Abstract
Aim
To test how the presence of peripheral arterial disease predicted mortality of middle-aged and elderly residents of Metlika county, a rural area in southeastern Slovenia.
Methods
In 1987, we interviewed and examined a representative cohort of 646 subjects aged 45-80 years at inclusion without overt coronary or cerebrovascular disease, for cardiovascular risk factors and measured the ankle-brachial pressure index (ABPI). Peripheral arterial disease was defined as ABPI<0.90. The subjects were followed up 15 years or until death. All-cause mortality and cardiovascular mortality were assessed and compared between subjects with and without peripheral arterial disease in a multivariate model.
Results
There were 580 subjects with normal ABPI and 66 subjects with peripheral arterial disease, among which 49 were asymptomatic and 17 had intermittent claudication. Because subjects with peripheral arterial disease were on average 10 years older than those without peripheral arterial disease, the mere presence of peripheral arterial disease was not an independent predictor of mortality. However, there was a significant interaction of peripheral arterial disease with age, with a more pronounced adverse prognostic effect of peripheral arterial disease in younger than in older age groups. For a 55-year-old subject with peripheral arterial disease, the hazard ratio of dying from any cause in the follow-up period was 2.44 (95% confidence interval [CI], 1.15-4.96) in comparison to an age-matched subject without peripheral arterial disease, but at 75 years of age, the hazard ratio decreased to only 0.71 (95% CI, 0.46-1.09). For cardiovascular mortality, the hazard ratio in the presence of peripheral arterial disease was 6.05 (95% CI, 1.87-16.27) at 55 years and 0.92 (95% CI, 0.54-1.52) at 75 years. Among patients with peripheral arterial disease, each decrement of ABPI at inclusion by 0.10 significantly increased the cardiovascular mortality after 15 years by 30% (P = 0.038).
Conclusion
Peripheral arterial disease, even asymptomatic, is an important predictor of adverse cardiovascular prognosis in relatively young patients. Reduced ABPI is a strong, independent predictor of cardiovascular mortality in all patients with peripheral arterial disease.
Clinical manifestation of peripheral arterial disease confirmed by reduced ankle-brachial pressure index (ABPI) ranges from the common asymptomatic disease, to the less prevalent intermittent claudication, to the relatively rare critical limb ischemia with rest pain, ulceration or gangrene (1). Even if peripheral arterial disease does not cause typical claudication, it reduces walking speed and walking endurance (2). Regardless of the clinical symptoms, reduced ABPI is a sign of hemodynamic disturbance in the arterial supply of the lower limbs, which is strongly associated with atherosclerosis in the coronary and carotid territories (3). Thus, peripheral arterial disease is associated with increased mortality due to myocardial infarction and ischemic stroke. Several studies described 2-3-fold greater mortality in patients with peripheral arterial disease in comparison with age-matched controls with normal ABPI; patients with peripheral arterial disease had a 5-year mortality of about 30% (4-9). Increasing severity of peripheral arterial disease, expressed as diminishing ABPI, progressively reduces survival (6-9).
Most previously studied cohorts were either patients referred for non-invasive vascular testing (6) or patients with known risk factors such as hypertension (8) or hyperlipidemia (4). There is still little data on the prognostic value of largely asymptomatic early-stage peripheral arterial disease in patients free of overt coronary or cerebrovascular disease in a community setting. We have focused on a representative sample of residents of Metlika county, a rural area in southeastern Slovenia to test how the presence of peripheral arterial disease, defined as ABPI<0.90, and the severity of peripheral arterial disease at inclusion, expressed as diminishing ABPI, affected all-cause mortality and cardiovascular mortality in a community setting.
Subjects and methods
Subjects
The study was carried out in Metlika county, a predominantly rural area in southeastern Slovenia. In 1987, the county had 8322 residents, of whom 3216 were aged between 45 and 80 years at the time of recruitment (10). From this age group, 708 were randomly selected from the list of appropriately aged residents by use of tables of random numbers.
Interviews and physical examinations were performed from January to March 1987. All subjects continued using the usual medical care provided by their general physicians. Survival of our subjects was followed up for 15 years until study completion in 2002.
Systolic blood pressure in the ankles and arms of supine subjects was measured at baseline by sphygmomanometer cuffs and Doppler flow detector (11,12). ABPI was calculated by dividing the average ankle arterial pressure (mean of posterior and anterior tibial artery) with the arm pressure. Values of ABPI of 0.91-1.50 were considered normal, whereas ABPI>1.50 indicatied rigid arterial walls and those subjects were excluded from further analysis. Subjects with ABPI<0.90 were considered to have peripheral arterial disease. Clinical symptoms of peripheral arterial disease were evaluated by the Rose questionnaire (13).
Clinically overt coronary or cerebrovascular disease was an exclusion criterion at baseline. Coronary heart disease was defined by a history of a myocardial infarction or angina pectoris according to the Rose questionaire (13), by evidence of an old myocardial infarction on the electrocardiogram on the basis of Minnesota codes (14), or by a history of coronary surgery or coronary angioplasty. Overt cerebrovascular disease, ie, stroke or transitory ischemic attack (TIA) was defined by an interviewer-administered questionnaire, or as a report of stroke or TIA by a physician.
Major cardiovascular risk factors were evaluated in the subjects. Hypertension was defined as a positive history of antihypertensive medication or 3 casual blood pressure measurements exceeding 140 mm Hg of systolic pressure or 90 mm Hg of diastolic pressure. Serum cholesterol was determined by the standard cholesterol oxidase-peroxidase aminophenazone phenol (CHOD-PAP) enzymatic method (15). Hypercholesterolemia was defined as serum cholesterol >5.2 mmol/L. Smokers were defined as those currently smoking one or more cigarettes per day. Diabetes mellitus was defined as fasting hyperglycemia >8 mmol/L or by being included in the registry of patients with diabetes mellitus in the area. Weight and height were measured indors, in clothing without shoes, and the body mass index (BMI) was calculated as the weight (kg) divided by the squared height (m). Overweight was defined as BMI>25.
Ascertainment of deaths
All deaths were ascertained through the Registry of the Republic of Slovenia, issued by the Institute of Health Protection of the Republic of Slovenia. A printed copy of this index, sorted by year of death, county of residence and name, was checked against the list of included patients without knowledge of their peripheral arterial disease status. The death certificate for each match of names was than reviewed to assure the accuracy of identification. Analysis of the causes of death included data from hospital autopsies when available. The underlying cause of death was coded according to the International Classification of Diseases, version 10 (ICD-10) (16), for each deceased subject. Cardiac causes of death were defined as diagnoses with ICD-10 codes I11-I25 (hypertensive and ishemic heart disease) and I44-I50 (cardiac conduction disturbances, arrhytmias, cardiac arrest, and heart failure). Cerebrovascular causes of death were defined as diagnoses with ICD codes I61-I64 (intracerebral bleeding and ischemic stroke). Cardiac and cerebrovascular diagnoses together comprised cardiovascular causes of death. Data analysis was completed in 2003.
Statistical analysis
Differences in the baseline characteristics of subjects with and those without peripheral arterial disease were evaluated by χ2 test for nominal variables and by two-tailed t-test for continuous variables. Overall survival of the the two groups was shown by Kaplan-Meier curves. Survival curves of the two groups (with and without peripheral arterial disease) were compared with the log-rank test, but since the two groups differed significantly in many variables affecting survival, these confounding variables were taken into account by using the Cox proportional hazards regression model (17). With the regression model, the effect of the presence of peripheral arterial disease and the effect of ABPI on all-cause mortality and cardiovascular mortality was determined, whereas all other variables were considered constant. In other words, the Cox model estimated the ratio of hazards for two hypothetical individuals with equal values of all confounding variables, one with and the other without peripheral arterial disease, and tested for the trend of linear lowering of ABPI in comparison with normal ABPI. In comparing the survival of our cohort with the general population of Slovenia, we used the transformation approach (18) based on the Slovene mortality tables (10). Statistical analysis was performed with R 2.1.0 statistical software, a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria). P<0.05 was considered statistically significant.
Results
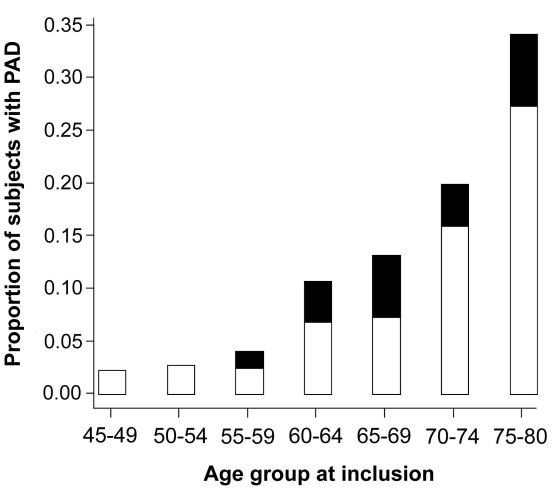
Of 708 randomly selected subjects, 685 (96.7%) participated in the study. Of those, 39 were excluded for mediocalcinosis and ABPI>1.50 (n = 14), clinically overt coronary disease (n = 17), and overt cerebrovascular disease (n = 8). The remaining 646 participants still represented 20% of the population aged 45-80 years in Metlika county. In the studied cohort, there were 66 patients with peripheral arterial disease, among which 49 were asymptomatic and 17 had claudication. The prevalence of peripheral arterial disease increased with age from <5% in 55-59-year age group to about 20% in 70–74-year age group and almost 35% in 75-80-year age group (Figure 1).
Figure 1.
Prevalence and symptoms of peripheral arterial disease (PAD) in the cohort stratified by age. Open bar – asymptomatic PAD, closed bar – claudication.
There was a predominance of women in our cohort, which was in accordance with the sex distribution in the population aged 45-80 years in Metlika county (Table 1). The subjects with peripheral arterial disease were on average 10 years older than their counterparts with normal ABPI, and had a significantly higher prevalence of smoking, arterial hypertension, hypercholesterolemia and diabetes (Table 2). About three-quarters of subjects in both groups were overweight, with a BMI>25 kg/m2. The mean (±standard deviation) body mass index was 28.1 ± 4.4 in subjects without peripheral arterial disease and 28.0 ± 5.3 in subjects with peripheral arterial disease (P = 0.92).
Table 1.
Baseline characteristics of participants with and without peripheral arterial disease (PAD)
| No. (%) of participants |
||||
|---|---|---|---|---|
| without PAD |
with PAD |
|||
| Characteristic | men (n = 211) | women (n = 369) | men (n = 39) | women (n = 27) |
| Age at inclusion (y)* | 58.0 ± 8.8 | 59.2 ± 9.2 | 66.4 ± 10.2 | 72.1 ± 5.2 |
| Overweight | 159 (75.4) | 276 (74.8) | 26 (66.7) | 22 (81.5) |
| Hypercholesterolemia | 36 (17.1) | 47 (12.7) | 16 (41.0) | 12 (44.4) |
| Hypertension | 83 (39.9) | 169 (45.8) | 18 (46.2) | 22 (81.5) |
| Diabetes mellitus | 24 (11.4) | 69 (18.7) | 10 (25.6) | 9 (33.3) |
| Smoking | 100 (47.4) | 14 (3.8) | 34 (87.2) | 2 (7.4) |
*Mean±standard deviation.
Table 2.
Comparison of risk factors in participants with normal ankle-brachial pressure index and without peripheral arterial disease (PAD)
| No. (%) of participants |
|||
|---|---|---|---|
| Risk factor | without PAD | with PAD | P |
| Overweight | 435 (75.0) | 48 (72.7) | 0.687* |
| Hypercholesterolemia | 83 (14.3) | 28 (42.4) | <0.001* |
| Hypertension | 252 (43.4) | 40 (60.6) | 0.008* |
| Diabetes | 93 (16.0) | 19 (28.8) | 0.010* |
| Smoking | 114 (19.7) | 36 (54.5) | <0.001* |
| Age at inclusion (y) | 58.8 ± 9.0 | 68.7 ± 8.0 | <0.001† |
*χ2 test.
†t test, mean±standard deviation.
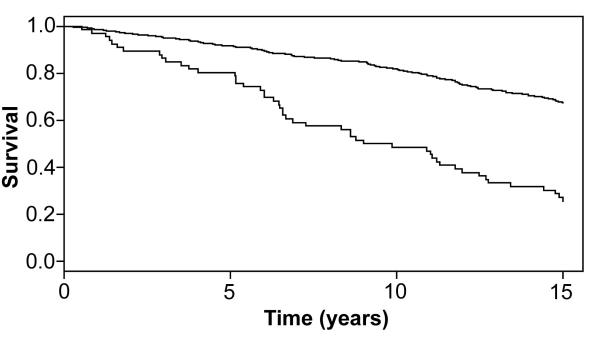
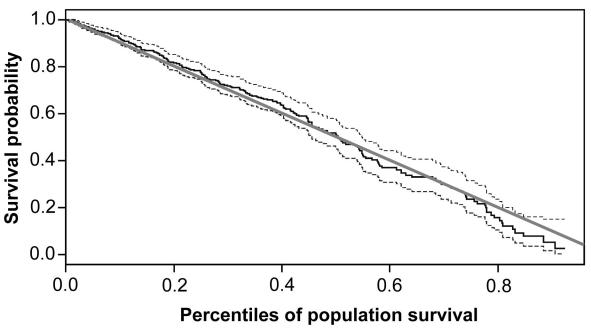
Mortality was much higher in subjects with peripheral arterial disease than in those without peripheral arterial disease (Figure 2). The difference by direct comparison would have been highly significant (P<0.001), but such a comparison was not justified because the subjects with peripheral arterial disease were on average 10 years older at inclusion and also differed from the control group in all other risk factors except BMI (Table 2). Overall, the survival of our cohort, ie, subjects with and without peripheral arterial disease, did not differ from survival of the whole Slovene population in the same calendar period (Figure 3).
Figure 2.
Kaplan-Meier estimates of the survival of the participants with (lower line) and those without peripheral arterial disease (upper line). The participants with peripheral arterial disease were on average 10 years older at inclusion than subjects with normal ankle-brachial pressure index (ABPI).
Figure 3.
Comparison by the transformation approach (16) of the observed survival in the Metlika cohort (subjects with and without peripheral arterial disease taken together). Gray line – survival of the Slovene population in the same calendar period; full line – the cohort; dashed lines – 95% confidence intervals for the observed survival in the cohort.
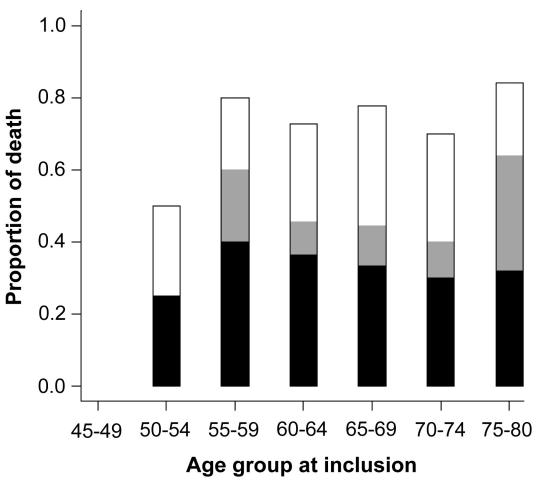
The causes of death in patients with peripheral arterial disease were predominantly cardiovascular with cardiac causes and cerebrovascular causes together accounting for 67% of deaths, as opposed to 45% of cardiovascular deaths among subjects without peripheral arterial disease (P = 0.007). Cardiac causes, mainly myocardial infarction and heart failure, accounted for 43% or deaths among patients with peripheral arterial disease and 31% in patients without peripheral arterial disease (P = 0.130). Cerebrovascular causes, mainly cerebrovascular stroke, accounted for 24% of deaths in patients with peripheral arterial disease and 14% in patients without peripheral arterial disease (P = 0.090). The causes of death among patients with peripheral arterial disease were stratified according to age at inclusion (Figure 4).
Figure 4.
Causes of death in patients with peripheral arterial disease over 15 years of follow up. None of the subjects in the 45-50-year age group at inclusion died, but a large proportion of the older participants died. Cardiac and cerebrovascular causes, together denoted as cardiovascular deaths, accounted for two-thirds of mortality among patients with peripheral arterial disease. Malignancies were the most common cause of non-cardiovascular death. Open bar – other; gray bar – cerebrovascular causes; closed bar – cardiac causes.
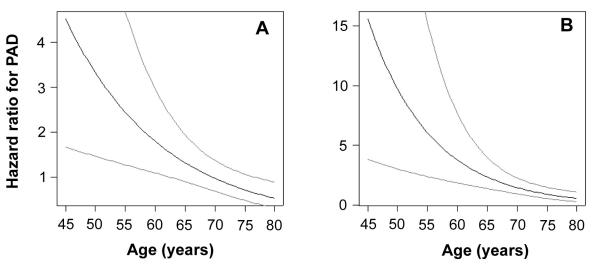
Since the subjects with peripheral arterial disease were significantly older than the subjects without peripheral arterial disease, we expected that a large proportion of the mortality difference (Figure 2) was due to age. Indeed, in the multivariate model that included age in years over 45 and the presence of peripheral arterial disease, the effect of peripheral arterial disease was no longer significant, with a regression coefficient of 0.235 ± 0.17 (P = 0.177). There was also no significant interaction of peripheral arterial disease and male sex, indicating that peripheral arterial disease affected both sexes equally in terms of cardiovascular prognosis.On the other hand, the prognostic effect of peripheral arterial disease changed with age, being more much important for survival of younger subjects. When the interaction of age and peripheral arterial disease was included in the model, the effect of peripheral arterial disease was significant even after including all other covariates in the model. From the interaction of age and peripheral arterial disease (Table 3), we calculated that the hazard ratio of dying in the next 15 years was 2.44 (95% confidence interval [CI], 1.15–4.96) for a 55-year-old subject with peripheral arterial disease in comparison with a subject without peripheral arterial disease; for a subject aged 75 years, the hazard ratio was reduced to 0.71 (95% CI, 0.46–1.09) (Figure 5A).
Table 3.
All-cause mortality analyzed by the Cox proportional hazards model that included the interaction of peripheral arterial disease (PAD) and age*
| Factor | β | HR | SE(β) | Z | P |
|---|---|---|---|---|---|
| PAD |
1.51 |
4.51 |
0.51 |
2.96 |
0.003 |
| Age |
0.12 |
1.12 |
0.01 |
13.19 |
<0.001 |
| Hypertension |
0.34 |
1.40 |
0.13 |
2.51 |
0.012 |
| Smoking |
0.85 |
2.33 |
0.15 |
5.73 |
<0.001 |
| Age and PAD interaction | -0.06 | 0.94 | 0.02 | -3.16 | 0.002 |
*Age was measured in number of years over 45. Abbreviations: HR – hazard ratio, SE – standard error, Z – normal deviate equal to β/SE(β).
Figure 5.
The risk of dying in the next 15 years in the presence of peripheral arterial disease (PAD) in comparison with risk of an age-matched and other risk factor-matched person with normal ankle-brachial pressure index, as a function of age at inclusion. The hazard ratios (full line) for all-cause mortality (A) and cardiovascular mortality (B) are shown with 95% confidence intervals (dashed lines).
Similarly, in analyzing cardiovascular mortality by the Cox proportional hazards model, the same variables had a significant adverse effect on survival (Table 4). Again, the negative prognostic effect of peripheral arterial disease diminished with advancing age. The hazard ratio for dying of cardiovascular causes in the observed period was 6.05 (95% CI, 1.87–16.27) for a 55-year-old subject with peripheral arterial disease in comparison with a subject without peripheral arterial disease, but the hazard ratio decreased to only 0.92 (95% CI, 0.54–1.52) at age of 75 years (Figure 5B).
Table 4.
Cardiovascular mortality analyzed by the Cox proportional hazards model that included the interaction of peripheral arterial disease (PAD) and age*
| Factor | β | HR | SE(β) | Z | P |
|---|---|---|---|---|---|
| PAD |
2.74 |
15.53 |
0.72 |
3.82 |
<0.001 |
| Age |
0.15 |
1.16 |
0.01 |
10.83 |
<0.001 |
| Hypertension |
0.42 |
1.53 |
0.19 |
2.18 |
0.029 |
| Smoking |
0.46 |
1.58 |
0.22 |
2.05 |
0.040 |
| Age and PAD interaction | -0.09 | 0.91 | 0.03 | -3.54 | 0.004 |
*Age was measured in number of years over 45. Abbreviations: HR – hazard ratio, SE – standard error, Z – normal deviate equal to β/SE(β).
When actual values of ABPI were analyzed, we found a significant association between the value of ABPI at inclusion and subsequent cardiovascular mortality among the peripheral arterial disease patients with a regression coefficient of -2.44 ± 1.18 (P = 0.038). In a multivariate model, even age was no longer a significant independent predictor of cardiovascular mortality when ABPI was taken into account, but each decrement of ABPI by 0.1 increased the hazard of dying from cardiovascular causes in the next 15 years by a factor of 1.3.
Discussion
We found that the incidence of peripheral arterial disease notably increased after the age of 55-60 years in a representative sample of residents of Metlika county. This finding was in accordance with the published epidemiological data for intermittent claudication (19). In all age groups, asymptomatic peripheral arterial disease was more prevalent than intermitent claudication, as reported previously in the PARTNERS study (1). In our cohort, there were no cases of critical limb ischemia at inclusion or as a cause of death, emphasizing that patients with peripheral arterial disease predominantly die from associated coronary and cerebrovascular atherothrombosis and not from limb gangrene (3).
The novel finding of our study was that the adverse prognostic effect of peripheral arterial disease strongly depended on subjects’ age at inclusion in the study. For a person found to have peripheral arterial disease at 55 years of age, even though peripheral arterial disease was largely still asymptomatic, the hazard of dying from any cause in the next 15 years was more than double that of a risk factor-matched person without peripheral arterial disease, and the hazard ratio for cardiovascular mortality was more than 6-times greater. With advancing age, the hazard ratios of peripheral arterial disease for all cause mortality and cardiovascular mortality diminished, and the risk of dying in the next 15 years was approximately equal – within 95% confidence intervals – among 75-year-old subjects with or without peripheral arterial disease. The most obvious reason for the diminishing adverse prognostic effect of peripheral arterial disease with advancing age is that the closer people get to the end of their predicted life span, the less any adverse prognostic sign matters. However, there may be additional reasons for the greater adverse prognostic effect of peripheral arterial disease at a younger age. The first possibility is that premature coronary and carotid atherosclerosis in conjunction with peripheral arterial disease carries a worse prognosis than coronary and carotid atherosclerosis that develops in older individuals irrespective of peripheral arterial disease. The second possibility is that younger people with peripheral arterial disease were less aggressively treated against atherothrombotic events than older ones. It is noteworthy that we found no differences between men and women regarding the prognostic impact of peripheral arterial disease.
The severity of peripheral arterial disease, measured as diminishing ABPI, was a strong, independent predictor of cardiovascular death in our study, which is in accordance with findings published previously (6-9,20,21). Each decrement of ABPI by 0.1 at inclusion increased the risk of subsequent cardiovascular death by 30%. It is well known that with increasing severity of peripheral arterial disease, the atherosclerotic burden is increased also in the coronary and in the carotid vascular bed (3,22,23).
To prevent premature disability and mortality from cardiovascular diseases, early screening is recommended together with assessment of total cardiovascular risk (24). Measurement of ABPI should become a component of cardiovascular risk assessment, especially as we now have effective pharmacological treatment for cardiovascular risk reduction in patients with peripheral arterial disease. Antiplatelet treatment (25), lipid-lowering statins (26), and antihypertensive therapy, especially ramipril (27), have been shown to reduce cardiovascular mortality by at least 20% each, so the combined pharmacological approach should reduce the risk of premature death by about 50%. Since its is no longer ethical to perform a placebo-controlled trial of the recommended pharmacological prevention of atherothrombosis in patents with peripheral arterial disease, a prospective follow-up of adequately treated patients with peripheral arterial disease in comparison to apparently healthy controls will be welcome.
In conclusion, the presence of peripheral arterial disease, even asymptomatic, is an important predictor of adverse cardiovascular prognosis in relatively young patients, and our study has confirmed that diminishing values of ABPI are strong independent predictors of cardiovascular mortality. Since the overall mortality of our cohort did not differ from mortality of the whole Slovenia in the corresponding calendar years (10), our results might be relevant for the whole country and possibly at the international level, because cardiovascular morbidity and mortality of Slovenia corresponds to that in Central Europe (10).
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Liu K, Guralnik JM, Mehta S, Criqui MH, Martin GJ, et al. The ankle brachial index independently predicts walking velocity and walking endurance in peripheral arterial disease. J Am Geriatr Soc. 1998;46:1355–62. doi: 10.1111/j.1532-5415.1998.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 3.Clement DL, Boccalon H, Dormandy J, Durand-Zaleski I, Fowkes G, Brown T. A clinical approach to the management of the patient with coronary (Co) and/or carotid (Ca) artery disease who presents with leg ischaemia (Lis). Int Angiol. 2000;19:97–125. [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 5.Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality. The Whitehall study. Circulation. 1990;82:1925–31. doi: 10.1161/01.cir.82.6.1925. [DOI] [PubMed] [Google Scholar]
- 6.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–28. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Tyrrell KS, Kuller LH. Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc. 1997;45:1472–8. doi: 10.1111/j.1532-5415.1997.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Shemanski L, Manolio T, Cushman M, Mittelmark M, Polak JF, et al. Ankle-arm index as predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 10.Statistical yearbook, Republic of Slovenia, 1982-2003. Ljubljana: Statistical Office of the Republic of Slovenia, 1982-2003. [Google Scholar]
- 11.Marinelli MR, Beach KW, Glass MJ, Primozich JF, Strandness DE., Jr Noninvasive testing vs clinical evaluation of arterial disease. A prospective study. JAMA. 1979;241:2031–4. [PubMed] [Google Scholar]
- 12.Hiatt WR, Marshall JA, Baxter J, Sandoval R, Hildebrandt W, Kahn LR, et al. Diagnostic methods for periph R - a language and environment for statistical computing, eral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]
- 13.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977;31:42–8. doi: 10.1136/jech.31.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackburn H, Keys A, Simonson E, Rautaharju P, Punsar S. The electrocardiogram in population studies. A classification system. Circulation. 1960;21:1160–75. doi: 10.1161/01.cir.21.6.1160. [DOI] [PubMed] [Google Scholar]
- 15.Röschlau P, Bernt E, Gruber W. Z Klin Chem Klin Biochem. 1974;12:403–7. Enzymatic determination of total cholesterol in serum [in German]. [PubMed] [Google Scholar]
- 16.International statistical classification of diseases and related health problems. 10th revision. Geneva: World Health Organisation; 1992. [Google Scholar]
- 17.Cox DR. Regression models and life tables. J Roy Statist Soc Ser B Methodological. 1972;34B:187–220. [Google Scholar]
- 18.Stare J, Henderson R, Pohar M. An individual measure of relative survival. J R Stat Soc Ser C Appl Stat. 2005;54:115–26. [Google Scholar]
- 19.Management of peripheral arterial disease (PAD). TransAtlantic Inter-Society Consensus (TASC). Int Angiol. 2000;19(1) Suppl 1:I–XXIV, 1-304. [PubMed] [Google Scholar]
- 20.Vogt MT, McKenna M, Anderson SJ, Wolfson SK, Kuller LH. The relationship between ankle-arm index and mortality in older med and women. J Am Geriatr Soc. 1993;41:523–30. doi: 10.1111/j.1532-5415.1993.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 21.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ. 1996;313:1440–4. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, et al. Associations of ankle brachial index with clinical coronary heart disease, stroke, and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–25. doi: 10.1016/s0021-9150(97)06089-9. [DOI] [PubMed] [Google Scholar]
- 23.Papamichael CM, Lekakis JP, Stamatelopoulos KS, Papaioannou TG, Alevizaki MK, Cimponeriu AT, et al. Ankle brachial index as a predictor of the extent of coronary atherosclerosis and cardiovascular events in patients with coronary artery disease. Am J Cardiol. 2000;86:615–8. doi: 10.1016/s0002-9149(00)01038-9. [DOI] [PubMed] [Google Scholar]
- 24.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2003;10:S1–S10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- 25.Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 27.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]