Abstract
Aim
To present a summary of the lifestyle, genetic origin, diet, and disease in the population of Sami, indigenous people of northern Fennoscandia.
Method
A survey of the available scientific literature and preliminary results from our own study of the Swedish Sami population.
Results
The Sami probably have a heterogeneous genetic origin, with a major contribution of continental or Eastern European tribes and a smaller contribution from Asia. The traditional Sami diet, high in animal products, persists in Sami groups still involved with reindeer herding, but others have adopted a diet typical of Western cultures. Early reports indicated a lower prevalence of heart disease and most cancers, except stomach cancer. Recent studies have not found a lower risk of heart disease, but have consistently shown an overall reduced cancer risk. Sami have been reported to share some specific health-related genetic polymorphisms with other European populations, but none that would explain the observed differences in disease risk.
Conclusion
The genetic structure of the Sami population makes it suitable for studies of the genetic and environmental factors influencing the development of common diseases. The difference in incidence of heart disease between studies may reflect the ongoing transition from a traditional to a more Westernized lifestyle. The ability to compare population segments with different lifestyles, combined with the genetic structure of the population, creates unusual possibilities for studies of the genetic and environmental factors involved in the development of common disease.
Sami are the indigenous people of the northernmost parts of Sweden, Finland, Norway, and the Kola Peninsula of Russia. The Sami speak a language belonging to the Finno-Ugric branch of the Uralic language family with Finns, Karelians, and Estonians as their closest linguistic neighbors. The Sami languages can be further divided into 10 distinct extant languages. Sami are believed to have been present in the area from soon after glacial ice-sheet retreated. The Sami where initially hunters (mainly of reindeer and moose), but over time they domesticated the reindeer and became reindeer herders. Today, the Sami population is estimated to be less than 100 000 individuals (1). Some of the Sami are still reindeer herders and maintain a traditional lifestyle linked to the annual migration of the reindeer between summer and winter grazing areas, while the rest have other occupations. The Sami population is not known to have experienced any dramatic population changes and until recent, marriages between Sami and non-Sami families have been infrequent.
Origin of the Sami
The Sami are recognized as genetically distinct from other European populations but their origin is enigmatic (2). Modern humans first colonized the European continent during the early Upper Paleolithic period, about 45 000 years before present (3). During the last glaciation, which peaked about 18 000-20 000 years before present (4), hunter-gatherers still populated small refuges in warmer climates from which they expanded as the climate improved during the late upper Paleolithic or Mesolithic period. The most northerly parts of Europe were populated soon after the retreat of the glacial ice, about 10 000 years before present. Human artifacts from the west coast of Sweden have been dated at 10 200 years before present (5) and radiocarbon dating indicates that northern Fennoscandia was populated as early as 9000 years before present (6). The first immigrants are believed to have arrived to Finland about 7000 years before present and it has been suggested that during the same time period there was an influence from southeast Mongolian and Finno-Ugric people in the area around Lake Ladoga and Lake Onega (7). This area has been suggested as a possible place where the Sami population could have originated from and subsequently spread throughout Fennoscandia.
In the 1950s, the first studies of genetic markers in the Sami population were performed in order to investigate their origin (8-10). During the following decades, a large number of genetic markers, such as blood groups, enzyme polymorphisms, and serum proteins were investigated (11). Most of these markers showed differences between the Sami and the general Swedish population, and a higher similarity between the Sami and Finns. Some markers, such as GC (12), C3 and BF (13), ORM1 (14), TF (15), the (CGG)n alleles in the FMR1 gene (16) and HLA (17) showed some similarity in allele frequencies between Sami and Asian populations, although most indicated the Sami to be of European origin.
Since the mid 1990s, a number of studies of polymorphism in the maternally inherited mitochondrial genome and the paternally inherited Y-chromosome among Sami and between Sami and other populations have been performed. Studies of the mitochondrial genome have indicated that two mitochondrial haplogroups, V and U5b1b1, account for 80% of the Sami population (18,19). A study of the V haplogroup in 56 populations from different geographic regions found that it was relatively common in Europe (20). Estimates of the age of this haplogroup vary from 16 000 years before present in Western Europe to 8000 years before present in Eastern Europe (20), indicating that the lineage originated in Western Europe after the last glacial maxim and subsequently spread throughout Europe by human re-colonization from glacial refugia. Studies of the other major Sami mitochondrial haplogroup (U5b1b1) have indicated that it is either absent or only found at very low frequencies in many European populations (21), but found at the highest frequencies in the populations of Eastern and Northern Europe (21). However, the U5b1b haplogroup, which includes the U5b1b1 subclade, is present across Europe. The higher diversity of the U5b1b clade in Western and Southern Europe led to the proposal that while U5b1b1 has arisen in Eastern Europe, U5b1b is likely to be of southwestern European origin (21). Two minor mitochondrial haplogroups may be indicative of a genetic contribution from Asia (D5 and Z), but this influx of people appears to have been more recent (22). The Y chromosome tells a similar story with 3 haplogroups (N3, I, and R1a) accounting for 80% of the Y-chromosomal diversity in the Sami (21). The most common haplogroup in Sami (N3) is common in Eastern Europe and Northern Asia, and is almost absent in Western Europe. This polymorphism is at its highest frequency in the Yakuts, Buryats, and Finns (23) and has been dated to between 3140-6200 years before present (24). The other main Y-chromosome haplogroups in the Sami are all common in other European populations.
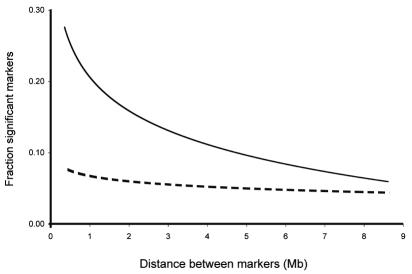
The Sami exhibit reduced genetic heterogeneity compared with other European populations. A few studies have suggested a population bottleneck followed by exponential growth, but the pattern of pairwise differences between mitochondrial DNA (mtDNA) sequences in Sami agree with the prediction of a limited population size that has been maintained over a long period of time (18,25). Microsatellite diversity is also lower in the Sami, compared with the general Swedish population (26). A characteristic of a small population with constant population size is the sensitivity to random genetic drift, whereby the frequency of an allele or alleles at linked sites can increase or decrease in a population by chance. The effect of random genetic drift can be studied by screening for increased levels of linkage disequilibrium, the non-random association of alleles at different sites (27). However, few human populations have a demographic history that could lead to high linkage disequilibrium generated by genetic drift. A number of studies based on single nucleotide polymorphisms (SNPs), and microsatellite markers have shown the high linkage disequilibrium among the Sami (26,28,29). The pattern has been shown to be quite similar across studies with up to 5 times higher linkage disequilibrium in the Sami compared with the general Swedish population (Figure 1). Linkage disequilibrium created by genetic drift in populations such as the Sami represents a valuable asset in mapping disease genes at the population level (27).
Figure 1.
Linkage Disequilibrium in the Sami population. Full line – Sami population, broken line – general Swedish population. Fraction of microsatellite marker pairs that are in linkage disequilibrium (P<0.05) in Sami compared to the general Swedish population. Data from Johansson (26).
In summary, genetic studies indicate that the Sami population has a heterogeneous origin and is the result of several migration events into Fennoscandia. A large portion of the genetic background is likely to be of Continental or Eastern European origin and reflects a migration of early hunter-gatherer tribes that arrived in the area soon after deglaciation. There is a small but detectable influence from Eastern Asian that may reflect people migrating into the region after it was already colonized by way of Central Asia and the Volga-Ural region. Of note is that while the Sami bear a genetic similarity to the Finns and some Eastern European populations, they are quite distinct from the Swedish and Norwegian populations, to which they are often compared for the purpose of studies of disease incidence. The Sami are also characterized by high linkage disequilibrium due to a long history of small population size, making them well suited to studies of genetic risk factors.
Dietary trends in Sami
Before the 20th century, the traditional Sami diet was composed almost exclusively of foods of animal origin (mainly reindeer) with the addition of fish and plant foods (eg, berries) when available (30). During the last century, the diet of many Sami has become progressively more westernized, with an increase in the intake of carbohydrates from plant foods and a decrease in meat protein, but it is still high in protein and low in carbohydrate compared to the diet of non-Sami in the same area (30,31). A summary of studies on the nutrient intake of the Sami over the past 25 years is shown in Table 1. This dietary change has been accelerated by the Chernobyl disaster in 1986. In order to minimize the intake of radiation via the lichen-reindeer-human chain, the Sami were advised to avoid eating too much reindeer meat, especially the organs that concentrated radioactive elements, such as liver (36).
Table 1.
Summary of studies on the nutrient intake in Sami compared with geographically matched control populations*†
| Country | Control population | Gender | No. of Sami individuals | Total energy | Carbohydrates | E% protein | E% fat | E% carbohydrate | Vitamin B12 | Niacin | Folate | Iron | Calcium | Zinc | Selenium | Sodium | Fiber |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sweden (30) | location matched controls | male | 25 | - | D | nr | nr | nr | I | - | D | - | D | I | I | D | |
| female | 23 | - | - | nr | nr | nr | - | - | - | I | - | I | I | ||||
| Sweden (31) | location matched lumberjacks | male | 31 | I | D | I | I | D | I | - | D | - | D | - | I | nr | nr |
| female | 25 | I | D | I | - | - | I | - | - | - | D | - | I | nr | nr | ||
| Finland (32) | Finnish reindeer herders | male | 56 | - | nr | I | D | - | nr | I | nr | I | D | nr | nr | nr | nr |
| Norway (33) | location matched controls | male | 45 | I | - | - | I | D | I | I | - | - | - | I | I | I | D |
| female | 30 | - | - | - | - | - | I | I | - | - | - | - | I | I | D | ||
| Norway (34) | adolescents vs costal controls | male | 16 | - | - | I | - | - | nr | nr | nr | - | - | nr | nr | nr | |
| female | 26 | - | - | I | - | D | nr | nr | - | - | - | nr | nr | nr | |||
| Sweden (35) | reindeer herding Sami vs location matched controls | male | 45 | - | I | - | - | D | I | - | - | I | D | - | I | I | |
| female | 32 | - | - | - | - | - | - | - | - | - | - | - | - |
*Abbreviations: minus – no difference between Sami and control population; I – increased in Sami compared to control population (P<0.05); D – decreased in Sami compared to control population (P<0.05); nr – not reported/not measured; E% – percent energy from that source.
†Only nutrients where differences have been found in two or more studies are included. For other common nutrients, no differences have been found, or differences were found in one study only.
Local groups of Sami differ in their diet depending on location and migration habits. The Sami living in costal regions have a higher intake of fish compared to those living inland, who tend to get most of their protein from reindeer meat supplemented by lake fish (30). Because the traditional Sami dietary pattern emphasizes animal products, their diet has been regarded as having too much fat and being deficient in fruit and cereal grains. Norwegian Sami were found to have a higher intake of fat, table sugar, and coffee compared to Norwegian controls (33) and both Swedish and Norwegian Sami have a low intake of fruit and vegetables compared to Swedish and Norwegian controls, with the exception of berries when they are available (30,31,33). Their consumption of dairy products is also low, mirrored by a low intake of calcium (Table 1). Sami also breastfeed their babies longer after birth – on average one year (37,38).
A diet high in meat and low in fruit and vegetables is contrary to most national dietary recommendations and may be expected to be deficient in certain nutrients, particularly those that mostly come from plant foods (eg, fiber and some water soluble vitamins). Several studies have found that in general, the Sami have an adequate intake of all micronutrients except for folic acid, fiber, and calcium and iron for women (31-33) (Table 1). However, differences in dietary intake do not appear to have led to any differences in serum lipid profiles (32,39), which could explain differences in cardiovascular disease risk.
The Sami involved in the traditional occupation of reindeer herding tend to have a traditional diet, characterized by a relatively higher intake of vitamins B12, D, niacin, iron, selenium, and sodium (35), compared with the Sami in other occupations, whose diet is similar to that of other Western people. The indigenous people of Yakutia in Siberia are another population making the transition from a hunter-gatherer society to a settled “modern” society (40). In this population, three types of diet were identified: market (modern), mixed foods, and subsistence (hunter-gatherer). The subsistence diet was associated with higher total cholesterol and low-density lipoprotein (LDL), while the market diet was associated with higher adiposity and obesity (40). While the traditional diet in this case may lead to increased biomarkers of cardiovascular disease risk, the change to a modern diet may also predispose this population to increased risk of other diseases associated with obesity. These health problems seen in other indigenous populations are strong evidence that the change of dietary habits is not a trivial issue.
Some studies have noted that the Sami have a higher alcohol intake, which may be a factor in the development of disease, as compared with the surrounding populations (35,41). However, aside from ethnicity, factors such as age, region, and marital status are also important and alcohol intake have not been found to be related specifically to occupation (reindeer herding) (41). Some studies have also suggested that the prevalence of alcohol abuse among the Sami is lower relative to other ethnic minorities around the world (37,42). Ethnicity is not a predictor of the use of tobacco products (smoking or chewing tobacco) in populations of northern Scandinavia and Finland (35,43).
Physical activity
Before motorized transport and snowmobiles became available, reindeer herding was a very labor-intensive occupation, often carried out in hostile environmental conditions and required a nomadic lifestyle. Due to this, many aspects of the traditional Sami diet (high protein, high fat) could be considered to not only arise out of availability but also from the need for a high energy diet to carry out their work. With the availability of motorized transport, the amount of physical work has been reduced and Sami reindeer herders now live in permanent settlements for most of the year and lead a lifestyle more similar to those with other occupations. Nevertheless, occupation is still an important factor for determining the physical activity, and reindeer herding Sami males have a higher self-reported physical activity compared with non-reindeer herding Sami and location-matched controls (35). In Norway, both Sami men and women reported a higher physical activity during work time than location-matched controls, and Sami women had lower amounts of leisure time than controls (44).
Lifestyle-related diseases
Several studies found that Sami differ from other populations with respect to some diseases for which diet and lifestyle are major risk factors (Table 2). This has lead to the idea that, in some cases, their traditional lifestyle may even protect against some lifestyle-related diseases. It should be noted when discussing these differences, that work-related accidents are still a major cause of death among the Sami and may be a confounder in some studies (1).
Table 2.
Mortality from cardiovascular disease among Sami in comparison to the location-matched control populations*
| Disease studied | Country | Population | Period | Control | Gender | No. of Sami | Person years† | Cardiovascular disease‡ | Stroke‡ | Subarachnoid hemorrhage‡ |
|---|---|---|---|---|---|---|---|---|---|---|
| Coronary heart disease (45) | Finland | male reindeer herders | 1981-1990 | non-Sami Lappland area | male | 5600 | 0.71-0.96 | |||
| Ischemic heart disease (46) | Finland | Sami regions vs non-Sami regions | 1961-1990 | non-Sami Lappland area | male | 743 | 8700 | 0.53-0.92 | ||
| female | 641 | 7500 | 0.32-0.86 | |||||||
| Cardiovascular disease (47) | Norway | Sami from Finnmark | 1974-1992 | non-Sami from the same area | male | 551 | 8201 | 0.25-0.73 | ||
| female | 552 | 8344 | 0.22-1.60 | |||||||
| Cardiovascular disease and stroke (48) | Sweden | Sami from Northern Sweden | 1961-2000 | non-Sami from the same area | male | 21 867 | 493 159 | 0.95-1.08 | 0.90-1.15 | 1.02-2.38 |
| female | 19 854 | 475 803 | 1.07-1.29 | 0.92-1.21 | 1.02-2.24 | |||||
| Cardiovascular disease and stroke (49) | Sweden | Sami from Northern Sweden | 1987-2002 | non-Sami from the same area | male | 8271 | 109 840 | 0.99-1.21 | 0.88-1.13 | 0.84-2.69 |
| female | 3344 | 110 112 | 1.05-1.38 | 0.87-1.14 | 0.69-2.65 |
*Information on the cause of death was derived from death certificates.
†The total number of at risk years for the study population. Calculated from the total number of years each person was included in the study.
‡95% confidence interval of standardized mortality rate in Sami population for disease and country indicated.
Cardiovascular diseases
Some early studies indicated that the Sami have a lower mortality from cardiovascular disease than other populations in the same area (45,46,50,51) (Table 2). The traditional Sami diet contains high amounts of animal protein and fat and low levels of fiber, which are considered to be risk factors for cardiovascular disease (52). More recent studies found that Sami, and in particular Sami women, have a similar incidence of cardiovascular disease as non-Sami from the same geographic region (48,49). Differences in the incidence of acute myocardial infarction, stroke, and subarachnoid hemorrhage among the Swedish Sami and demographically matched controls could not be explained by traditional socioeconomic factors (49). Instead, it was suggested that factors such as diet, behavior, or psychosocial factors are responsible. The differences between the earlier and more recent studies may be due to the fact that larger data sets have become available or that the more recent studies cover the period from 1990-2000 (Table 2). During this decade, there was a rapid shift toward the lifestyle factors that are associated with higher cardiovascular disease risk, such as the major dietary changes that were recommended after the Chernobyl disaster in 1986 (53). Another possible confounding factor is disease diagnosis. Monolingual Sami have a higher rate of dissatisfaction with primary health care services than other populations in the area, which may lead to an avoidance of public health services and problems with disease diagnosis (54).
Luoma et al (45) found that a decreased risk of coronary heart disease among Sami was correlated with increased serum concentrations of α-tocopherol, albumin, and selenium, and concluded that serum antioxidant status is an important risk factor for coronary heart disease. There is no clear evidence that an elevated serum antioxidant level is protective against coronary heart disease but it may reflect a high intake of reindeer meat. Reindeer meat has two times higher amount of α-tocopherol and four times higher amount of selenium than both pork and beef (α-tocopherol: 0.84mg/100g vs 0.40mg/100g; selenium 25μg/100g vs 6 μg/100g) (55), and also a fatty acid composition that is suggested to be favorable for cardiovascular protection (56). Björksten et al (57) found similar mean serum cholesterol levels in all populations from the Nordic countries, including the Sami, despite the large differences in the incidence of coronary heart disease. However, a Norwegian study showed that once major risk factors have been adjusted for, the difference in risk factors between ethnic groups was small and the Sami had a similar risk of cardiovascular disease and diabetes as the other studied populations (58). In the most recent study, the risk factors for coronary heart disease, including the plasma cholesterol profile, were found not to differ between the Swedish Sami and demographically matched controls (39). The difference between earlier findings of a decreased risk of cardiovascular disease in the Sami and the more recent studies showing no difference or even greater risk than in other populations may be in part due to the different cohorts used or the lag in the development of cardiovascular disease. A possible interpretation is that the traditional lifestyle has a protective effect, but that, due to increased urbanization and the transition toward a more westernized lifestyle and diet, the protective effect is disappearing.
Cancer
Despite the fact that the Sami have been exposed to high levels of radioactivity since the 1950s due to nuclear testing by the former Soviet Union and the 1986 Chernobyl disaster (via direct contact with radioactive fallout and indirectly via the lichen-reindeer-Sami food chain), they appear to have a lower risk of most types of cancer compared with the general Swedish and Finnish population (Table 3). These include leukemia and thyroid cancer, where exposure to radioactivity is a major risk factor (36). A study of the incidence of cancer over the period between 1961 and 1984 found that the Sami had a lower risk of cancer of the colon, respiratory organs, female breast, male genital organs, kidneys, as well as of malignant lymphomas than the general Swedish population (59). They showed an increased risk only of stomach cancer, which is often linked to diet. The suggested reason for the increased stomach cancer risk was the high intake of smoked reindeer meat and low intake of foods containing fiber. Reindeer meat is often preserved by smoking, a process that increases the number of carcinogens in meat and has been linked to cancers of the gut. Frying of reindeer meat also causes the formation of a higher amount of heterocyclic amines (carcinogens) than in other types of meat (62). On the other hand, reindeer meat also contains high amounts of selenium, which has been suggested to be protective against cancer in population-based studies (63). A recent study compared the Sami population to the general Swedish population and to a geographically matched reference population and similarly found that the Sami have a higher risk of stomach cancer and a decreased risk of prostate cancer and malignant lymphoma (60). Overall, the Sami have a 40% lower cancer risk than the general Swedish population. However, some factors, including the apparently higher rates of physical activity among reindeer herders compared with the general population, have not been accounted for in this study. Similar findings of a decreased risk of cancer were also seen in the Finnish Sami, especially for prostate and breast cancer. However, a group of Sami, the Skolt Sami, have 4 times higher risk of cancer than the Finnish population and 5-16 times higher risk than two Finnish Sami populations (61), although the incidence of cancer that could be related to nuclear fallout is low, in spite of up to 100 times higher exposure than in Finns living in the south of Finland (61). A recent study on Sami across northern Norway, Sweden, and Finland found a slightly lower incidence of thyroid cancer and leukemia than in the controls, as well as a decreased incidence of prostate, lung, breast, and colorectal cancer (64). While this study also concurred on the higher risk of stomach cancer in the Sami, the pattern of decreased risk of most cancers among the Sami is consistent across several studies (Table 2) and indicates that as yet unidentified genetic and lifestyle or diet factors, either alone or in combination, are important in determining the cancer risk.
Table 3.
Risk of cancer among Sami in comparison to the location-matched control populations
| Country | Population | Period | Method | Control | Sample size | Person years* | All cancers† | Breast† | Prostate† | Bladder† | Skin† | Basal cell carcinoma† | Stomach† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sweden (59) | reindeer herding Sami | 1961-1984 | cancer register | non-Sami from Northern Sweden | 2034 | 44 859 | 0.56-0.83 | 0.15-1.06 | 0.13-0.68 | 0.24-1.75 | 0.12-1.76 | 1.05-2.11 | |
| Sweden (60) | reindeer herding Sami | 1961-1997 | cancer register and death certificates | non-Sami from the same area | 2033 | 63 752 | 0.57-0.89 | 0.35-1.13 | 0.25-0.65 | 0.23-1.20 | 0.18-1.30 | 1.05-2.11 | |
| Finland (61) | Sami from Northern Finland | 1979-1998 | registered cases/deaths | non-Sami from the same area | 2100 | 37 415 | 0.53-0.76 | 0.14-0.73 | 0.08-0.58 | 0.03-0.99 | 0.00-0.63 | 0.03-0.30 | 0.61-2.0 |
*The total number of at risk years for the study population. Calculated from the total number of years each person was included in the study.
†95% confidence intervals of standardized incidence ratios in Sami population for disease and country indicated.
Other diseases
The incidence of obesity and adult-onset diabetes was increasing among the people of northern Norway, including the Sami (65). Little data exist on the prevalence of diabetes in the Sami, but a study of the Greenland Inuit found that diabetes is increasing and appears to be associated with a family history of diabetes, obesity, a sedentary lifestyle, and alcohol consumption, while a frequent intake of fresh fruit and seal meat were inversely associated with diabetes (66). Many other indigenous and minority populations have a higher prevalence of diabetes and obesity (39) and it is likely that these diseases will become more prevalent in the Sami unless successful public health measures tailored for the Sami are introduced. Sami are twice as likely to have asthma and other allergic conditions than the general Norwegian population (67) and the prevalence of allergic rhino conjunctivitis, atopic dermatitis, diagnosed asthma, and symptoms of asthma was found to be higher in Sami than in Norwegian school children (68).
Genetic risk factors in the Sami
Compared to the large number of studies on the genetic origin of the Sami, only a few studies have been performed to investigate health-related genes. Most have focused on alleles that are linked to easily measurable biomarkers, such as plasma lipid profile. The apoE protein plays a key role in plasma lipoprotein metabolism and in lipid transport within tissues (69). The APOE gene encodes three alleles – APOE*2, APOE*3, and APOE*4. APOE*2 is associated with low levels of total plasma cholesterol, LDL cholesterol, and apolipoprotein B. APOE*4 shows the opposite pattern and is associated with their increased levels (69). APOE*4 is a major risk factor for susceptibility to coronary heart disease and Alzheimer disease, particularly when combined with a western diet. In the Sami and several other indigenous populations, the frequency of APOE*4 is high (31% in the Sami), while in populations with a long history of agriculture, such as the Greeks, the frequency is low (5.2%) (69). APOE*4 is also relatively common (17.4%-20.8%) in Swedes, Danes, and Finns. The APOE genotype has been shown to influence plasma antioxidant status, with increased antioxidant levels for APOE*2 (70). The frequency of this allele among the Sami is 5% (71). The high frequency of the APOE*4 allele in the Sami contributes to their susceptibility to coronary heart disease, given exposure to the appropriate environmental factor. The lower coronary heart disease risk observed in earlier studies is likely to be the result of the lifestyle, so with the transition to a more Westernized lifestyle, the high frequency of the APOE*4 will contribute to even higher coronary heart disease risk than for other populations in the area.
The gene encoding apolipoprotein A4 has also been studied in the Sami. There are two common alleles at this locus, the frequencies of which differ between the Sami and the Finns (10.6% and 5.6%, respectively) (72). In the Sami, heterozygote for these two alleles had a higher high-density lipoprotein (HDL) than APOA4*1 homozygotes (72). They also found slightly higher levels of total cholesterol, LDL, HDL, and triglyceride levels in the Sami than in the Finns. However, more recent studies on the plasma lipid profile of the Sami have not found any differences from geographically matched controls (39). More detailed analyses of the genotype/phenotype combinations are needed to identify specific risk groups in the Sami population.
Lactose tolerance in the Sami varies between 40 and 75% for different subpopulations (73,74), which is much lower than in the general Swedish population (91%). The ability to digest lactose as an adult has been associated with two different mutations located upstream of the lactase gene, LCT (75). The haplotype with these two mutations was shown to have been under positive selection during the last 5000-10 000 years before present in the Europeans, consistent with its selective advantage in dairy farming cultures (76). The Sami have been involved in reindeer herding during the last 1000 years (73) and used reindeer milk, which is very low in lactose (2.4%) on a limited basis (30) until the 1920s. The high frequency of lactose tolerance in Sami, given their relatively short exposure to dairy products with a high lactase level, is either a reflection of admixture with the European farming population or strong genetic drift (73).
It has been hypothesized that a polymorphism in the gene encoding alanine glyoxylate aminotransferase (AGT) could be advantageous for individuals with a diet rich in meat and disadvantageous for those with a low meat diet. In humans, AGT catalyzes the detoxification of the intermediary metabolite glyoxylate to glycine. A reduced enzyme activity will result in glyoxylate being oxidized to oxalate. This can cause a poorly soluble calcium salt, which crystallizes out in the kidney and urinary tract (77). There is a clear relationship between organelle distribution of AGT and diet, suggesting AGT to be peroxisomal in herbivores, mitochondrial in carnivores, and both peroxisomal and mitochondrial in omnivores (78,79). This relocalization of AGT due to diet has been explained by the dietary precursor of glyoxylate in herbivores is thought to be glycolate, which is metabolized to glyoxylate in the peroxisomes, whereas in carnivores it is more likely to be hydroxyproline that is converted to glyoxylate in mitochondria (80). By comparing different species, a number of studies has found evidence for adaptive response to episodic changes in dietary selection pressure (81-83), and large changes in AGT distribution has occurred at least 11 times during the evolution of mammals (82). In humans, one polymorphism in the AGT, Pro11Leu, has been associated with miss-targeting of about 5% of the AGT that is routed toward the mitochondria (84), as well as a reduced catalytic activity (85). The frequency of the Pro11Leu polymorphism varies between human populations, with the highest frequency in the Sami (27.9%) and the lowest in the Chinese (2.3%), who have a more mixed ancestral diet (86). The high frequency in the Sami has been suggested to result from a significant advantage for people with a diet containing a high proportion of meat, as they could detoxify glyoxylate derived from hydroxyproline with greater efficiency.
The role of genetic adaptations to different diets in the development of disease is currently unknown. However, it is clear that genes do affect both diet and health. In laboratory experiments with mice, there are both strong genetic and environmental bases for macronutrient selection (87). Also, high heritability for eating behavior was seen in humans (88). Such findings indicate a link between genetics and ancestral lifestyle, including diet. While relatively few of these links are known, the implications for health due to the dietary change that has occurred over the last half-century is clearer in the light of genetic adaptation. Indigenous (homogeneous) populations, such as the Sami, who have specific genetic adaptations compared to more mixed populations, may cope less well with this rapid change in diet and lifestyle. Current knowledge on the nutrient intake of Sami suggests that they are comparable with other geographically matched populations, and that lifestyle (eg, physical activity) as well as genetic differences may be responsible for the difference in the risk of some cancers.
Potential for population-based epidemiology
Studies of the Sami provide unusual opportunities to understand the causes of common diseases. Comparisons between different population strata can, in principle, be used to disentangle the effect of genetic and environmental factors. At least three population groups can be distinguished: Sami reindeer herders, Sami with other occupations, and non-Sami living in the same geographic region (Figure 2). The effect of environmental factors can be addressed by comparison between groups with the same genetic background, such as diet (eg, meat intake in reindeer herding Sami compared with Sami with other occupations) or occupation (eg, reindeer herding vs other occupations). Also, the effect of genetic factors can be similarly addressed by comparison of non-reindeer herding Sami with those of other genetic backgrounds. Sami maintaining a traditional lifestyle are also likely to be exposed to less environmental variation, as compared to the multicultural population of southern and central Sweden. Less environmental variance will result in higher heritability and more power to identify genetic components influencing a complex phenotype. Preliminary analyses showed that the heritability (h2) for a number of quantitative traits in the Sami, calculated using SOLAR software (89) on the basis of 121 families and a total of 427 individuals, was similar to or higher than the values reported for other populations (Table 4). A very high heritability is seen for stature, which is interesting in the context of the large variation seen among the Sami for this trait.
Figure 2.
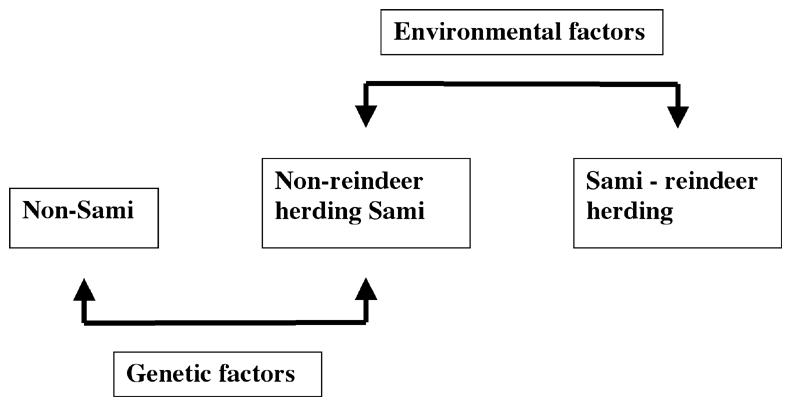
Comparing environmental and genetic factors. At least three population groups can be distinguished in our study: Sami reindeer herders, Sami with other occupations, and non-Sami living in the same geographic region. The effect of environmental factors can be addressed by comparison between groups with the same genetic background (eg, meat intake between reindeer herding Sami and Sami with other occupations) or occupation (eg, reindeer herding vs other occupations). The effect of genetic factors can be addressed by comparison of non-reindeer herding Sami with those with other genetic backgrounds.
Table 4.
Heritability for a number of traits in Sami*
| Trait | Heritability† |
|---|---|
| Fasting blood glucose | 0.34‡ |
| BMI | 0.44‡ |
| Body stature | 0.90‡ |
| Diastolic blood pressure | 0.26‡/0.30§ |
| Systolic blood pressure | 0.13‡/0.16§ |
| HDL | 0.43‡/0.46§ |
| LDL | 0.52‡/0.52§ |
| Serum total cholesterol | 0.44‡/0.49§ |
| Triglycerides | 0.37‡/0.41§ |
*Abbreviation: BMI – body mass index; HDL – high-density lipoprotein; LDL – low-density lipoprotein.
†Preliminary analyses of heritability were calculated for 121 families consisting of a total of 427 individuals of Sami origin.
‡Sex and age were used as covariates.
§Sex, age, and BMI were used as covariates.
The availability of genealogical data over a 200-300-year period and the high endogamy level in the Sami population permits an approach based on identity-by-descent (IBD) of chromosome segments between and within individuals, rather than identity-by-state (IBS). In Sweden, population-based genealogical information is available through digitized parish records, making it possible to construct population pedigrees going as far back as about 1700 AD. This allows for powerful studies of the effective population size, migration, and marriage patterns. Our study of northern Swedish populations is part of the EUROSPAN project and the five populations included in this project encompass much of the genetic diversity present in Europe and a wide range of environmental exposures. Contrasting different environmental conditions is a powerful strategy used in genetic epidemiology and we will employ cross-population mapping of data from these five European populations to compare genetic (quantitative trait loci and known genetic risk factors) and environmental risk factors between populations differing widely in both origin and environmental conditions.
Acknowledgments
We would like to thank all participants from the southern Sami villages and from Karesuando. This study is part of the EUROSPAN project number 018947: “European Special Populations Research Network: Quantifying and Harnessing Genetic Variation for Gene Discovery.” The study was also supported by grants from the Swedish Foundation for Strategic Research (SSF), the National Swedish Research Council (VR-M, N) and Mĺl 1 Sápmi (dnr. SN 1.42-11/00).
References
- 1.Hassler S, Sjolander P, Ericsson AJ. Construction of a database on health and living conditions of the Swedish Sami population. In: Lantto P, Skold P, editors. People and settlements in north – etnicity, identity and boundaries in the light of history [in Swedish]. Centre for Sami Research, Umea: Umea University; 2004. p. 107-124. [Google Scholar]
- 2.Cavalli-Sforza LL, Piazza A. Human genomic diversity in Europe: a summary of recent research and prospects for the future. Eur J Hum Genet. 1993;1:3–18. doi: 10.1159/000472383. [DOI] [PubMed] [Google Scholar]
- 3.Cavalli-Sforza LL, Menozzi P, Piazza A. The history and geography of human genes. Princeton: Princeton University Press; 1994. [Google Scholar]
- 4.Mellars P. The upper Paleolithic revolution. In: Cunliffe B, editor. The Oxford illustrated prehistory of Europe. Oxford: Oxford University Press; 1994. p. 42-78. [Google Scholar]
- 5.Nordqvist B. Coastal adaptations in the Mesolitic. A study of costal sites with organic remains from the Boreal and Atlantic periods in Western Sweden. GOTARC. Series B 13. Goteborg: Department of Archeology, Gothenburg University; 2000. [Google Scholar]
- 6.Bergman I. Deglaciation and colonization: pioneer settlements in northern Fennoscandia. Journal of World Prehistory. 2004;18:155–77. [Google Scholar]
- 7.Eriksson AW. Anthropology and health of Lapps. Coll Antropol. 1988;12:197–235. [Google Scholar]
- 8.Allison AC, Broman B, Mourant AE, Ryttinger L. The blood groups of Swedish Lapps. J R Anthropol Inst. 1956;86:87–94. [Google Scholar]
- 9.Allison AC, Hartmann O, Brendemoen OJ, Mourant AE. The blood groups of the Norwegian Lapps. Acta Pathol Microbiol Scand. 1952;31:334–8. doi: 10.1111/j.1699-0463.1952.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 10.Beckman L, Broman B, Jonsson B, Mellbin T. Further data on the blood groups of the Swedish Lapps. Acta Genet Stat Med. 1959;9:1–8. [PubMed] [Google Scholar]
- 11.Beckman L. Sami a genetically unique indigenous population [in Swedish]. Umel: Solfjädern Offset AB; 1996. [Google Scholar]
- 12.Beckman L, Beckman G, Nylander PO. Gc subtypes in Finns, Swedes and Swedish Lapps. Hum Hered. 1988;38:18–21. doi: 10.1159/000153748. [DOI] [PubMed] [Google Scholar]
- 13.Beckman G, Beckman L, Sikstrom C. Serum complement (C3, BF, C4) types in Swedish Saamis. Hum Hered. 1993;43:362–5. doi: 10.1159/000154160. [DOI] [PubMed] [Google Scholar]
- 14.Fan C, Sikstrom C, Beckman G, Beckman L. Orosomucoid polymorphism in Finns, Swedes and Swedish Saamis. Hum Hered. 1993;43:272–5. doi: 10.1159/000154144. [DOI] [PubMed] [Google Scholar]
- 15.Sikstrom C, Nylander PO. Transferrin C subtypes and ethnic heterogeneity in Sweden. Hum Hered. 1990;40:335–9. doi: 10.1159/000153957. [DOI] [PubMed] [Google Scholar]
- 16.Larsen LA, Vuust J, Nystad M, Evseeva I, Van Ghelue M, Tranebjaerg L. Analysis of FMR1 (CGG)(n) alleles and DXS548-FRAXAC1 haplotypes in three European circumpolar populations: traces of genetic relationship with Asia. Eur J Hum Genet. 2001;9:724–7. doi: 10.1038/sj.ejhg.5200697. [DOI] [PubMed] [Google Scholar]
- 17.Evseeva I, Spurkland A, Thorsby E, Smerdel A, Tranebjaerg L, Boldyreva M, et al. HLA profile of three ethnic groups living in the North-Western region of Russia. Tissue Antigens. 2002;59:38–43. doi: 10.1034/j.1399-0039.2002.590107.x. [DOI] [PubMed] [Google Scholar]
- 18.Sajantila A, Lahermo P, Anttinen T, Lukka M, Sistonen P, Savontaus ML, et al. Genes and languages in Europe: an analysis of mitochondrial lineages. Genome Res. 1995;5:42–52. doi: 10.1101/gr.5.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Delghandi M, Utsi E, Krauss S. Saami mitochondrial DNA reveals deep maternal lineage clusters. Hum Hered. 1998;48:108–14. doi: 10.1159/000022789. [DOI] [PubMed] [Google Scholar]
- 20.Torroni A, Bandelt HJ, Macaulay V, Richards M, Cruciani F, Rengo C, et al. A signal, from human mtDNA, of postglacial recolonization in Europe. Am J Hum Genet. 2001;69:844–52. doi: 10.1086/323485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tambets K, Rootsi S, Kivisild T, Help H, Serk P, Loogvali EL, et al. The western and eastern roots of the Saami – the story of genetic "outliers" told by mitochondrial DNA and Y chromosomes. Am J Hum Genet. 2004;74:661–82. doi: 10.1086/383203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingman M, Gyllensten U. A recent genetic link between Sami and the Volga-Urallic region of Russia. Eur J Hum Genet. doi: 10.1038/sj.ejhg.5201712. Forthcoming 2006. [DOI] [PubMed] [Google Scholar]
- 23.Zerjal T, Dashnyam B, Pandya A, Kayser M, Roewer L, Santos FR, et al. Genetic relationships of Asians and Northern Europeans, revealed by Y-chromosomal DNA analysis. Am J Hum Genet. 1997;60:1174–83. [PMC free article] [PubMed] [Google Scholar]
- 24.Lahermo P, Savontaus ML, Sistonen P, Beres J, de Knijff P, Aula P, et al. Y chromosomal polymorphisms reveal founding lineages in the Finns and the Saami. Eur J Hum Genet. 1999;7:447–58. doi: 10.1038/sj.ejhg.5200316. [DOI] [PubMed] [Google Scholar]
- 25.Lahermo P, Sajantila A, Sistonen P, Lukka M, Aula P, Peltonen L. The genetic relationship between the Finns and the Finnish Saami (Lapps): analysis of nuclear DNA and mtDNA. Am J Hum Genet. 1996;58:1309–22. [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson A, Vavruch-Nilsson V, Edin-Liljegren A, Sjolander P, Gyllensten U. Linkage disequilibrium between microsatellite markers in the Swedish Sami relative to a worldwide selection of populations. Hum Genet. 2005;116:105–13. doi: 10.1007/s00439-004-1213-8. [DOI] [PubMed] [Google Scholar]
- 27.Terwilliger JD, Zollner S, Laan M, Paabo S. Mapping genes through the use of linkage disequilibrium generated by genetic drift: 'drift mapping' in small populations with no demographic expansion. Hum Hered. 1998;48:138–54. doi: 10.1159/000022794. [DOI] [PubMed] [Google Scholar]
- 28.Kaessmann H, Zollner S, Gustafsson AC, Wiebe V, Laan M, Lundeberg J, et al. Extensive linkage disequilibrium in small human populations in Eurasia. Am J Hum Genet. 2002;70:673–85. doi: 10.1086/339258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laan M, Paabo S. Demographic history and linkage disequilibrium in human populations. Nat Genet. 1997;17:435–8. doi: 10.1038/ng1297-435. [DOI] [PubMed] [Google Scholar]
- 30.Haglin L. Nutrient intake among Saami people today compared with an old, traditional Saami diet. Arctic Med Res. 1991;(Suppl):741–6. [PubMed] [Google Scholar]
- 31.Haglin L. The nutrient density of present-day and traditional diets and their health aspects: the Sami- and lumberjack families living in rural areas of Northern Sweden. Int J Circumpolar Health. 1999;58:30–43. [PubMed] [Google Scholar]
- 32.Laitinen J, Nayha S, Sikkila K, Hassi J. Diet and cardiovascular risk factors among Lapp and Finnish reindeer herders. Nutr Res. 1996;16:1083–93. [Google Scholar]
- 33.Nilsen H, Utsi E, Bonaa KH. Dietary and nutrient intake of a Sami population living in traditional reindeer herding areas in north Norway: comparisons with a group of Norwegians. Int J Circumpolar Health. 1999;58:120–33. [PubMed] [Google Scholar]
- 34.Brox J, Bjornstad E, Olaussen K. Hemoglobin, iron, nutrition and life-style among adolescents in a coastal and an inland community in northern Norway. Int J Circumpolar Health. 2003;62:130–41. doi: 10.3402/ijch.v62i2.17547. [DOI] [PubMed] [Google Scholar]
- 35.Ross AB, Johansson A, Vavruch-Nilsson V, Hassler S, Sjolander P, Edin-Liljegren A, et al. Nutrient intake of non-reindeer herding Sami in Sweden does not differ from other Swedish populations. Br J Nutr. Forthcoming 2006. [Google Scholar]
- 36.Mehli H, Skuterud L, Mosdol A, Tonnessen A. The impact of Chernobyl fallout on the Southern Saami reindeer herders of Norway in 1996. Health Phys. 2000;79:682–90. doi: 10.1097/00004032-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Larsson E, Ogaard B, Lindsten R. Rearing of Swedish, Norwegian, and Norwegian Sami children. Scand J Dent Res. 1993;101:382–5. doi: 10.1111/j.1600-0722.1993.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 38.Larsson E. Orthodontic aspects on feeding of young children. 1. A comparison between Swedish and Norwegian-Sami children. Swed Dent J. 1998;22:117–21. [PubMed] [Google Scholar]
- 39.Edin-Liljegren A, Hassler S, Sjolander P, Daerga L. Risk factors for cardiovascular diseases among Swedish Sami – a controlled cohort study. Int J Circumpolar Health. 2004;63(Suppl 2):292–7. doi: 10.3402/ijch.v63i0.17922. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen MV, Snodgrass JJ, Leonard WR, Tarskaia A, Ivanov KI, Krivoshapkin VG, et al. Health consequences of postsocialist transition: dietary and lifestyle determinants of plasma lipids in Yakutia. Am J Hum Biol. 2005;17:576–92. doi: 10.1002/ajhb.20426. [DOI] [PubMed] [Google Scholar]
- 41.Poikolainen K, Nayha S, Hassi J. Alcohol consumption among male reindeer herders of Lappish and Finnish origin. Soc Sci Med. 1992;35:735–8. doi: 10.1016/0277-9536(92)90011-e. [DOI] [PubMed] [Google Scholar]
- 42.Larsen S, Nergard R. Cultural background and drinking patterns in problem drinkers in northern Norway. Br J Addict. 1990;85:1469–73. doi: 10.1111/j.1360-0443.1990.tb01630.x. [DOI] [PubMed] [Google Scholar]
- 43.Spein AR, Sexton H, Kvernmo S. Predictors of smoking behaviour among indigenous sami adolescents and non-indigenous peers in north Norway. Scand J Public Health. 2004;32:118–29. doi: 10.1177/140349480403200206. [DOI] [PubMed] [Google Scholar]
- 44.Hermansen R, Njolstad I, Fonnebo V. Physical activity according to ethnic origin in Finnmark county, Norway. The Finnmark Study. Int J Circumpolar Health. 2002;61:189–200. doi: 10.3402/ijch.v61i3.17452. [DOI] [PubMed] [Google Scholar]
- 45.Luoma PV, Nayha S, Sikkila K, Hassi J. High serum alpha-tocopherol, albumin, selenium and cholesterol, and low mortality from coronary heart disease in northern Finland. J Intern Med. 1995;237:49–54. doi: 10.1111/j.1365-2796.1995.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 46.Nayha S. Low mortality from ischaemic heart disease in the Sami district of Finland. Soc Sci Med. 1997;44:123–31. [Google Scholar]
- 47.Tverdal A. Cohort study of ethnic group and cardiovascular and total mortality over 15 years. J Clin Epidemiol. 1997;50:719–23. doi: 10.1016/s0895-4356(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 48.Hassler S, Johansson R, Sjolander P, Gronberg H, Damber L. Causes of death in the Sami population of Sweden, 1961-2000. Int J Epidemiol. 2005;34:623–9. doi: 10.1093/ije/dyi027. [DOI] [PubMed] [Google Scholar]
- 49.Sjolander P, Hassler S, Janlert U. Incidence ratios of stroke and acute myocardial infarction in the Swedish Sami population – effects of socioeconomic risk factors. Eur J Cardiov Prev R. Forthcoming 2006. [Google Scholar]
- 50.Thelle DS, Forde OH. The cardiovascular study in Finnmark county: coronary risk factors and the occurrence of myocardial infarction in first degree relatives and in subjects of different ethnic origin. Am J Epidemiol. 1979;110:708–15. doi: 10.1093/oxfordjournals.aje.a112851. [DOI] [PubMed] [Google Scholar]
- 51.Luoma P, Nayha S, Hassi J. High serum concentrations of vitamin E and cholesterol and low mortality from ischaemic heart disease in northern Finland. Arctic Med Res. 1995;54(Suppl 2):26–8. [PubMed] [Google Scholar]
- 52.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–21. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 53.Strand P, Selnaes TD, Boe E, Harbitz O, Andersson-Sorlie A. Chernobyl fallout: internal doses to the Norwegian population and the effect of dietary advice. Health Phys. 1992;63:385–92. doi: 10.1097/00004032-199210000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Nystad T, Melhus M, Lund E. The monolingual Sami population is less satisfied with the primary health care. Tidsskr Nor Laegeforen. 2006;126:738–40. [in Norwegian]. [PubMed] [Google Scholar]
- 55.Livsmedelsverket. Swedish Food Database [in Swedish]. Available from: http://www.slv.se; 2002; version 02.2. Accessed: January 22, 2004.
- 56.Sampels S, Pickova J, Wiklund E. Fatty acids, antioxidants and oxidation stability of processed reindeer meat. Meat Sci. 2004;67:523–32. doi: 10.1016/j.meatsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Bjorksten F, Aromaa A, Eriksson AW, Maatela J, Kirjarinta M, Fellman J, et al. Serum cholesterol and triglyceride concentrations of Finns and Finnish Lapps. II. Interpopulation comparisons and occurrence of hyperlipidemia. Acta Med Scand. 1975;198:23–33. doi: 10.1111/j.0954-6820.1975.tb19501.x. [DOI] [PubMed] [Google Scholar]
- 58.Njolstad I, Arnesen E, Lund-Larsen PG. Cardiovascular diseases and diabetes mellitus in different ethnic groups: the Finnmark study. Epidemiology. 1998;9:550–6. [PubMed] [Google Scholar]
- 59.Wiklund K, Holm LE, Eklund G. Cancer risks in Swedish Lapps who breed reindeer. Am J Epidemiol. 1990;132:1078–82. doi: 10.1093/oxfordjournals.aje.a115750. [DOI] [PubMed] [Google Scholar]
- 60.Hassle S, Sjolander P, Barnekow-Bergkvist M, Kadesjo A. Cancer risk in the reindeer breeding Saami population of Sweden, 1961-1997. Eur J Epidemiol. 2001;17:969–76. doi: 10.1023/a:1016232606232. [DOI] [PubMed] [Google Scholar]
- 61.Soininen L, Jarvinen S, Pukkala E. Cancer incidence among Sami in Northern Finland, 1979-1998. Int J Cancer. 2002;100:342–6. doi: 10.1002/ijc.10486. [DOI] [PubMed] [Google Scholar]
- 62.Skog K, Augustsson K, Steineck G, Stenberg M, Jagerstad M. Polar and non-polar heterocyclic amines in cooked fish and meat products and their corresponding pan residues. Food Chem Toxicol. 1997;35:555–65. doi: 10.1016/s0278-6915(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 63.Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–42. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 64.Auvinen A, Haldorsen T, Hall P, Hassler S, Pukkala E, Sjolander P, et al. Cancer incidence among reindeer herding Saami in the Nordic Countries. Vilhelmina (Sweden): Southern Lapland Research Department; 2002. [Google Scholar]
- 65.Midthjell K, Kruger O, Holmen J, Tverdal A, Claudi T, Bjorndal A, et al. Rapid changes in the prevalence of obesity and known diabetes in an adult Norwegian population. The Nord-Trondelag Health Surveys: 1984-1986 and 1995-1997. Diabetes Care. 1999;22:1813–20. doi: 10.2337/diacare.22.11.1813. [DOI] [PubMed] [Google Scholar]
- 66.Jorgensen ME. Glucose intolerance and its relation to cardiovascular risk factors among Greenland Inuit. The Greenland Population Study. Int J Circumpolar Health. 2004;63:286–8. doi: 10.3402/ijch.v63i3.17738. [DOI] [PubMed] [Google Scholar]
- 67.Selnes A, Bolle R, Holt J, Lund E. Atopic diseases in Sami and Norse schoolchildren living in northern Norway. Pediatr Allergy Immunol. 1999;10:216–20. doi: 10.1034/j.1399-3038.1999.00032.x. [DOI] [PubMed] [Google Scholar]
- 68.Selnes A, Bolle R, Holt J, Lund E. Cumulative incidence of asthma and allergy in north-Norwegian schoolchildren in 1985 and 1995. Pediatr Allergy Immunol. 2002;13:58–63. doi: 10.1034/j.1399-3038.2002.01009.x. [DOI] [PubMed] [Google Scholar]
- 69.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Ortega H, Castilla P, Gomez-Coronado D, Garces C, Benavente M, Rodriguez-Artalejo F, et al. Influence of apolipoprotein E genotype on fat-soluble plasma antioxidants in Spanish children. Am J Clin Nutr. 2005;81:624–32. doi: 10.1093/ajcn/81.3.624. [DOI] [PubMed] [Google Scholar]
- 71.Comas D, Reynolds R, Sajantila A. Analysis of mtDNA HVRII in several human populations using an immobilised SSO probe hybridisation assay. Eur J Hum Genet. 1999;7:459–68. doi: 10.1038/sj.ejhg.5200326. [DOI] [PubMed] [Google Scholar]
- 72.Lehtinen S, Luoma P, Nayha S, Hassi J, Ehnholm C, Nikkari T, et al. Apolipoprotein A-IV polymorphism in Saami and Finns: frequency and effect on serum lipid levels. Ann Med. 1998;30:218–23. doi: 10.3109/07853899808999407. [DOI] [PubMed] [Google Scholar]
- 73.Kozlov A, Lisitsyn D. Hypolactasia in Saami subpopulations of Russia and Finland. Anthropol Anz. 1997;55:281–7. [PubMed] [Google Scholar]
- 74.Sahi T. Genetics and epidemiology of adult-type hypolactasia. Scand J Gastroenterol Suppl. 1994;202:7–20. doi: 10.3109/00365529409091740. [DOI] [PubMed] [Google Scholar]
- 75.Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Jarvela I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30:233–7. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- 76.Bersaglieri T, Sabeti PC, Patterson N, Vanderploeg T, Schaffner SF, Drake JA, et al. Genetic signatures of strong recent positive selection at the lactase gene. Am J Hum Genet. 2004;74:1111–20. doi: 10.1086/421051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danpure CJ. Variable peroxisomal and mitochondrial targeting of alanine: glyoxylate aminotransferase in mammalian evolution and disease. Bioessays. 1997;19:317–26. doi: 10.1002/bies.950190409. [DOI] [PubMed] [Google Scholar]
- 78.Danpure CJ, Fryer P, Jennings PR, Allsop J, Griffiths S, Cunningham A. Evolution of alanine:glyoxylate aminotransferase 1 peroxisomal and mitochondrial targeting. A survey of its subcellular distribution in the livers of various representatives of the classes Mammalia, Aves and Amphibia. Eur J Cell Biol. 1994;64:295–313. [PubMed] [Google Scholar]
- 79.Danpure CJ, Guttridge KM, Fryer P, Jennings PR, Allsop J, Purdue PE. Subcellular distribution of hepatic alanine:glyoxylate aminotransferase in various mammalian species. J Cell Sci. 1990;97:669–78. doi: 10.1242/jcs.97.4.669. [DOI] [PubMed] [Google Scholar]
- 80.Takayama T, Fujita K, Suzuki K, Sakaguchi M, Fujie M, Nagai E, et al. Control of oxalate formation from L-hydroxyproline in liver mitochondria. J Am Soc Nephrol. 2003;14:939–46. doi: 10.1097/01.asn.0000059310.67812.4f. [DOI] [PubMed] [Google Scholar]
- 81.Birdsey GM, Lewin J, Cunningham AA, Bruford MW, Danpure CJ. Differential enzyme targeting as an evolutionary adaptation to herbivory in carnivora. Mol Biol Evol. 2004;21:632–46. doi: 10.1093/molbev/msh054. [DOI] [PubMed] [Google Scholar]
- 82.Birdsey GM, Lewin J, Holbrook JD, Simpson VR, Cunningham AA, Danpure CJ. A comparative analysis of the evolutionary relationship between diet and enzyme targeting in bats, marsupials and other mammals. Proc Biol Sci. 2005;272:833–40. doi: 10.1098/rspb.2004.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holbrook JD, Birdsey GM, Yang Z, Bruford MW, Danpure CJ. Molecular adaptation of alanine:glyoxylate aminotransferase targeting in primates. Mol Biol Evol. 2000;17:387–400. doi: 10.1093/oxfordjournals.molbev.a026318. [DOI] [PubMed] [Google Scholar]
- 84.Purdue PE, Takada Y, Danpure CJ. Identification of mutations associated with peroxisome-to-mitochondrion mistargeting of alanine/glyoxylate aminotransferase in primary hyperoxaluria type 1. J Cell Biol. 1990;111:2341–51. doi: 10.1083/jcb.111.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lumb MJ, Danpure CJ. Functional synergism between the most common polymorphism in human alanine:glyoxylate aminotransferase and four of the most common disease-causing mutations. J Biol Chem. 2000;275:36415–22. doi: 10.1074/jbc.M006693200. [DOI] [PubMed] [Google Scholar]
- 86.Caldwell EF, Mayor LR, Thomas MG, Danpure CJ. Diet and the frequency of the alanine:glyoxylate aminotransferase Pro11Leu polymorphism in different human populations. Hum Genet. 2004;115:504–9. doi: 10.1007/s00439-004-1191-x. [DOI] [PubMed] [Google Scholar]
- 87.Smith BK, Andrews PK, West DB. Macronutrient diet selection in thirteen mouse strains. Am J Physiol Regul Integr Comp Physiol. 2000;278:R797–805. doi: 10.1152/ajpregu.2000.278.4.R797. [DOI] [PubMed] [Google Scholar]
- 88.Tholin S, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. Am J Clin Nutr. 2005;81:564–9. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- 89.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]