
| 10 | 49 | 1 | right | SAH in basal cisterns and cortical sulci | 2 |
*Abbreviations: d – days; CT – computerized tomography; SAH – subarachnoid hemorrhage (Fisher grade of subarachnoid hemorrhage; ref. 22); CSF – cerebrospinal fluid.
The postoperative course was uneventful in all patients. They had no neurological deficits at discharge, except for patient No. 7, who developed transient palsy of the cranial nerve III that disappeared several months after the operation. The postoperative CT scans of the head showed improvement in all patients, with the resolution of blood. No hydrocephalus developed, and none of the patients needed ventricular drainage.
During the course of the study, 7 patients were receiving antihypertensive medications (patients No. 3-8 and 10), 2 were on oral antidiabetic medications (patients No. 1 and 3), and one patient was taking thyroid replacement therapy due to the hypothyroidism (patient No. 9). Blood glucose concentration was well-controlled and within normal limits for the two patients on antidiabetic medications, and so was the concentration of thyroid hormones in one patient treated for hypothyroidism. The levels of blood pressure were also well-controlled and within normal limits. None of the patients was receiving medications for depression or anxiety disorders. No major effect was expected from the medications on the cognitive functioning of patients.
The group of relatives consisted of patients’ husbands or wives (one relative of each patient), aged between 43 and 71 years (median, 57). Patient No. 8 lived alone and no relative’s report was obtained for him.
For neurophysiological measurements, a control group of 10 healthy individuals (6 men and 4 women) was used for comparison. The median age of healthy controls was 57.5 years (range, 49-73) and median duration of education was 12 years (range, 8-16). There were no significant differences between the patient and control group in age and duration of education.
Community integration and cognitive deficits after subarachnoid hemorrhage
Patients’ employment after operation was noted on the initial interview before the neuropsychological and neurophysiological assessment. The patients graded the severity of their disease on a 4-point scale – mild, moderate, severe, or very severe. They filled out the Community Integration Questionnaire (CIQ) (23), a structured questionnaire with 15 items covering various aspects of home, social, educational, and work integration. The overall CIQ score is the sum of scores from the individual questions and can range from 0 to 29; a higher score indicates better quality of integration. The patients also evaluated the decrease in their ability to work after subarachnoid hemorrhage, because it was not addressed in the CIQ.
Patients and their relatives completed the Cognitive Symptom Checklists (24) for problems with executive functions, memory, and attention. Various everyday problems were listed on the checklist, and patients simply had to note whether or not they had a problem. The relatives did the same, evaluating patients’ problems. The total number of complaints on each of the 3 checklists was divided by the total number of possible complaints and expressed as a percentage of possible complaints.
Neuropsychological assessment
A battery of standardized neuropsychological tests was applied to all patients.
Wisconsin Card Sorting Test (25) involves sorting of cards according to one of three categories (color, form, or number). The test was used to estimate cognitive flexibility, problem-solving, set shifting, conceptualization, and perseveration. Total errors, perseverative responses, perseverative errors, and non-perseverative errors were evaluated.
On Tower of London Test (26), a subject has to match the model of configurations on the examiner’s tower board consisting of three pegs and three beads, taking into account some predefined rules. In particular, the ability to plan was assessed. The total move score, ie, minimum number of moves to accomplish the task, and total problem-solving time were evaluated.
A broad range of memory functions was assessed with Williams’ Memory Assessment Scales (27). Several subscales for verbal and visual attention were used. The following subscales were used to assess verbal attention: verbal span (to remember a series of numbers and repeat them in the exact and reverse orders); list acquisition (to remember a list of words across multiple trials); list recall (to recall the list of words after a short period of time); delayed list recall (to recall the list of words after a longer period of time); immediate prose recall (to recall details from a short story after a brief period of time), and delayed prose recall (to recall details from a short story after a longer period of time). Visual memory was assessed with the following subscales: visual span (to recall the position and correct order of the objects on the stimulus card); immediate names-faces (to remember the names of people in a series of ten photographs); delayed names-faces (to choose the proper names for people from among three offered names for a series of previously presented photographs); visual reproduction (to draw previously presented designs); immediate visual recognition (to decide whether a given design is similar to or different from one memorized previously) and delayed visual recognition (to recognize previously presented designs from a larger series after a longer period).
The following tests were used to evaluate attention: Stroop Color and Word test (28), D2 Test of Attention (29) to assess selective attention, and Color Trail Test (30) to assess the ability to maintain sustained visual attention.
All test results were compared to the published norms, taking into account both the age and educational level of the patient, and were expressed on a percentile scale. Years of formal education also served as an adjunct to determine the approximate level of premorbid cognitive functioning.
Event-related potential recordings
A three-stimulus auditory oddball paradigm was used (31-33) according to the standard protocol used at the Institute of Clinical Neurophysiology, University Medical Center, Ljubljana. Event-related potentials (ERPs) were recorded at the time of neuropsychological testing, using 10 scalp electrodes: a ground electrode referenced to balanced linked-earlobe electrodes, three electrodes placed at Fz, Cz, and Pz, and two electrodes on each side for elimination of eye artifacts. The auditory oddball paradigm consisted of three tones administered randomly: frequent stimuli of 1000 Hz, rare non-target stimuli of 500 Hz, and rare target stimuli of 2000 Hz. Patients had to press the button whenever 2000 Hz stimuli appeared. One trial consisted of 150 stimuli. The recordings for each subject were repeated twice.
Each ERP component was identified according to its polarity, latency, and scalp distribution. The components of the early waves were measured in the Cz band of the ERP for the target stimuli. The first positive deflection was labeled P1, and the first negative deflection was labeled N1. The first positive deflection following N1 was labeled P2, and the second negative deflection was labeled N2. A positive deflection recorded in the Fz band of the non-target stimuli that occurred after N2 was labeled P3a. A positive deflection recorded in the Pz band of the target stimuli that occurred after N2 was labeled P3b. The amplitudes of the ERP waves were determined with respect to the mean EEG baseline, and peak-to-peak values were calculated. P3a or P3b waves were considered non-identifiable when they could not be clearly distinguished. The latencies and amplitudes of the ERPs in our group of patients were compared to the control group described before.
Statistical analysis
Due to the small sample size, median and range were used as measures of central tendency and variability. Mann-Whitney U test was used for comparison between two different groups of subjects and Wilcoxon Signed Ranks Test was used for comparison between pairs of variables in the same group of subjects. Spearman’s rho (ρ) correlation coefficients were calculated to access association between pairs of variables. P<0.05 was considered statistically significant. Statistical analysis was performed with Statistical Package for Social Sciences, version 10.0 (SPSS Inc., Chicago, IL, USA) for Windows.
Results
Neither the craniotomy side nor the time from the onset of subarachnoid hemorrhage to the operation had a major impact on postoperative cognitive performance. We expected that patients with left-sided (dominant side) craniotomy (patients No. 2 and 8; Table 1) would have worse cognitive performance, but we did not prove this, since both patients had impaired results only on a few of the neuropsychological tests. The correlations between the time from the onset of subarachnoid hemorrhage to the operation and the neuropsychological test results were all not significant (ρ range, -0.42 to 0.66). Patient No. 6 was excluded from this analysis, since he was not operated in the acute period after hemorrhage. No significant correlation between the length of time from the operation to testing (see Table 2) and the neuropsychological test results was found (ρ range, -0.36 to 0.56; Table 2).
Table 2.
Level of education of patients with subarachnoid hemorrhage and their relatives, employment after subarachnoid hemorrhage, and Community Integration Questionnaire results*
| Patient |
Relative |
|||||
|---|---|---|---|---|---|---|
| Patient No. | education level | employment | CIQ | operation-to-testing time (mo) | relation | education level |
| 1 | 11 | unemployed before SAH | 6 | 37 | wife | 11 |
| 2 | 12 | unemployed before SAH | 12 | 43 | wife | 12 |
| 3 | 12 | retired before SAH | 15 | 36 | husband | 11 |
| 4 | 10 | retired before SAH | 19 | 35 | husband | 11 |
| 5 | 12 | retired before SAH | 17 | 57 | wife | 16 |
| 6 | 11 | retired before SAH | 11 | 44 | wife | 14 |
| 7 | 8 | retired after SAH | 9 | 60 | wife | 8 |
| 8 | 12 | same employment | 22 | 39 | single | |
| 9 | 12 | retired before SAH | 18 | 28 | husband | 12 |
| 10 | 8 | retired before SAH | 14 | 43 | husband | 8 |
*Abbreviations: CIQ – Community Integration Questionnaire (23); SAH – subarachnoid hemorrhage.
The median duration of formal education of patients was 11.5 years (range, 8-12), similar to that of their relatives (Table 2). Most patients were either retired or unemployed before subarachnoid hemorrhage. Only one patient (patient No. 5) reported undiminished work capacity after subarachnoid hemorrhage; all others reported it to be decreased. The CIQ score ranged between 6 and 22 (median, 14.5), showing a variable level of community integration after subarachnoid hemorrhage. Of the two patients who had been employed before the event, one retained the same position at work and had the highest CIQ score in the group, while the other who had to retire after the subarachnoid hemorrhage had the second lowest CIQ score in the group (Table 2). This showed that their work status was related to their level of community integration.
Most patients self-evaluated their disease as severe or very severe (Table 3). Only patient No. 2 graded the severity of his disease as moderate, and patient No. 9 graded it as mild. Great variability in the patients’ and relatives’ reporting of problems was found, from none to many. Both patients and relatives most frequently reported problems with attention. Patients had more complaints than their relatives for all cognitive domains. The correlation between patients’ and relatives’ reports was not significant (ρ = 0.48, P = 0.191 for executive functions; ρ = 0.47, P = 0.202 for memory; and ρ = 0.34, P = 0.376 for attention). The correlations between the results on cognitive symptom checklists of both patients and relatives and the degree of community integration according to the results on CIQ ranged between ρ=-0.22 and ρ=-0.75. Patients reported significantly more attention problems than their relatives (P = 0.019).
Table 3.
Patients’ and relatives’ reports on the patients’ cognitive problems after subarachnoid hemorrhage
| Report (%)* | |||||||
|---|---|---|---|---|---|---|---|
| patients |
relatives |
||||||
| Patient No. | Self-evaluated severity of disease | executive function | memory | attention | executive function | memory | attention |
| 1 | severe | 45 | 59 | 73 | 21 | 22 | 32 |
| 2 | moderate | 7 | 7 | 31 | 8 | 5 | 34 |
| 3 | very severe | 7 | 1 | 34 | 0 | 11 | 0 |
| 4 | severe | 17 | 14 | 25 | 1 | 0 | 5 |
| 5 | moderate | 0 | 0 | 2 | 5 | 4 | 2 |
| 6 | severe | 0 | 0 | 2 | 0 | 0 | 20 |
| 7 | very severe | 12 | 22 | 46 | 0 | 6 | 12 |
| 8 | very severe | 4 | 14 | 27 | single | single | single |
| 9 | mild | 5 | 4 | 17 | 0 | 0 | 0 |
| 10 | very severe | 12 | 6 | 27 | 18 | 5 | 10 |
| Median (range) | 7 (0-45) | 6.5 (0-59) | 27 (2-73) | 1 (0-21) | 5 (0-22) | 10 (0-34) | |
*Percentage of the total number of possible complaints.
Neuropsychological testing
Visual memory was most frequently affected, followed by verbal memory and executive functions (Table 4). Severe neuropsychological dysfunctions across all cognitive domains were found in only a single patient (patient No. 1). Additionally, prominent dysfunction with impairments on many tests was present in 4 patients. Taking into consideration the years of education, a clear general decline in cognitive functioning could be observed only in patients 1, 3, and 5.
Table 4.
Findings of different neuropsychological tests in patients surgically treated for subarachnoid hemorrhage
| Neuropsychological aspect* | Median percentile (range) | No. of patient with impaired results ≤10th percentile |
|---|---|---|
| Executive functions: | ||
| WCST – total errors | 13.5 (1-90) | 1, 5, 9, 10 |
| WCST – perseverative responses | 24 (1-90) | 1, 5, 9, 10 |
| WCST – perseverative errors | 24 (1-87) | 1, 9 |
| WCST – non-perseverative errors | 9.5 (1-87) | 1, 4, 5, 9, 10 |
| TOL – total move score | 16 (1-94) | 1, 3, 5, 9 |
| TOL – total problem-solving time | 22.5 (1-66) | 1, 3, 7, 9 |
| Attention: | ||
| Color Trail Test (form A) | 5.5 (1-79) | 1, 5, 7, 9, 10 |
| Color Trail Test (form B) | 28 (1-73) | 1, 6, 9 |
| D2 – total number minus errors | 27 (2-68) | 1, 6, |
| D2 – errors (%) | 48 (4-79) | 3 |
| Stroop – words | 21 (1-50) | 1, 6, 7 |
| Stroop – colors | 22.5 (1-58) | 1, 6 |
| Stroop – interference | 46 (4-88) | 1, 3 |
| Verbal memory: | ||
| verbal span | 16 (0-63) | 1, 2, 3, 4 |
| list acquisition | 12.5 (2-63) | 1, 3, 5, 7, 8 |
| list recall | 12.5 (1-84) | 1, 3, 4, 5, 7 |
| delayed list recall | 12.5 (0-95) | 1, 3, 4, 5, 7 |
| immediate prose recall | 31 (2-91) | 1, 3, 5 |
| delayed prose recall | 20.5 (2-91) | 1, 3, 5 |
| Visual memory: | ||
| visual span | 12.5 (1-50) | 1, 4, 5, 8, 10 |
| immediate names-faces | 9 (0-91) | 1, 3, 4, 5, 7, 8, 10 |
| delayed names-faces | 9 (1-50) | 1, 3, 5, 6, 7, 9, 10 |
| visual reproduction | 12.5 (0-75) | 1, 3, 4, 7, 10 |
| immediate visual recognition | 25 (1-50) | 1, 7, 9 |
| delayed visual recognition | 25 (0-99) | 1, 3 |
Abbreviations: WCST – Wisconsin Card Sorting Test; TOL – Tower of London Test; D2 – D2 Test of Attention.
To test if patients’ memory performance would improve when provided with organizational strategies, list recall was compared with cued list recall, but no significant differences were found (Wilcoxon signed-rank test; P = 0.655). Additionally, results showed significantly better scores for list recognition than for list recall (Wilcoxon signed-rank test; P = 0.035).
The correlation between the neuropsychological test results and patients’ reports on frequency of cognitive symptoms showed high negative correlations for executive functions between patients’ reports and the total time on Tower of London Test (ρ = -0.51). Negative correlations were also obtained between patients’ reports and Stroop test results for attention (ρ range, -0.40 to -0.44), and between patients’ reports and verbal span results (ρ = -0.41) and visual reproduction (ρ = -0.41) for memory. However, none of the correlations reached statistical significance. Other correlations were low. Overall, no association could be observed between the results of the neuropsychological tests and patients’ reports from the cognitive symptom checklists.
ERP results
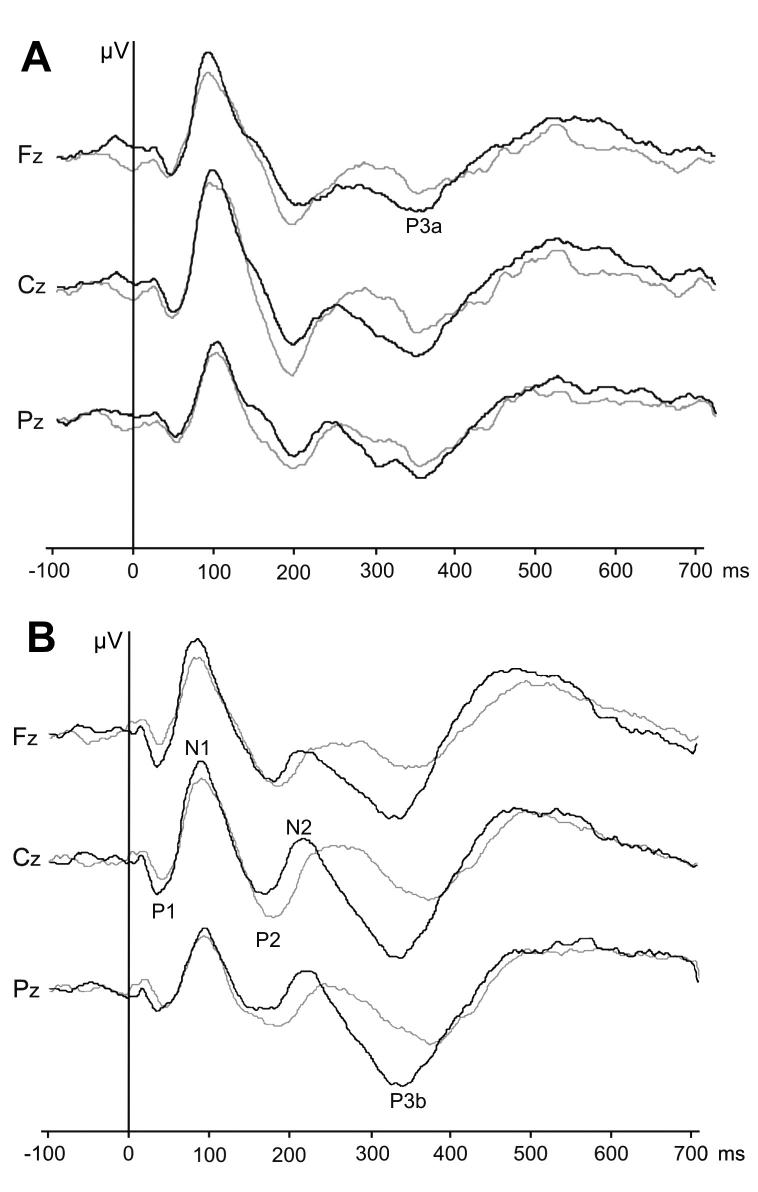
ERPs from scalp electrodes for non-target and target stimuli in patients and healthy controls were grand-averaged (Figure 1). Patients reaction times for pressing the button when target stimuli were presented were not significantly longer than times when non-target stimuli were presented (441 vs 374 ms, respectively; Mann-Whitney U-test; P = 0.131). Early ERP components (waves N1, P2, and N2) could be identified in all controls and patients, but there were no significant differences in early ERP components (amplitudes and latencies of waves N1, P2, and N2).
Figure 1.
Grand-averaged event-related potentials for patients after subarachnoid hemorrhage (gray line) and controls (black line) for non-target (A) and target (B) stimuli. The abscise shows time (ms) from the onset of stimuli; ordinate shows voltage (1 mm = 0.5 μV) in the three bands according to the baseline (at time 0 ms the voltage is 0 μV in each band).
P3a wave could not be identified in one subject in the control group, but P3b wave was identified in all subjects in the control group. Neither P3a wave nor P3b wave could be identified in 3 patients (patients No. 3, 7, and 10). P3a latencies were not significantly longer in patients when compared with healthy controls (333 vs 322 ms). In contrast, P3b latencies were significantly longer in the patients (364 vs 334 ms; Mann-Whitney U-test; P = 0.025) than in the control group. Differences in peak-to-peak amplitudes of the P3a and P3b waves between groups were not statistically significant.
The results of the neuropsychological tests (percentile values) and the ERP results were compared. The three patients in whom neither P3a nor P3b waves could be observed had prominent cognitive deficits (patients No. 3, 7, and 10). Prominent, although statistically insignificant, were negative correlations between the result on the form A (a series of numbers that had to be connected in ascending order) of the Color Trail Test and P3a latencies (ρ = -0.58; P = 0.172) and P3b waves (ρ = -0.58; P = 0.172). Many positive, although insignificant, higher correlations were also observed between the P3a amplitude and the results on both tests for executive functions. All other correlations between neuropsychological test results and late ERP components (P3a and P3b latencies and amplitudes) were mostly low and statistically insignificant. The lack of statistical significance for some higher correlations is attributable to the small sample size used.
Discussion
Our group of patients showed a mild degree of long-term cognitive impairment. A clear cognitive decline could be confirmed in only three patients. Deficits in memory and executive functions were observed most frequently. The patients’ and relatives’ evaluation of cognitive dysfunction was similar. Both patients and relatives reported problems with attention most frequently, but their reports also showed the greatest variability in this domain. The reason could be that the symptoms listed on a checklist for attention were not described clearly enough and were only moderately related to everyday attention problems of our patients. Besides, neuropsychological results on the attention tests were actually better than for other cognitive domains, confirming the differences between listed symptoms and actual attention problems. The association between self-rated cognitive dysfunction and neuropsychological test results was weak. A more profound self-rated cognitive dysfunction was associated with weaker community integration.
Impaired cognitive functioning was also evident in neurophysiological measures where patients had significantly longer P3b latencies than controls. Most correlations between neuropsychological and neurophysiological results were low and insignificant, which is probably a consequence of many uncontrollable confounding factors that can influence performance on neuropsychological tests. Prominent (although insignificant) correlations were found only between one test of sustained attention and P3a and P3b latencies, and many positive (although insignificant) correlations were observed between P3a amplitudes and tests of executive functions.
Other studies found cognitive impairments to be more prevalent (9,10,18,34,35). It might be that our group of patients had greater advantages in terms of clinical characteristics and outcome. Moreover, our patients were tested at least 2 years after subarachnoid hemorrhage, and it has been established that the cognitive profile following ACoA rupture improves with time (36). Other studies that examined similar groups of patients also reported neuropsychological consequences to be more favorable (37). A wide range of cognitive impairments was also a common finding in other studies, both for subarachnoid hemorrhage of various aneurysmal origins (6,9,34,38) and for subarachnoid hemorrhage after ACoA rupture (39,40), although some studies failed to support this (41,42).
Subarachnoid blood and its toxic products are thought to be the primary factors for cognitive dysfunction (7-9,43). The surgical treatment itself may also be a potential cause of local structural brain damage, since patients having endovascular treatment for ruptured aneurysms have more favorable cognitive outcomes than those having their aneurysms surgically clipped (6,44-46). No general association between the degree of subarachnoid hemorrhage and cognitive dysfunction could be observed in our group of patients, although there was a trend toward worse cognitive performance with more extensive subarachnoid hemorrhage.
In cases of subarachnoid hemorrhage from ACoA rupture, it was proposed that damage to the specific brain regions might be responsible for the deficits (17). One specific area could be the basal forebrain, which is supplied by perforating branches of the ACoA (47,48). It has been suggested that in patients with ACoA aneurism rupture, the cerebral areas traditionally associated with memory functioning are spared (14,15,17) and that damage to the basal forebrain and frontal lobes is responsible for memory dysfunction. This was also confirmed by the fact that amnesic patients improved in their memory performance when an organizational strategy was provided (49). This could not be confirmed in our group of patients, since they did not score any better on cued list recall. Still, they performed better on list recognition than on list recall, indicating more problems in recall than in storage. The latter to some degree confirms the involvement of frontal lobe damage in at least some of the memory deficits. On the other hand, studies that found more prevalent deficits in patients with ACoA aneurism rupture suggested that such deficits were the consequence of a more diffuse cerebral damage (9,18,19,50).
Although the role of the P3 wave in cognition is not clearly understood, it was found to be smaller and to peak later in patients with decreased cognitive abilities (51). The P3a wave probably reflects the processing of novel stimuli and orientation of attention and the P3b wave is associated with cognitive contextual integration (52). Differences in the late ERP components reflect cortical damage that was still present in patients. Longer P3b latencies in this study probably reveal difficulties in evaluation of the stimulus and response to it. The results on tests of the frontal lobe functions (attention and executive functions) were expected to correlate particularly with ERP measures, which was in our study confirmed only partially. Another study on the relationship between neuropsychological and ERP findings in patients after aneurysmal subarachnoid hemorrhage following ACoA aneurism rupture, although investigating only short-term consequences, led to a conclusion that ERPs might be an objective parameter in the follow-up of cases with cognitive impairment (53). Given our results, we can agree with this position.
A clear limitation of our study is a small and selected number of patients, which precluded generalization of the results and could not explain the role of different clinical factors and treatment modalities. Another limitation was that symptoms of depression were not evaluated in our patients. Depression is a common condition in patients who suffered from subarachnoid hemorrhage (54) and can have a major impact on functional status (55-57). Its effect could explain the lack of significant correlation between neuropsychological and neurophysiological measures.
In conclusion, we showed that cognitive deficits were still present in a subset of patients with good outcomes several years after subarachnoid hemorrhage from ACoA rupture. They were confirmed by both neuropsychological and neurophysiological investigations. Appropriate long-term evaluation is more precise by combining both methods. Future studies could focus on patients who received endovascular treatment in order to more purely study the effect of subarachnoid hemorrhage itself and evaluation of emotional problems should be part of the evaluation. Neurophysiological evaluation should be performed at different time points to more objectively evaluate its clinical utility. Longitudinal studies with a larger number of patients exhibiting a more diverse clinical course and receiving different type of treatment are needed.
References
- 1.Longstreth WT, Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43:712–8. doi: 10.1212/wnl.43.4.712. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan KM, Elias LJ, Goplen GB. Differing perspectives on outcome after subarachnoid hemorrhage: the patient, the relative, the neurosurgeon. Neurosurgery. 2000;46:831–8. doi: 10.1097/00006123-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002;33:200–8. doi: 10.1161/hs0102.101080. [DOI] [PubMed] [Google Scholar]
- 4.Hütter BO, Gilsbach JM. Which neuropsychological deficits are hidden behind a good outcome (Glasgow=I) after aneurysmal subarachnoid hemorrhage? Neurosurgery. 1993;33:999–1006. doi: 10.1227/00006123-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Hillis AE, Anderson N, Sampath P, Rigamonit D. Cognitive impairments after surgical repair of ruptured and unruptured aneurysms. J Neurol Neurosurg Psychiatry. 2000;69:608–15. doi: 10.1136/jnnp.69.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadjivassiliou M, Tooth CL, Romanowski CAJ, Byrne J, Battersby RDE, Oxbury S, et al. Cognitive outcome and structural damage after clipping or coiling. Neurology. 2001;56:1672–7. doi: 10.1212/wnl.56.12.1672. [DOI] [PubMed] [Google Scholar]
- 7.Hutter BO, Kreitschmann-Andermahr I, Mayfrank L, Rohde V, Spetzger U, Gilsbach JM. Functional outcome after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 1999;72:157–74. doi: 10.1007/978-3-7091-6377-1_13. [DOI] [PubMed] [Google Scholar]
- 8.Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654–61. doi: 10.1227/00006123-198804000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ogden JA, Mee EW, Henning M. A prospective study of impairment of cognition and memory and recovery after subarachnoid hemorrhage. Neurosurgery. 1993;33:572–87. doi: 10.1227/00006123-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Tidswell P, Dias PS, Sagar HJ, Mayes AR, Battersby RDE. Cognitive outcome after aneurysm rupture: Relationship to aneurysm site and perioperative complications. Neurology. 1995;45:875–82. doi: 10.1212/wnl.45.5.876. [DOI] [PubMed] [Google Scholar]
- 11.Stenhouse LM, Knight RG, Longmore BE, Bishara SN. Long-term cognitive deficits in patients after surgery on aneurysms of the anterior communicating artery. J Neurol Neurosurg Psychiatry. 1991;54:909–14. doi: 10.1136/jnnp.54.10.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talland GA, Sweet WH, Ballantine HT. Amnestic syndrome with anterior communicating artery aneurysm. J Nerv Ment Dis. 1967;145:179–92. doi: 10.1097/00005053-196709000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Volpe BT, Hirst W. Amnesia following the rupture and repair of an anterior communicating artery aneurysm. J Neurol Neurosurg Psychiatry. 1983;46:704–9. doi: 10.1136/jnnp.46.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damasio AR, Graff-Radford NR, Esling PJ, Damasio H, Kassell N. Amnesia following basal forebrain lesions. Arch Neurol. 1985;42:263–71. doi: 10.1001/archneur.1985.04060030081013. [DOI] [PubMed] [Google Scholar]
- 15.Phillips S, Sangalang V, Sterns G. Basal forebrain infarction: a clinicopathologic correlation. Arch Neurol. 1987;44:1134–8. doi: 10.1001/archneur.1987.00520230024008. [DOI] [PubMed] [Google Scholar]
- 16.Alexander MP, Freedman M. Amnesia after anterior communicating artery aneurysm rupture. Neurology. 1984;34:752–4. doi: 10.1212/wnl.34.6.752. [DOI] [PubMed] [Google Scholar]
- 17.DeLuca J. Cognitive dysfunction after aneurysm of the anterior communicating artery. J Clin Exp Neuropsychol. 1992;14:924–34. doi: 10.1080/01688639208402544. [DOI] [PubMed] [Google Scholar]
- 18.Laiacona M, De Santis A, Barbarotto R, Basso A, Spagnoli D, Capitani E. Neuropsychological follow-up of patients operated for aneurysms of anterior communicating artery. Cortex. 1989;25:261–73. doi: 10.1016/s0010-9452(89)80042-5. [DOI] [PubMed] [Google Scholar]
- 19.Böttger S, Prosiegel M, Steiger HJ, Yassouridis A. Neurobehavioural disturbances, rehabilitation outcome, and lesion site in patients after rupture and repair of anterior communicating artery aneurysm. J Neurol Neurosurg Psychiatry. 1998;65:93–102. doi: 10.1136/jnnp.65.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985–6. doi: 10.3171/jns.1988.68.6.0985. [DOI] [PubMed] [Google Scholar]
- 21.Jennett B, Bond M. Assessment of outcome after severe brain damage. A practical scale. Lancet. 1975;1:480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 22.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Willer B, Offenbacher KJ, Coad ML. The community integration questionnaire: A comparative examionation. Am J Phys Med Rehabil. 1994;73:103–11. doi: 10.1097/00002060-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 24.O'Hara C, Harrell M, Bellingrath E, Lisicia K. Cognitive symptom checklists. Odessa (FL): Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- 25.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtidd G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa (FL): Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- 26.Culbertson WC, Zillmer EA. Tower of London -Drexel University. North Tonawanda (NY): MHS Multi-Health Systems Inc.; 2001. [Google Scholar]
- 27.Williams JM. The Memory Assessment Scales (MAS): Professional manual. Odessa (FL): Psychological Assessment Resources, Inc.; 1991. [Google Scholar]
- 28.Golden C. Stroop Color and Word test: A manual for clinical and experimental uses. Wood Dale (IL): Stoelting Co; 1978. [Google Scholar]
- 29.Brickenkamp R, Zillmer E. The d2 Test of Attention. Seattle: Hogrefe & Huber Publishers; 1998. [Google Scholar]
- 30.D'Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test: Professional Manual. Odessa (Florida): Psychological Assessment Resources, Inc.; 1996. [Google Scholar]
- 31.Katayama J, Polich J. P300 from one-, two-, and three-stimulus auditory paradigms. Int J Psychophysiol. 1996;23:33–40. doi: 10.1016/0167-8760(96)00030-x. [DOI] [PubMed] [Google Scholar]
- 32.Demiralp T, Ademoglu A, Comechero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topogr. 2001;13:251–67. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- 33.Knight RT. Aging decreases auditory event-related potentials to unexpected stimuli in humans. Neurobiol Aging. 1987;8:109–13. doi: 10.1016/0197-4580(87)90019-4. [DOI] [PubMed] [Google Scholar]
- 34.Saciri BM, Kos N. Aneurysmal subarachnoid haemorrhage: outcomes of early rehabilitation after surgical repair of ruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2002;72:334–7. doi: 10.1136/jnnp.72.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fertl E, Killer M, Eder H, Linzmayer L, Richling B, Auff E. Long-term functional effects of aneurysmal haemorrhage with special emphasis on the patient’s view. Acta Neurochir (Wien) 1999;141:571–7. doi: 10.1007/s007010050345. [DOI] [PubMed] [Google Scholar]
- 36.D’Esposito M, Alexander MP, Fischer R, McGlinchey-Berroth R, O’Connor M. Recovery of memory and executive function following anterior communicating artery aneurysm rupture. J Int Neuropsychol Soc. 1996;2:565–70. doi: 10.1017/s1355617700001740. [DOI] [PubMed] [Google Scholar]
- 37.Uski TK, Lilja A, Säveland H, Ekman R, Sonesson B, Brandt L. Cognitive functioning and cerebrospinal fluid concentrations of neuropeptides for patients with good neurological outcomes after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2000;47:812–8. doi: 10.1097/00006123-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Hütter BO, Gilsbach JM, Kreitschmann I. Quality of life and cognitive deficits after subarachnoid haemorrhage. Br J Neurosurg. 1995;9:465–75. doi: 10.1080/02688699550041106. [DOI] [PubMed] [Google Scholar]
- 39.Van der Linden M, Bruyer R. Memory disorders and symptoms of frontal dysfunction in 29 patients operated on for anerysm of the anterior communicating artery. Acta Neurol Belg. 1992;92:255–77. [PubMed] [Google Scholar]
- 40.Mavaddat N, Sahakian BJ, Hutchinson PJ, Kirkpatrick PJ. Cognition following subarachnoid hemorrhage from anterior communicating artery aneurysm: relation to timing of surgery. J Neurosurg. 1999;91:402–7. doi: 10.3171/jns.1999.91.3.0402. [DOI] [PubMed] [Google Scholar]
- 41.Richardson JT. Cognitive performance following rupture and repair of intracranial aneurysm. Acta Neurol Scand. 1991;83:110–22. doi: 10.1111/j.1600-0404.1991.tb04659.x. [DOI] [PubMed] [Google Scholar]
- 42.McKenna P. Willison Jr, Phil B, Lowe D, Neil-Dwyer G. Cognitive outcome and quality of life one year after subarachnoid haemorrhage. Neurosurgery. 1989;24:361–7. doi: 10.1227/00006123-198903000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Hütter BO, Kreitschmann-Andermahr I, Gilsbach J. Cognitive deficits in the acute stage after subarachnoid hemorrhage. Neurosurgery. 1998;43:1054–65. doi: 10.1097/00006123-199811000-00030. [DOI] [PubMed] [Google Scholar]
- 44.Bellebaum C, Schafers L, Schoch B, Wanke I, Stolke D, Forsting M, et al. Clipping versus coiling: neuropsychological follow up after aneurismal subarachnoid hemorrhage (SAH). J Clin Exp Neuropsychol. 2004;26:1081–92. doi: 10.1080/13803390490515342. [DOI] [PubMed] [Google Scholar]
- 45.Fontanella M, Perozzo P, Ursone R, Garbossa D, Bergui M. Neuropsychological assessment after microsurgical clipping or endovascular treatment for anterior communicating artery aneurysm. Acta Neurochir (Wien) 2003;145:867–72. doi: 10.1007/s00701-003-0111-5. [DOI] [PubMed] [Google Scholar]
- 46.Chan A, Ho S, Poon WS. Neuropsychological sequelae of patients treated with microsurgical clipping or endovascular embolization for anterior communicating artery aneurysm. Eur Neurol. 2002;47:37–44. doi: 10.1159/000047945. [DOI] [PubMed] [Google Scholar]
- 47.Dunker RO, Harris AB. Surgical anatomy of the proximal anterior cerebral artery. J Neurosurg. 1976;44:359–67. doi: 10.3171/jns.1976.44.3.0359. [DOI] [PubMed] [Google Scholar]
- 48.DeLuca J, Cicerone KD. Confabulation following aneurysm of the anterior communicating artery. Cortex. 1991;27:417–23. doi: 10.1016/s0010-9452(13)80036-6. [DOI] [PubMed] [Google Scholar]
- 49.Diamond BJ, DeLuca J, Kelley SM. Memory and executive functions in amnesic and non-amnesic patients with aneurysms of the anterior communicating artery. Brain. 1997;120:1015–25. doi: 10.1093/brain/120.6.1015. [DOI] [PubMed] [Google Scholar]
- 50.Richardson JTE. Performance in free recall following rupture and repair of intracranial aneurysm. Brain Cogn. 1989;9:210–26. doi: 10.1016/0278-2626(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 51.Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–79. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in the auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–64. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 53.Fontanella MM, Bergamasco L, Perozzo P, Priano L, Vighetti S, Griva F, et al. Neuropsychological and neurophysiological evaluation after anterior communicating artery (ACoA) aneurysm surgery. J Neurosurg Sci. 2000;44:61–6. [PubMed] [Google Scholar]
- 54.Carter BS, Buckley D, Ferraro R, Rordorf G, Ogilvy CS. Factors associated with reintegration to normal living after subarachnoid hemorrhage. Neurosurgery. 2000;46:1326–33. doi: 10.1097/00006123-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 55.King JT, Jr, Kassam AB, Yonas H. Horowitz Mb, Roberts MS. Mental health, anxiety, and depression in patients with cerebral aneurysms. J Neurosurg. 2005;103:636–41. doi: 10.3171/jns.2005.103.4.0636. [DOI] [PubMed] [Google Scholar]
- 56.Fertl E, Killer M, Eder H, Linzmayer L, Richling B, Auff E. Long-term functional effects on aneurysmal subarachnoid haemorrhage with specific emphasis on the patient’s view. Acta Neurochir (Wien) 1999;141:571–7. doi: 10.1007/s007010050345. [DOI] [PubMed] [Google Scholar]
- 57.Morris PG, Wilson JT, Dunn L. Anxiety and depression after spontaneous subarachnoid hemorrhage. Neurosurgery. 2004;54:47–52. doi: 10.1227/01.neu.0000097198.94828.e1. [DOI] [PubMed] [Google Scholar]