Abstract
Aim
To determine possible nerve conduction changes in the somatosensory pathway in children with brain ventricular dilatation and to estimate the relation between the ventricular size and somatosensory evoked potentials (SEP).
Methods
Twelve children with ventricular dilatation (frontal and occipital horn ratios >0.44) and 19 children without ventricular dilatation (control group), aged between 2 and 15 years, were included in the study. Somatosensory evoked responses to median nerve stimulation were recorded in both groups. Evoked potentials were recorded by silver/silver-chloride cup electrodes from Erb’s point in the supraclavicular fossa (wave N9), the cervical spine at the C7 vertebral prominence (wave N13), and the scalp above the contralateral sensory cortex at the point C3’ or C4’, 1 cm behind the C3 or C4 site in the standard 10-20 system (wave N19). Computed tomography scanning was performed to estimate ventricular dilatation.
Results
The conduction time of the central somatosensory pathway (N19-N13 interwave latency) was significantly longer in the children with ventricular dilatation than in the control group (P = 0.046). A statistically significant but weak correlation was found between the frontal and occipital horn ratio values and the N19-N13 interwave latencies in the subjects with enlarged ventricles (r = 0.579, P = 0.045)
Conclusion
Ventricular dilatation is associated with prolonged conduction of the central part of the somatosensory pathway in children. Early detection and treatment of hydrocephalus could be useful in preventing long-term consequences of high intraventricular pressure.
Testing of somatosensory evoked potentials (SEP) is a noninvasive, objective method for evaluating the central and peripheral nervous systems, and can also generate information about the maturation of the human afferent sensory system (1,2). This testing is particularly useful in infants and children because the clinical sensory neurological examination in these patients is often difficult and unreliable. In infants with hydrocephalus, both visual and auditory brainstem evoked potentials have already been studied (3,4). However, a limited number of studies have been done to assess SEPs in such infants (5).
Hydrocephalus is characterized by diffuse cortical and subcortical damage and therefore differs from other pathologies that result from discrete lesions. Neurophysiological consequences of hydrocephalus and their influence on the function of different neuroanatomic structures are not well known. In children, hydrocephalus compromises normal neurological development and can also cause permanent neurocognitive sequels (6). Therefore, special attention should be paid to the early detection and timely treatment of hydrocephalus.
The aim of our study was to determine the possible nerve conduction changes in the somatosensory pathway in children with ventricular dilatation and to estimate the relation between the ventricular size and SEPs latencies.
Patients and methods
Patients
All children between 2 and 15 years of age with radiologically diagnosed brain ventricular dilatation admitted to the Department of Neurosurgery, University Medical Center Ljubljana, between April 1999 and April 2001 were included in the study. Of 12 children with enlarged ventricles, 7 were girls and 5 were boys aged 24-188 months (median, 66 months). Computed tomography (CT) scanning of the brain was performed in all the children due to clinical signs of hydrocephalus. All children had pathological values of frontal and occipital horn ratios >0.44 and closed anterior and posterior fontanels (7). We decided to use the frontal and occipital horn ratio to evaluate ventricular size because it is a simple method and the ratio has an excellent correlation with the exact ventricular volume (7). This ratio also takes into account the size of the occipital horns of the lateral ventricles. The increase in ventricular size in children is often more pronounced in the posterior than the anterior brain regions, because the occipital horns of the lateral ventricles enlarge at a faster rate than other ventricular areas (8,9). Sometimes the occipital horns are the only portions of the lateral ventricles which enlarge (10). The exclusion criteria were malformations detected by prenatal morphological ultrasound or after delivery and any evidence of a degenerative neurological disease. In the studied group, aqueductal stenosis was detected in 2 children, hydrocephalus due to perinatal hemorrhage was diagnosed in 3 children, previous traumatic subarachnoid hemorrhage was present in 2 children, whereas in 5 children the etiology of hydrocephalus was unknown. All children presented with a headache and some of them also had occasional nausea and vomiting.
The control group consisted of 10 girls and 9 boys aged 2-15 years (median, 74 months; range 26-184 months) who were admitted to our hospital due to suspect head trauma. All of them had minimal or mild head trauma, with a Glasgow Coma Score of 14 or 15. Brain CT scan performed in all children did not reveal any intracranial lesions (contusion or hemorrhage), edema or ventricular dilatation. In 2 children, only a linear skull fracture was detected, but operative treatment was not required. None of them had any neurological deficits. Otherwise, the infants were full-term and delivered after an uncomplicated pregnancy. They all had an Apgar score of at least 8 at birth and 10 at five minutes, birth weight of at least 2500 g, and normal head circumference. None showed any evidence of a previous neurological disease, ventricular dilatation or hydrocephalus. All children in this group had a frontal and occipital horn ratio <0.44, which is the upper limit of normal range (7).
The study protocol was approved by the Ethics Committee and the children’s parents gave an informed consent to all the procedures.
Methods
Evoked potentials to median nerve stimulation were recorded by silver/silver chloride cup electrodes from Erb’s point in the supraclavicular fossa (wave N9), the cervical spine at the C7 vertebral prominence (wave N13), and the scalp above the contralateral sensory cortex at the point C3’or C4’, 1 cm behind the C3 or C4 site in the standard 10-20 system (wave N19) referenced to Fz of 12 children with enlarged ventricles and 19 children in the control group. The recordings were made with Medelec Sensor equipment (Medelec Ltd Old Woking, Surrey, UK) using an amplifier with a filter band-pass of 10-3000 Hz. An analysis time of 50 ms was used and responses to 256 stimuli were summated.
The study was performed in a quiet room at a constant temperature of 20-25°C, and all subjects were calm. The median nerve at the wrist was stimulated by an electric square-wave pulse of 0.3 ms duration, which was of sufficient intensity to produce a noticeable movement of the thumb (11). The positioning of the recording electrodes on the scalp was carefully selected, taking into account the differences in the size of the skull at different ages.
Cranial CT scanning was performed on a Somatom Plus 4 scanner (Siemens AG, Forchheim, Germany). The CT slices were parallel to the orbitomeatal plane, with axial slices from the foramen magnum to the vertex. For each procedure, 15 continuous slices were obtained, consisting of six 5-mm-thick scans in the posterior fossa and nine 10-mm-thick scans through the cerebrum to the vertex. Linear measurements were made by means of a standard ruler, graded in millimeters. Maximum bifrontal horns width of the lateral ventricles (A), maximum width of both occipital horns of the lateral ventricles (B) and maximal interparietal diameter (C) were measured and the frontal and occipital horn ratio was calculated as A+B/2C (7).
Statistical analysis
In all children, the difference between the latencies of N19 and N13 (N19-N13) and of N13 and N9 (N13-N9) were calculated and used for statistical analysis. In this way the sensory conduction time of different parts of the somatosensory pathway was estimated. These differences are independent of the length of the arm and of the subject’s body (12). Mann-Whitney test was used to compare the latency intervals in children with ventricular dilatation and control children. Spearman’s correlations were calculated between the differences in N19-N13 latency and the frontal and occipital horn ratio. All values were expressed as the median value and range, unless otherwise indicated. P<0.05 was considered statistically significant. The analysis was performed with Statistical Package for Social Sciences 9.0 (SPSS Inc. Chicago, IL, USA).
Results
Somatosensory evoked responses were recorded in all children included in the study. Similar SEP latencies of the N9 and N13 waves were detected in both control children and children with ventricular dilatation (Table 1). No statistically significant differences were found between the groups. The latency of wave N19 was prolonged in the children with enlarged ventricles, but this prolongation was not statistically significant (P = 0.265).
Table 1.
Somatosensory evoked responses after median nerve stimulation in children with or without ventricular dilatation
| Somatosensory evoked responses (median, range; ms) |
|||
|---|---|---|---|
| Latency | control group (n = 19) | children with ventricular dilatation (n = 12) | P* |
| N9 | 7.7 (5.7-11.2) | 7.8 (5.8-10.4) | 0.570 |
| N13 | 10.1 (7.1-13.8) | 10.1 (7.2-13.4) | 0.839 |
| N19 | 16.2 (13.2-19.8) | 16.8 (14.4-22.4) | 0.265 |
*Mann-Whitney test.
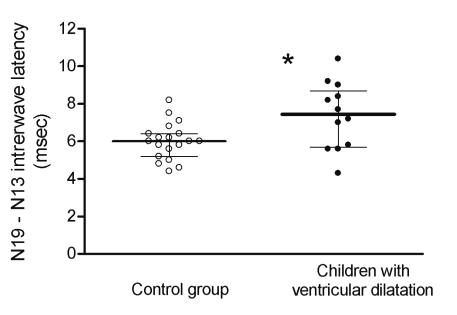
The conduction time of the central somatosensory pathway (N19-N13 interwave latency) was significantly longer in the group of children with ventricular dilatation compared with the control group (P = 0.046) (Figure 1). In the control children, the median value of N19-N13 latency was 6.0 ms (range, 4.4-8.2), compared with a median of 7.45 ms (range, 4.3-10.4) in children with ventricular dilatation.
Figure 1.
Differences between N19 and N13 somatosensory evoked responses (N19-N13 interwave latency, median ± quartiles) after median nerve stimulation in control children and children with ventricular dilatation. The difference between the groups was significant (P = 0.046).
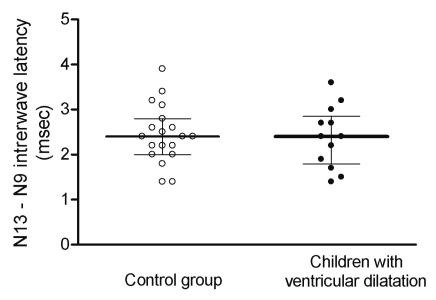
The median N13-N9 interwave latency was 2.4 ms (range, 1.4-3.6) in children with ventricular dilatation, and also 2.4 ms (range, 1.4-3.9) in the control group. No statistically significant difference was found between the groups (Figure 2).
Figure 2.
Differences between N13 and N9 somatosensory evoked responses (N19-N13 interwave latency, median ± quartiles) after median nerve stimulation in control children and children with ventricular dilatation.
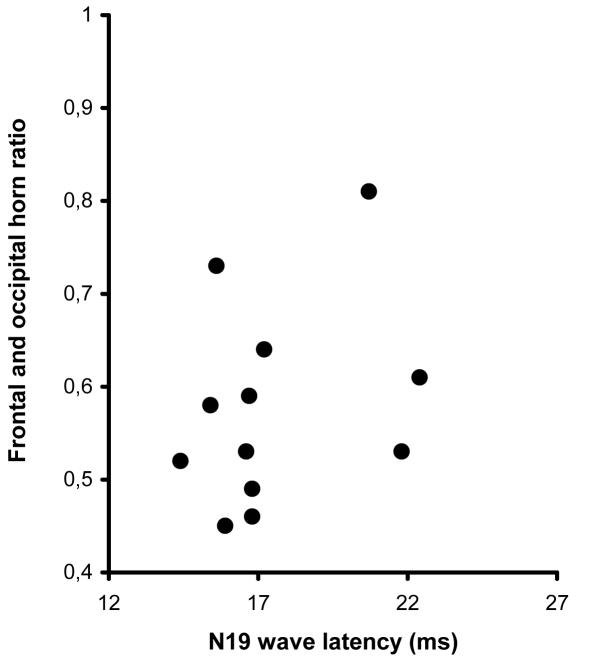
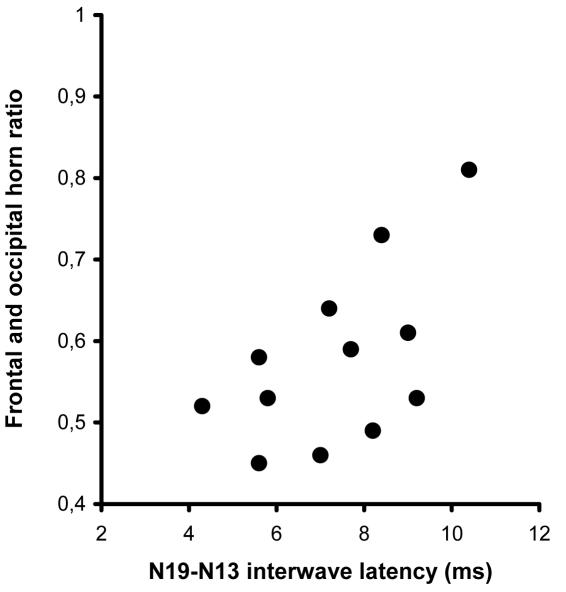
The median value of frontal and occipital horn ratio was 0.36 (range, 0.29-0.39) and 0.55 (range, 0.45-0.81) in the control group and group of children with ventricular dilatation, respectively. No correlation was found between the N19 wave latencies and ventricular size in the children with ventricular dilatation (Figure 3). However, a weak but statistically significant correlation was found between the frontal and occipital horn ratio values and the N19-N13 interwave latencies in the children with enlarged ventricles (r = 0.579, P = 0.045) (Figure 4).
Figure 3.
Relationship between N19 somatosensory evoked response after median nerve stimulation and the frontal and occipital horn ratio in children with ventricular dilatation. There was no statistically significant correlation (r = 0.277, P = 0.364).
Figure 4.
Relationship between N19 and N13 somatosensory evoked responses (N19-N13 interwave latency) after median nerve stimulation and the frontal and occipital horn ratio in children with ventricular dilatation (r = 0.579, P = 0.045).
Discussion
Our results showed that the short latency somatosensory evoked responses (N19-N13 intervals) were significantly prolonged in children with ventricular dilatation. A significant correlation was also found between the increase in the latency and the size of the ventricles.
However, the N19 wave latency did not discriminate significantly between the control children and children with ventricular dilatation, most probably because N19 depends on the subject’s height (12,13). Therefore, the N19-N13 interval was used for further analysis, and the results showed that the central conduction time was significantly longer in the group with higher frontal and occipital horn ratios. An abnormal interpeak latency between N13 and N19 waves suggests a conduction defect in the large-fiber sensory system above the lower medulla (14).
In an animal study, the auditory evoked response and somatosensory evoked response were recorded in 16 cats as a baseline, after which hydrocephalus was provoked (animals received a solution of kaolin) and the change in evoked potentials assessed (15). However, the measurements did not show any significant differences in the latencies. The study revealed a reduction in the amplitude of the negative wave rather than an increase in the latency following an artificially induced increase in the intracranial pressure. Pathophysiology of hydrocephalus induced by kaolin was discussed in another study also on cats (16).
The effect of hydrocephalus on SEPs was also estimated in newborns (5). In contrast to the previously described animal model, an increase in the latency was found in infants studied longitudinally during a period of progressive ventricular dilatation. Also a correlation was found between cerebrospinal fluid pressure and the delay in SEP latency.
In our study, older children with closed fontanels were studied. Intracranial pressure was not measured, but a correlation was found between the ventricular size and the N19-N13 latency. However, this correlation was not as high as we expected. In all 12 children with clinically and radiologically diagnosed hydrocephalus, ventriculoperitoneal shunt was inserted. During the surgery, increased pressure of the cerebrospinal fluid was found, but it was not precisely measured in all cases. A recent study has shown that intracranial pressure and ventricular size are not well correlated (17).
The SEP testing as a diagnostic method for ventricular dilatation is probably not very useful because of a considerable overlap between the results of control children and children with enlarged ventricles. Although the recorded values of the N19-N13 interwave latencies were significantly different in the two groups, the SEP results were not clinically very helpful in discerning whether a child belonged to the control group or group of children with enlarged ventricles.
Children with hydrocephalus also have a complex neuropsychological profile (6). The most notable neuropsychological deficits in these children are related to motor, visuoperceptual, and visuomotor functions. Common causes of deficits are corpus callosum abnormalities that result from stretching of the callosal fibers and other white matter tracts important for motor and sensory functions (18). In one study, children with hydrocephalus had a smaller callosal size relative to control children, and the smaller size correlated with more severe fine motor deficits (19). Visuoperceptual deficits are primarily due to enlarged ventricles that cause compression of parietal cortical areas (20). The combination of motor and visuoperceptual deficits makes the execution of visuomotor skills difficult for children with hydrocephalus.
In conclusion, our results showed that ventricular dilatation (as a consequence of hydrocephalus) was associated with prolonged conduction of the central part of the somatosensory pathway in children. However, this finding should be confirmed in a larger study sample and also in relation to intracranial pressure. Although ventricular size is not very well correlated with cerebrospinal fluid pressure, a weak and statistically significant correlation was found between ventricular dilatation and SEP central conduction time. The raised cerebrospinal fluid pressure probably causes compression of the transmission fibers, which may result in their atrophy (21). Stretching of different white matter tracts is also the result of ventricular enlargement. Early detection and treatment of hydrocephalus could be important in the prevention of long-term consequences of high intraventricular pressure. Since CT scan could not predict increased intracranial pressure and functional damage of the brain, SEP could be useful in detecting functional impairments caused by hydrocephalus. However, the value of SEP in routine clinical practice as a noninvasive method for assessing the need for treatment of hydrocephalus is questionable because of considerable overlap between the results of children with and those without ventricular dilatation.
References
- 1.Laureau E, Majnemer A, Rosenblatt B, Riley P. A longitudinal study of short latency somatosensory evoked responses in healthy newborns and infants. Electroencephalogr Clin Neurophysiol. 1988;71:100–8. doi: 10.1016/0168-5597(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 2.Sitzoglou C, Fotiou F. A study of the maturation of the somatosensory pathway by evoked potentials. Neuropediatrics. 1985;16:205–8. doi: 10.1055/s-2008-1059538. [DOI] [PubMed] [Google Scholar]
- 3.Ehle A, Sklar F. Visual evoked potentials in infants with hydrocephalus. Neurology. 1979;29:1541–4. doi: 10.1212/wnl.29.11.1541. [DOI] [PubMed] [Google Scholar]
- 4.Kraus N, Ozdamar O, Heydemann PT, Stein L, Reed NL. Auditory brain-stem responses in hydrocephalic patients. Electroencephalogr Clin Neurophysiol. 1984;59:310–7. doi: 10.1016/0168-5597(84)90048-0. [DOI] [PubMed] [Google Scholar]
- 5.De Vries LS, Pierrat V, Minami T, Smet M, Casaer P. The role of short latency somatosensory evoked responses in infants with rapidly progressive ventricular dilatation. Neuropediatrics. 1990;21:136–9. doi: 10.1055/s-2008-1071480. [DOI] [PubMed] [Google Scholar]
- 6.Erickson K, Baron IS, Fantie BD. Neuropsychological functioning in early hydrocephalus: review from a developmental perspective. Neuropsychol Dev Cogn C Child Neuropsychol. 2001;7:199–229. doi: 10.1076/chin.7.4.199.8737. [DOI] [PubMed] [Google Scholar]
- 7.O'Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: A linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245–9. doi: 10.1159/000028730. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JM, McCauley SR, Brandt ME, Bohan TP, Kramer LA, Francis DJ, et al. Regional brain tissue composition in children with hydrocephalus. Relationships with cognitive development. Arch Neurol. 1996;53:549–57. doi: 10.1001/archneur.1996.00550060093022. [DOI] [PubMed] [Google Scholar]
- 9.Brann BS, IV, Qualls C, Wells L, Papile L. Asymmetric growth of the lateral cerebral ventricle in infants with posthemorrhagic ventricular dilation. J Pediatr. 1991;118:108–12. doi: 10.1016/s0022-3476(05)81859-1. [DOI] [PubMed] [Google Scholar]
- 10.Reeder JD, Kaude JV, Setzer ES. The occipital horn of the lateral ventricles in premature infants. An ultrasonographic study. Eur J Radiol. 1983;3:148–50. [PubMed] [Google Scholar]
- 11.Burke D, Skuse NF, Lethlean AK. Cutaneous and muscle afferent components of the cerebral potential evoked by electrical stimulation of human peripheral nerves. Electroencephalogr Clin Neurophysiol. 1981;51:579–88. doi: 10.1016/0013-4694(81)90202-9. [DOI] [PubMed] [Google Scholar]
- 12.Hume AL, Cant BR. Conduction time in central somatosensory pathways in man. Electroencephalogr Clin Neurophysiol. 1978;45:361–75. doi: 10.1016/0013-4694(78)90188-8. [DOI] [PubMed] [Google Scholar]
- 13.Chu NS. Somatosensory evoked potentials: correlations with height. Electroencephalogr Clin Neurophysiol. 1986;65:169–76. doi: 10.1016/0168-5597(86)90051-1. [DOI] [PubMed] [Google Scholar]
- 14.Chiappa KH. Short-latency somatosensory evoked potentials: interpretation. In: Chiappa KH, editor. Evoked potentials in clinical medicine. 2nd edition. New York (NY): Raven Press; 1990. p. 371-437. [Google Scholar]
- 15.Sutton LN, Cho BK, Jaggi J, Joseph PM, Bruce DA. Effects of hydrocephalus and increased intracranial pressure on auditory and somatosensory evoked responses. Neurosurgery. 1986;18:756–61. doi: 10.1227/00006123-198606000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Mise B, Klarica M, Seiwerth S, Bulat M. Experimental hydrocephalus and hydromyelia: a new insight in mechanism of their development. Acta Neurochir (Wien) 1996;138:862–8. doi: 10.1007/BF01411265. [DOI] [PubMed] [Google Scholar]
- 17.Eide PK. The relationship between intracranial pressure and size of cerebral ventricles assessed by computed tomography. Acta Neurochir (Wien) 2003;145:171–9. doi: 10.1007/s00701-002-1062-y. [DOI] [PubMed] [Google Scholar]
- 18.Jinkins JR. Clinical manifestations of hydrocephalus caused by impingement of the corpus callosum on the falx: an MR study in 40 patients. AJNR Am J Neuroradiol. 1991;12:331–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher JM, Bohan TP, Brandt ME, Kramer LA, Brookshire BL, Thorstad K, et al. Morphometric evaluation of the hydrocephalic brain: relationships with cognitive development. Childs Nerv Syst. 1996;12:192–9. doi: 10.1007/BF00301250. [DOI] [PubMed] [Google Scholar]
- 20.Ito J, Saijo H, Araki A, Tanaka H, Tasaki T, Cho K, et al. Neuroradiological assessment of visuoperceptual disturbance in children with spina bifida and hydrocephalus. Dev Med Child Neurol. 1997;39:385–92. [PubMed] [Google Scholar]
- 21.Bothe HW, Lee SW, Samii M. Somatosensory evoked potentials and intracranial pressure during chronic dilatation of an artificial extraparenchymal space-occupying lesion in cats. Acta Neurochir (Wien) 1993;122:105–12. doi: 10.1007/BF01446995. [DOI] [PubMed] [Google Scholar]