Abstract
Aim
To investigate the relation between metabolic parameters of the brain tissue, as direct indicators of real metabolic conditions within the brain, and intracranial pressure, as the consequence of pathophysiological changes.
Methods
Twelve patients with closed head injuries were followed up for 24 hours after injury. A Codman parenchymal intracranial pressure and a Neurotrend electrode were inserted within 3 hours after injury to monitor parenchymal intracranial pressure, brain tissue partial oxygen pressure (PbrO2), brain tissue partial carbon dioxide pressure (PbrCO2), pH, and brain tissue temperature. Data detected at 8-hourly intervals were compared with repeated measures analysis of variance.
Result
At the initial observation, the mean value of intracranial pressure was 22.2 ± 3.2 mm Hg. Although it increased at the second and decreased at the third measurement, the differences between the measurements were not significant (P = 0.320). The value of PbrCO2 was increased from the beginning (63.3 ± 6.0 mm Hg), whereas PbrO2 was within the normal range at the first measurement (38.9 ± 6.9 mm Hg), but significantly decreased after 8 hours (P = 0.004), remaining low at later time points.
Conclusion
After brain injury, changes in PbrCO2 are visible earlier than those in PbrO2. Improvement in intracranial pressure values did not necessary mean improvement in the brain tissue oxygenation. In addition to intracranial pressure, PbrO2, PbrCO2 and pH should also be monitored, as they directly reflect the real metabolic conditions within brain tissue and may be used in predictions about the outcome and possible therapeutic approaches.
In head injuries, the brain is often threatened by various secondary pathophysiological processes leading to hypoxia or ischemia of the brain tissue (1). In the acute phase after head injury, the cerebral blood flow decreases, while the oxygen consumption in the brain tissue markedly increases (2-4). Secondary neurocytotoxic processes cause brain edema and increase the intracranial pressure, which further worsens cerebral metabolism, causing new neurocytotoxic complications. To prevent this vicious circle of pathophysiological processes, it is necessary to maintain adequate oxygenation of the brain tissue, which depends on the cerebral perfusion pressure and cerebral blood flow (5).
Cerebral perfusion pressure equals the difference between the mean arterial pressure and intracranial pressure. Continuous measurement of the mean arterial pressure is always possible in intensive care units (ICU), and the treatment of head injuries today routinely includes monitoring of intracranial pressure. A cerebral perfusion pressure of about 70 mm Hg can optimally supply the brain with blood; if the brain tissue partial oxygen pressure is above 35 mm Hg, normal oxygenation of the brain tissue should be assured (5,6). Continuous assessment of brain oxygenation is possible with the use of commercial sensors.
It is generally accepted that intracranial pressure is the key parameter in the assessment of patients with brain injuries. Moreover, patient treatment is based on the value of intracranial pressure (7). However, biochemical parameters in the brain tissue, such as the brain tissue partial oxygen pressure (PbrO2), brain tissue partial carbon dioxide pressure (PbrCO2), and pH, directly reflect the real metabolic conditions in the brain, whereas intracranial pressure is merely the consequence of pathophysiological changes.
In the early phase after the injury, the value of intracranial pressure may still be within the normal range, while biochemical parameters are already pathologically changed. Therefore, these metabolic parameters should be monitored in the acute phase of brain injury. Our hypothesis was that the variations of intracranial pressure values and variations of biochemical parameters were not closely related, even in cases of closed brain injury. To test this, we measured intracranial pressure, brain tissue partial oxygen (PbrO2) and carbon dioxide (PbrCO2) pressure, pH, and temperature of the brain tissue at different time points within the first 24 hours after injury. We also investigated the relation between the changes of intracranial pressure values and variations of each of these metabolic parameters.
Patients and methods
Patients
All patients with isolated brain injuries admitted to the Central ICU at the Department of Anesthesiology and Intensive Care between September 2004 and February 2005 were included in our study. All patients were admitted directly to our Center within the first three hours after the injury. On admission, the patients were clinically assessed by an anesthesiologist using Glasgow Coma Scale, and then treated according to the standardized protocol of the European Brain Injury Consortium (4,7,8). All patients were tracheally intubated and mechanically ventilated, sedated with continuous infusion of propofol and fentanyl, and paralyzed depending on clinical needs. Intracranial hypertension, defined as an intracranial pressure of >20 mm Hg for more than 10 minutes, was treated by head elevation, hyperventilation, and infusion of mannitol. We kept cerebral perfusion pressure in patients above 70 mm Hg, if possible, by reducing intracranial pressure and pharmacologically increasing mean arterial blood pressure with dopamine and noradrenalin.
Emergency neuroradiological diagnostic examinations were performed, including computed tomography (CT) of the brain. If emergency craniotomy was required, the intracranial pressure and Neurotrend sensors (Codman, Johnson & Johnson, Raynham, MA, USA) were inserted immediately after suturing of the skin flap. If emergency surgery was not necessary, the sensors were inserted across “cranial bolts” before the patients were admitted to the ICU. The parameters were monitored with original standard mobile monitors and computer recording units. Data on intracranial pressure, PbrO2, PbrCO2, pH, and brain temperature were then loaded onto the computer.
Twenty-two patients were eligible for the study. Three patients were excluded because the sensor was not inserted into the brain within the first three hours after the head injury. Two patients who underwent osteoclastic craniotomy were also excluded, because the intracranial pressure decreased as a consequence of the large bone defect. Two patients with multiple skull fractures were excluded, because the dura in this type of fracture sufficiently expands to enable intracranial pressure to decrease. One patient was excluded due to posttraumatic cerebrospinal fluid leaks, and two patients died a few hours after the sensor insertion.
The final sample thus included 12 patients, 3 women and 9 men. They suffered from cerebral contusions, cerebral edema, and traumatic intracranial hemorrhage. Seven of them underwent osteoplastic craniotomy due to epidural or subdural hematoma. The bone flap was firmly sutured back to the skull and the procedure did not have any lasting effect on the intracranial pressure. To avoid any possible intraoperative fall in intracranial pressure, the measurements were commenced one hour after the procedure, when stable values were obtained.
The study protocol was approved by the Ethics Committee and the patients’ relatives informally consented to the insertion of the sensors and participation in the study.
Methods
Intracranial pressure sensor was a commercial parenchymal Codman’s intracranial pressure monitoring system (Johnson & Johnson). The sensors were inserted 2.5 cm lateral to the sagittal suture and 1 cm anterior to the coronal suture on the contralateral side to the site of the brain damage.
The PbrO2, PbrCO2, pH, and temperature were measured with the Neurotrend (Johnson & Johnson) sensors in all 12 patients. The accuracy and reliability of the sensor was tested in vivo and in vitro before use in humans (9). Before being inserted into the brain parenchyma of a patient, the minimally invasive 0.5 × 35.0-mm multiparameter sterile sensor was placed in the tonometer for calibration, performed by the microprocessing system of the Neurotrend system. Then, the sensors were inserted through a double bolt into the frontal lobe thatdid not show any damage on the first CT brain scan.
Data were always collected from the white matter of the brain. The parameters (intracranial pressure, PbrO2, PbrCO2, pH, temperature) were measured three times, one hour, 9 hours, and 17 hours after the insertion of the sensors.
Statistical analysis
Normal distribution of the data was tested with the D'Agostino and Pearson omnibus test, and all measured values were thoroughly checked. The repeated measures analysis of variance was used to assess statistically significant differences between the measurements of each parameter. The Bonferroni test was used for a post hoc analysis. Pearson correlations were calculated between intracranial pressure values and PbrO2, PbrCO2, and pH. All values were presented as mean ± standard error if not otherwise indicated. P<0.05 was considered statistically significant. The analysis was performed with SPSS statistical package (version 9, SPSS Inc., Chicago, IL, USA).
Results
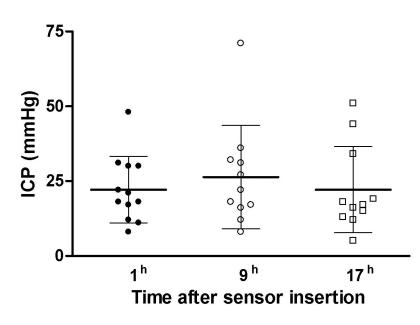
The median age of the 12 patients included in the study was 38 years, ranging from 15 to 79 years (Table 1). The median value of the Glasgow coma score on admission was 8 (range, 8-13). One hour after the insertion of the sensors, the mean value of intracranial pressure in the patients was 22.2 ± 3.2 mm Hg. Over the next 8 hours, intracranial pressure increased to 26.4 ± 5.2 mm Hg and then, 16 hours after the first measurement, decreased to 22.2 ± 4.3 mm Hg, ie, to the values almost equal to those recorded on the first measurement (Figure 1). No statistically significant difference was found among the three measurements (ANOVA, F = 1.203, P = 0.320).
Table 1.
Characteristics of 12 patients with brain injury included in the study*
| Demographic data | Brain injury features |
| Patient No. | age (years) | sex | GCS | ICP (mm Hg) 1 h after sensor insertion | sensor site (frontal lobe) | injury |
|---|---|---|---|---|---|---|
| 1 | 66 | m | 9 | 8 | right | SDH |
| 2 | 79 | m | 8 | 48 | right | SDH |
| 3 | 59 | f | 8 | 21 | left | DAI |
| 4 | 60 | m | 8 | 12 | right | SDH |
| 5 | 20 | m | 8 | 22 | left | EDH |
| 6 | 20 | f | 8 | 30 | right | DAI |
| 7 | 21 | m | 13 | 18 | right | contusions |
| 8 | 18 | m | 8 | 17 | right | contusions |
| 9 | 47 | m | 8 | 30 | right | contusions |
| 10 | 33 | f | 11 | 31 | left | EDH, contusions |
| 11 | 15 | m | 8 | 11 | right | EDH |
| 12 | 20 | m | 10 | 18 | left | SDH |
* Abbreviations: GCS - Glasgow Coma Score; ICP – intracranial pressure; m – male, f – female; SDH – subdural hematoma; DAI – diffuse axonal injury; EDH – epidural hematoma.
Figure 1.
Change in the intracranial pressure (ICP, mean ± standard error) after the brain injury.
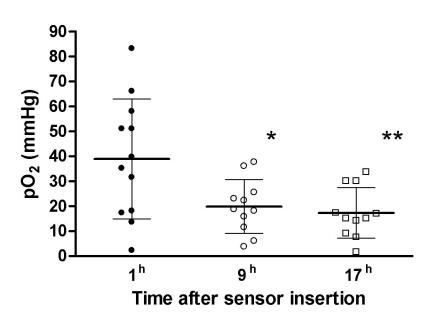
At the initial measurement, the mean value of PbrO2 (38.9 ± 6.9 mm Hg) was within the normal physiological range. Nine hours after the insertion of sensors, the value decreased to 19.8 ± 3.3 mm Hg and remained low (17.3 ± 3.1 mm Hg) 17 hours after the sensor insertion (Figure 2). The differences among the three measurements were statistically significant (ANOVA, F = 11.564, P = 0.004). Post hoc analysis revealed statistically significant differences between the values obtained one and 9 hours (P = 0.018), and one and 17 hours after the insertion of the sensors (P = 0.016). The differences between the second (after 9 hours) and the third (after 17 hours) measurement were not statistically significant. A negative correlation was found between the intracranial pressure and PbrO2 values (r = -0.387, P = 0.024)
Figure 2.
Dynamics of brain tissue partial oxygen pressure (PbrO2, mean ± standard error) during the monitoring. Asterisks indicate significant differences in comparison with values measured one hour after the sensor insertion. Repeated measures ANOVA and Bonferroni post hoc test.
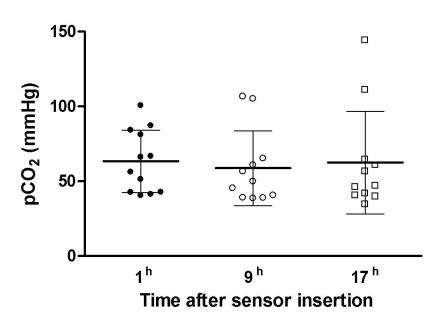
Initially, the mean value of PbrCO2 was as high as 63.3 ± 6.0 mm Hg, after which it decreased (Figure 3). No statistically significant differences were found between the values detected one hour, 9 hours, and 17 hours after the insertion of the sensors (ANOVA, F = 0.199, P = 0.713). However, there was a good correlation between intracranial pressure and PbrCO2 (r = 0.655, P<0.001).
Figure 3.
Dynamics of brain tissue partial carbon dioxide pressure (PbrCO2, mean ± standard error) during the monitoring.
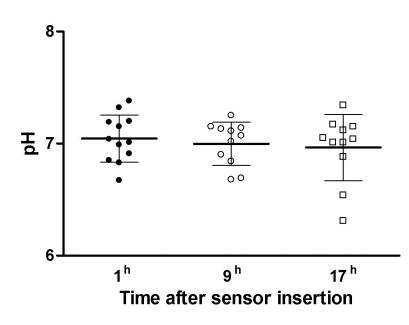
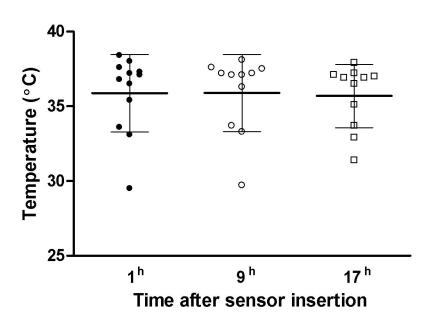
The mean pH value of 7.05 ± 0.06 on the first measurement did not statistically change on the next two measurements (ANOVA, F = 2.028, P = 0.176; Figure 4). No statistically significant differences were detected among the three measurements of brain intraparenchymal temperature (ANOVA, F = 0.361, P = 0.622; Figure 5). There was a good correlation between intracranial pressure and pH values (r = -0.609, P<0.001). The values of the brain parenchymal temperature were stable, showing only minimal variation.
Figure 4.
Dynamics of brain tissue pH (mean ± error) during the monitoring.
Figure 5.
Dynamics of brain tissue temperature (mean ± standard error) during the monitoring.
Two patients died within 24 hours after admission. They had the lowest first-measurement values of PbrO2 in the group, 2.2 and 13.5 mm Hg, respectively, as well as very high intracranial pressure (30 and 48 mm Hg, respectively).
Discussion
Our results revealed that variations of intracranial pressure values and variations of brain tissue partial oxygen pressure during the monitoring after brain injury were not closely related. Improvement in intracranial pressure values does not necessary mean improvement in brain tissue oxygenation. However, according to the literature, intracranial pressure is still the most important parameter that is monitored in brain injures (7). The main questions that need to be answered are how intracranial pressure correlates with metabolic variables within the injured brain tissue and if the monitoring of these biochemical parameters – PbrO2, PbrCO2, and pH – could be useful. The treatment of brain injury has to be implemented as soon as possible to minimize and prevent secondary brain lesions. Therefore, we monitored different variables in the acute phase after injury, ie, within 24 hours.
At the first measurement after admission, the mean intracranial pressure value was already high, as was the mean PbrCO2, while the mean PbrO2 was still within the normal range and the mean pH value was at the lower limit. In most cases, it seemed that the oxygenation of the brain tissue was still maintained at a physiological level within a short period of time after brain injury. However, pH was at the lower limit and PbrCO2 was already increased. Nine hours after the insertion of the sensors, significantly lower PbrO2 values were detected, although standardized therapy was implemented immediately after the admission. At that time, (the second measurement) the mean PbrCO2 was lower than at the initial observation, possibly due to a positive response to the treatment. However, this improvement in the PbrCO2 values was not statistically significant. Finally, 17 hours after admission to the ICU, the mean intracranial pressure decreased to almost the same value as that at the beginning of the study. However, contrary to expectations, PbrO2 also decreased. It is obvious that improvement in intracranial pressure did not necessarily mean improvement in brain tissue oxygenation. Thus, in addition to monitoring intracranial pressure, it is also necessary to monitor PbrO2, PbrCO2, and pH, because these parameters directly reflect the real metabolic conditions within brain tissue.
Because of the various types of head injury in our patients, there were differences in the basal values of the physiological parameters of the brain tissue. These values reflected their own individual values within the physiological limits. The degree of disturbance of autoregulation also varied among the patients, and over time in the same patient (10,11). The importance of absolute values is negligible in comparison with the dynamics of their alteration (12-14).
In this study, the mean value of intracranial pressure increased 9 hours after insertion of sensors and then decreased after 17 hours. However, one hour after the insertion of the sensors, brain tissue oxygenation was still maintained within the physiological range, probably as a result of the activity of autoregulatory mechanisms. Cerebral blood flow is controlled by autoregulation and vasoreactivity to adapt properly to changes in the oxygen demand of the brain. Loss of these regulatory capacities generally results in a poor outcome (15). In the two patients who died within 48 hours, the PbrO2 values were the lowest in the group, indicating that the breakdown of regulatory mechanisms had probably already occurred. The changes in PbrO2 seemed to start later than the changes in PbrCO2 and pH. At the onset of hypoxemia, PbrO2 changes only minimally for a certain period of time, whereas the accumulation of PbrCO2 is observed much earlier and to a greater extent (16). In our study, the mean PbrCO2 was already high at the first measurement. Thus, the sensor insertion and monitoring have to start as soon as possible after the brain damage.
Our study had several limitations. The number of patients was too small to determine more precisely the dynamics of PbrO2, PbrCO2, and pH in injured brain tissue. The period of assessment and follow-up was relatively short, and the values measured in different patients showed important range of variability.
In conclusion, our results, although on a small number of patients, suggest that intracranial pressure must not be the only relevant parameter for planning management of the patients with injured brain. Intracranial pressure values only reflect the consequences of pathological intracranial events (4). Real-time data obtained by measuring physiological parameters of brain tissue (PbrO2, PbrCO2, pH, and temperature) are important for planning intensive medical treatment and timely recognition of impending brain deterioration, as they change before intracranial pressure starts to rise as a final consequence of the metabolic brain disturbance. Thus, assessment of the severity of a head injury based only on the values of intracranial pressure could be misleading in treatment management and dangerous for the patient. However, further these findings should be confirmed by investigations on larger number of patients with brain injury.
References
- 1.Bruzzone P, Dionigi R, Bellinzona G, Imberti R, Stocchetti N. Effects of cerebral perfusion pressure on brain tissue pO2 in patients with severe head injury. Acta Neurochir Suppl. 1998;71:111–3. doi: 10.1007/978-3-7091-6475-4_33. [DOI] [PubMed] [Google Scholar]
- 2.Levasseur JE, Alessandri B, Reinert M, Bullock R, Kontos HA. Fluid percussion injury transiently increases then decreases brain oxygen consumption in the rat. J Neurotrauma. 2000;17:101–12. doi: 10.1089/neu.2000.17.101. [DOI] [PubMed] [Google Scholar]
- 3.Bergsneider M, Hovda D, Shalmon E, Kelly D, Vespa P, Martin N, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86:241–51. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 4.Servadei F, Murray GD, Penny K, Teasdale GM, Dearden M, Iannotti F, et al. The value of the »worst« computed tomographic scan in clinical studies of moderate and severe head injury. European Brain Injury Consortium. Neurosurgery. 2000;46:70–7. doi: 10.1097/00006123-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 5.The Brain Trauma Foundation The American Association of Neurological Surgeons. The joint section of neurotruma and critical care. Guidelines for cerebral perfusion pressure. J Neurotrauma. 2000;17:507–11. doi: 10.1089/neu.2000.17.507. [DOI] [PubMed] [Google Scholar]
- 6.Schroder M, Muizelaar JP, Kuta A, Choi S. Thresholds for cerebral ischemia after severe head injury: relationship with late CT findings and outcome. J Neurotrauma. 1996;13:17–23. doi: 10.1089/neu.1996.13.17. [DOI] [PubMed] [Google Scholar]
- 7.Maas AI, Dearden M, Teasdale GM, Braakman R, Cohadon F, Iannotti F, et al. EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien) 1997;139:286–94. doi: 10.1007/BF01808823. [DOI] [PubMed] [Google Scholar]
- 8.Procaccio F, Stocchetti N, Citerio G, Berardino M, Beretta L, Della Corte F, et al. Guidelines for the treatment of adults with severe head trauma (part I). Initial assessment; evaluation and pre-hospital treatment; current criteria for hospital admission; systemic and cerebral monitoring. J Neurosurg Sci. 2000;44:1–10. [PubMed] [Google Scholar]
- 9.Zauner A, Bullock R, Di X, Young HF. Brain oxygen, CO2, pH, and temperature monitoring: evaluation in the feline brain. Neurosurgery. 1995;37:1168–77. doi: 10.1227/00006123-199512000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Czosnyka M, Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral autoregulation following head injury. J Neurosurg. 2001;95:756–63. doi: 10.3171/jns.2001.95.5.0756. [DOI] [PubMed] [Google Scholar]
- 11.Stocchetti N, Chieregato A, De Marchi M, Croci M, Benti R, Grimoldi N. High cerebral perfusion pressure improves low values of local brain tissue O2 tension (PtiO2) in focal lesions. Acta Neurochir Suppl. 1998;71:162–5. doi: 10.1007/978-3-7091-6475-4_47. [DOI] [PubMed] [Google Scholar]
- 12.Doppenberg EM, Zauner A, Bullock R, Ward JD, Fatouros PP, Young HF. Correlations between brain tissue oxygen tension, carbon dioxide tension, pH, and cerebral blood flow–a better way of monitoring the severely injured brain? Surg Neurol. 1998;49:650–4. doi: 10.1016/s0090-3019(97)00355-8. [DOI] [PubMed] [Google Scholar]
- 13.Menzel M, Doppenberg E, Zauner A, Soukup J, Reinert M, Clausen T, et al. Cerebral oxygenation in patients after severe head injury - monitoring and effects of arterial hyperoxia on cerebral blood flow, metabolism and intracranial pressure. J Neurosurg Anesthesiol. 1999;11:240–51. doi: 10.1097/00008506-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Menzel M, Doppenberg E, Zauner A, Soukup J, Reinert M, Bullock R. Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J Neurosurg. 1999;91:1–10. doi: 10.3171/jns.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- 15.Dings J, Meixensberger J, Amschler J, Hamelbeck B, Roosen K. Brain tissue pO2 in relation to cerebral perfusion pressure, TCD findings and TCD-CO2-reactivity after severe head injury. Acta Neurochir (Wien) 1996;138:425–34. doi: 10.1007/BF01420305. [DOI] [PubMed] [Google Scholar]
- 16.Zauner A, Doppenberg EM, Woodward JJ, Choi SC, Young HF, Bullock R. Continuous monitoring of cerebral substrate delivery and clearance: initial experience in 24 patients with severe acute brain injuries. Neurosurgery. 1997;41:1082–93. doi: 10.1097/00006123-199711000-00011. [DOI] [PubMed] [Google Scholar]