Abstract
Aim
To evaluate the efficacy and toxicity of preoperative chemoradiotherapy with capecitabine in locally advanced rectal cancer.
Methods
Between June 2004 and January 2005, 57 patients with operable, clinical stage II-III adenocarcinoma of the rectum entered the prospective phase II study. Radiation dose was 45 Gy (25 × 1.8 Gy). Concurrent chemotherapy with a daily dose of 1650 mg/m2 capecitabine was administered orally, divided into two equal doses per day, including weekends. Patients were evaluated weekly for acute toxicity and compliance with the protocol. Surgery was scheduled 6 weeks after the completion of the chemoradiotherapy.
Results
A single female patient died after receiving 27 Gy, because of pulmonary embolism. All other patients completed the preoperative chemoradiotherapy according to the protocol and a definitive operation was performed in all but one of these patients. The complete pathological response was recorded in 5 patients (9.1%). Tumor (T), lymph nodes (N), and overall downstaging rates were 40%, 52.9%, and 49.1%, respectively. Total sphincter preservation rate was 65.5% (36 out of 55 patients) and the rate in 27 patients with tumors located within 5 cm of the anal opening was 37% (10 out of 27 patients). The most frequent side-effect of the combined therapy was dermatitis (grade 3 in 19 patients). After surgery, a single patient died due to sepsis during the early perioperative period. Nonlethal perioperative complications were recorded in 24/55 patients.
Conclusion
Preoperative chemoradiotherapy with oral capecitabine is safe and well tolerated. It has a downstaging potential and can increase the possibility for sphincter preservation surgery.
Preoperative chemoradiation has become a standard part of treatment protocols in stage II and III rectal cancer. Compared to postoperative chemoradiotherapy, the advantage of preoperative application of chemotherapeutics and irradiation includes improved compliance, reduced toxicity, and downstaging of the tumor in a substantial number of patients. The latter may enhance the rate of curative surgery, permit sphincter preservation in patients with low-sited tumors, and have a positive impact on the quality of life of these patients (1).
Although several innovative agents are being investigated in combination with radiotherapy, 5-fluorouracil (5-FU) in continuous infusion remains the gold standard in preoperative chemoradiotherapy schedules. The prolongation of exposure of cells to 5-FU results in improved antitumor activity, but requires prolonged infusion, usually through a vascular access port (VAP). Complications resulting from long-term venous access, such as bleeding and thrombosis, are not uncommon (2).
Orally administered capecitabine (Xeloda®, Hoffmann-La Roche Ltd, Basel, Switzerland) mimics the pharmacokinetics of continuous 5-FU infusion and makes chemoradiotherapy more patient-friendly. The mechanism of capecitabine activation, preferably in tumor cells, may further enhance its efficacy and tolerability, offering the potential for an enhanced therapeutic ratio (3-5). The use of capecitabine allows chronic dosing and, at the same time, avoids the discomfort and complications associated with prolonged intravenous infusion of 5-FU (2,6). Thus, the majority of patients prefer oral chemotherapy to 5-FU infusions (7).
The aim of the present prospective phase II study was to evaluate the efficacy and toxicity of preoperative chemoradiotherapy with capecitabine in patients with locally advanced rectal cancer. The primary endpoint of the study was a pathologically determined complete remission rate of the disease, locally and regionally. Secondary outcomes were the rate of sphincter preservation in low-sited tumors, overall downstaging rate, and toxicity.
Patients and methods
Patient inclusion criteria
The inclusion criteria were: histologically verified adenocarcinoma of the rectum, clinical TNM stage II or III (8); no prior radiotherapy and/or chemotherapy; World Health Organization (WHO) performance status <2 (9); age at diagnosis of 18 or older; and adequate bone marrow, liver, renal, and cardiac function (no history of ischemic heart disease). A history of prior malignancy other than non-melanoma skin cancer or in situ carcinoma of the cervix rendered the patient ineligible.
Before treatment, all patients received detailed oral and written information on the treatment protocol and possible side effects, and signed an informed consent. The trial was approved by the Independent Ethical Committee of the Institute of Oncology, Ljubljana, Slovenia.
Pre-treatment evaluation
The pre-treatment work-up comprised a complete history, physical examination, complete blood count, serum biochemistry, carcinoembryonic antigen (CEA), chest radiography, ultrasonography (US), and/or computer tomography (CT) of the whole abdomen, and colonoscopy with biopsy. Locoregionally, the extent of disease was determined by endoscopic US (67%), CT scan (23%), magnetic resonance imaging (MRI) of the abdomen (5%), or the combination of these methods (5%).
Treatment protocol
Radiotherapy was delivered using 15 MV photon beams and four-field box technique, once per day, 5 days a week. The small pelvis received 45 Gy in 25 fractions over 5 weeks. Three-dimensional CT-based treatment planning was performed. The clinical target volume was defined to cover the small pelvis from the L5-S1 interspace to 5 cm below the primary tumor. The lateral borders were 5 mm outside the true bony pelvis. The posterior margin covered the sacrum, and the anterior margin encompassed the posterior one-third to one-half of the bladder and/or vagina. An additional 1 cm in all directions was added to the clinical target volume to obtain the planning target volume. The dose was prescribed to cover the planning target volume with a 95% reference isodose (95% of the ICRU point dose). Patients were treated in the prone position. They were instructed to have a full bladder during irradiation, and no devices were used to displace the small bowel out of the irradiated volume. A multileaf collimator was used for shaping the fields and for the protection of normal tissues.
Chemotherapy was administered concomitantly with radiotherapy and consisted of capecitabine administered orally at a daily dose of 1650 mg/m2, divided into two equal doses given 12 hours apart. One of the doses was taken 2 hours prior to irradiation. The chemotherapy started on the first day of radiotherapy and finished on the last day of radiotherapy (including weekends). Whenever radiotherapy was interrupted (treatment-related toxicity, machine breakdown or any other reason), chemotherapy was not administered.
During treatment, patients were evaluated weekly to assess acute toxicity and compliance with the treatment schedule. Clinical examination and complete blood count were performed and body weight was measured. Toxic side effects were assessed according to National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 2.0 (10).
Definitive surgery was scheduled for 6 weeks after the completion of chemoradiotherapy. Surgical management included a sphincter preservation approach whenever possible, using the total mesorectal excision technique. After the operation, pathologic evaluation of the surgical specimen was performed in the hospitals where patients were operated on. The pathologists followed the uniform guidelines when examining the tissue specimen obtained during rectal surgery.
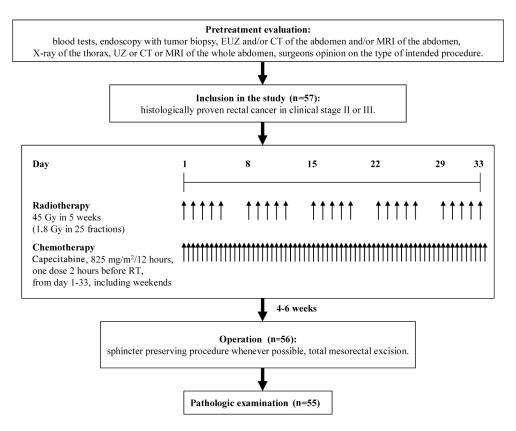
The effect of preoperative chemoradiotherapy on tumor downstaging was assessed by comparing the pretreatment radiologically determined TNM stage with the postoperative pathologic TNM stage (Figure 1).
Figure 1.
Study flowchart.
Statistical analysis
The pathologically determined complete remission rate was the main endpoint. According to the results of the NSABP R-03 (11) and the study by Sauer et al (1), a pathologically determined complete remission rate of 8% can be expected using infusional 5-FU and concomitant radiotherapy, while in a limited number of preliminary reports using capecitabine with radiotherapy, pathologically determined complete remission rates of 4%-31% were reported. We aimed to evaluate whether we could produce a 12% pathologically determined complete remission rate with our approach, but not lower than 4%. Setting 4% as the lowest pathologically determined complete remission rate of interest, with an alpha error of 5%, and a power of 80%, at least 55 evaluable patients were needed (calculated using power sample calculation, for single sample, percentages, α = 5%, 1-β = 20%, http://www.dssresearch.com/toolkit/sscalc/size_p1.asp).
Pathologically determined complete remission rates with 95% confidence intervals (CI) were reported. The dependence of pathologically determined complete remission rate and treatment intensity was tested using the two-tailed Fisher exact test. Statistical analysis was performed using the Statistical Package for the Social Sciences, version 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Between June 2004 and January 2005, 57 patients entered the study. There were 43 men and 14 women, with a median age of 67 years (range 34-81 years). The WHO performance status was graded as 0 in 52 patients and as 1 in 7 patients. According to the UICC TNM classification system, the extent of the disease was as follows: T3N0 – 20, T4N0 – 1, T1N1 – 1, T2N1 – 2, T3N1 – 26, T4N1 – 3, and T3N2 – 4 patients. The median distance of the tumor from the anal opening was 5.5 cm (range 1–12 cm). In 28 patients, the primary tumor was sited 5 cm or less from the anal opening (Table 1).
Table 1.
Characteristics of 57 patients and tumors included in prospective phase II trial on preoperative chemoradiotherapy with capecitabine in locally advanced rectal cancer
| Characteristics | No. (%) |
|---|---|
| Age (years; median and range) | 67 (34-81)* |
| Gender: | |
| male | 43 (75.4) |
| female | 14 (24.6) |
| WHO performance status:* | |
| stage 0 | 52 (91.2) |
| stage I | 7 (8.8) |
| Tumor differentiation (grade): | |
| well (G1) | 4 (7) |
| moderate (G2) | 21 (36.8) |
| poorly (G3) | 3 (5.3) |
| unknown or not stated (GX) | 29 (50.9) |
| Tumor distance from the anal opening (cm; median and range) | 5.5 (1-12) |
| Type of surgery:† | |
| anterior resection | 3 (5.3) |
| low anterior resection | 26 (45.6) |
| coloanal reconstruction | 3 (5.3) |
| abdominoperineal resection | 25 (43.9) |
*WHO performance status (9).
†As planned before the start of preoperative chemoradiotherapy.
Treatment compliance
Out of the 57 patients in the study, a single female patient died after receiving 27 Gy due to pulmonary embolism originating from deep venous thrombosis of the lower extremity, most probably not related to preoperative chemoradiotherapy. The other 56 patients (98%) completed the preoperative chemoradiotherapy according to the protocol. They all received the total dose of 45 Gy in 25 fractions of 1.8 Gy. Radiotherapy treatment was completed in the median time of 33 days (range 29-43 days). The median time of radiotherapy interruption was 2 days (range 1-6 days) and was recorded in 25 (45.6%) patients (machine breakdown, 21 patients; treatment-related toxicity, 4 patients). The median duration of chemotherapy was 33 days (range 8-50 days) and median time of chemotherapy interruption was 3 days (range 1-13 days). Because of poor compliance, 5/55 patients received less than 90% of the planned capecitabine dose, whereas in 3 additional patients the reasons were ischemic cardiac manifestation, nausea with vomiting, and low leukocyte count.
After chemoradiotherapy, 55 of 56 patients underwent definitive surgery performed by 24 surgeons in 10 hospitals all over Slovenia. In one patient, only explorative laparotomy was performed, as the tumor was deemed to be inoperable. Patients were operated on 13 to 87 days (median 45 days) from the last day of preoperative chemoradiotherapy. However, 93% (52/56) of the patients had surgery 5 to 7 weeks after finishing chemoradiation, whereas in other 4 patients the reasons for wide interval between chemoradiotherapy and surgery were ileus, additional preoperative diagnostics, or poor general condition of two patients. The types of operations were as follows: low anterior resection − 32 (58.2%); abdominoperineal resection − 17 (30.9%); anterior resection – 4 (7.3%); exenteration of the small pelvis −1 (1.8%); and Hartmann’s resection – 1 (1.8%). As determined by histopathological examination of surgical specimens, the resection was radical (R0) in 54 patients. In one patient, microscopic foci of cancer cells were found in the surgical margin (R1 resection).
Toxicity
Preoperative chemoradiotherapy was well tolerated in the majority of patients and no treatment-related mortality was observed, except for the patient who died because of pulmonary embolism. The frequency and intensity of treatment-related acute toxicities in 55 patients who underwent definitive surgery are listed in Table 2. The most frequent and serious side-effect of the combined therapy was dermatitis, occurring as grade 3 in 19 (34.5%) patients. During this period, 29 (52.7%) patients lost weight in comparison with the beginning of treatment. The maximum body weight loss was 17.5% (median 3.8%, range 1.2%-17.5%). Of the remaining patients, 19 (34.5%) succeed in maintaining a constant weight, and in 7 (12.7%) patients weight increased up to 11% (median 6.9%, range 1.1%-11%).
Table 2.
Acute toxicity of preoperative chemoradiotherapy according to the National Cancer Institute Common Toxicity Criteria (version 2.0) (8)
| Toxicity grade (No., %) |
|||
|---|---|---|---|
| Toxicity | 1 | 2 | 3 |
| Hematological: | |||
| anemia | 3 | 2 | |
| leukocytopenia | 5 | 2 | 1 |
| Non-hematological: | |||
| fatigue | 14 | 1 | |
| nausea | 11 | ||
| vomiting | 6 | ||
| hand and foot syndrome | 4 | ||
| diarrhea | 11 | 5 | 2 |
| infection | 4 | 1 | 1 |
| impaired heart function | 1 | ||
| radiodermatitis | 15 | 1 | 19 |
| cystitis | 5 | ||
| proctitis | 12 | 1 | 1 |
After surgery, one patient died due to sepsis during the early perioperative period. Non-lethal perioperative complications were recorded in 24 of 55 patients: delayed healing of postoperative wound in 12 (21.8%) patients, febrile episode in 5 (9.1%) patients, ileus in 3 (5.5%) patients, chronic diarrhea in 3 (5.5%) patients, and anastomotic leakage in 1 (1.8%) patient.
Tumor response
Tumor response was evaluated in 55 patients who had definitive surgery. In 2 of them, liver metastases were found during the operation. No signs of liver metastases were recorded in preoperative radiological studies in either of the two.
The overall downstaging rate was 49.1% (ie, in 27/55 patients): decrease in T-and N stage were observed in 22 (40.0%) and 18 (52.9%) patients, respectively. The increase in T- and/or N-stage (upstaging) was recorded in 6 patients (10.9%). Pathologic TNM stages, as assessed on histopathological examination of resected specimens, in relation to preoperative TNM status, are listed in Table 3. The complete pathological response was recorded in 5 patients (9.1%; 95% CI, 3%-20%). In an additional patient, only an isolated cluster of cancer cells was found in the extranodal perirectal fat tissue. All 5 patients with pathologically determined complete remission rate received more than 90% of the planned capecitabine dose and did not have any radiotherapy interruption. Whereas the difference in pathologically determined complete remission rate between patients with (none out of 25 patients) and without (5 out of 30 patients) radiotherapy interruptions was marginally statistically significant (Fisher exact test, P = 0.056), the capecitabine dose that patients received (more vs less than 90% of the planned dose) had no influence on the rate of pathologically determined complete remission.
Table 3.
Distribution of postoperative pathological TNM (pTNM) stages compared to pretreatment clinical stages (cTNM) in 55 patients who underwent definitive surgery*
| After surgery (pTNM) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before surgery (cTNM) | T0N0 | T0N1 | T1N0 | T2N0 | T2N1 | T3N0 | T3N1 | T3N2 | T4N0 | T4N1 | T4N2 | T3N2M1 |
| T1N1 | 1 | |||||||||||
| T2N1 | 1 | 1 | ||||||||||
| T3N0 | 1 | 1 | 7 | 7 | 3 | 1 | ||||||
| T3N1 | 4 | 1 | 1 | 4 | 1 | 4 | 4 | 5 | 1 | |||
| T3N2 | 2 | 1 | 1 | |||||||||
| T4N0 | 1 | |||||||||||
| T4N1 | 1 | 1 | ||||||||||
| Total (%) | 5 (9.1) | 1 (1.8) | 3 (5.4) | 13 (23.6) | 1 (1.8) | 12 (21.8) | 9 (16.4) | 6 (10.9) | 1 (1.8) | 1 (1.8) | 1 (1.8) | 2 (3.6) |
*Abbreviations: T – tumor, N – node, M – metastasis (8) .
Preservation of anal sphincter
Before therapy, abdominoperineal resection was planned in 24 out of 55 patients who had definitive surgery. After completion of chemoradiotherapy, sphincter-conserving surgery was successfully performed in 7 of these 24 patients. Among 31 patients in whom sphincter-conserving surgery was planned before having had any therapy, this was not possible in two patients, which resulted in an ultimate sphincter preservation rate of 65.5% (36/55). In the subgroup of 27 patients with tumors located within 5 cm of the anal opening, sphincter preservation was possible in 10 patients (37%).
Discussion
In the present prospective phase II study, capecitabine proved its safety and, from the viewpoint of organ preservation had considerable downstaging potential, when combined with concomitant radiotherapy in the preoperative setting in patients with stage II-III rectal cancer.
Currently, chemoradiotherapy using 5-FU is considered the gold standard in the treatment of locally advanced rectal cancer in the neoadjuvant setting (12). Given the short half-life of 5-FU in plasma and its intracellular metabolites, it should be administered in the form of prolonged intravenous infusions during the course of fractionated radiotherapy in order to act as a radiosensitizer (13-15). Moreover, infusional 5-FU is generally better tolerated than a bolus schedule (12,13). As oral capecitabine mimics continuous 5-FU infusion, both from the standpoint of locoregional control and treatment-related toxicity (16), we initiated this phase II study to evaluate its toxicity and efficacy in combination with preoperative radiotherapy in patients with locally advanced rectal cancer.
According to the results of randomized trials, short-course hypofractionated radiotherapy regimen (Swedish schedule, 5 fractions of 5 Gy delivered in 5 consecutive days) followed by immediate surgery also reduces local recurrence rate and improves survival compared to surgery alone (17). However, this approach has not been accepted worldwide, mainly because a short interval between radiotherapy and surgery allows neither tumor regression nor an increased ability of sphincter sparing surgery to be performed. In addition, the irradiation using 5 Gy fractions is associated with increased incidence of late effects, such as poor function of the anal sphincter and small bowel injury (18). Consequently, chemotherapy cannot be integrated into a large fraction regimen without excessive toxicity.
In the National Surgical Adjuvant Breast and Bowel Project Protocol R-03 (11) and in the German study by Sauer et al (1), which evaluated the effectiveness of the preoperative application of continuous 5-FU infusion and irradiation, pathologically determined complete remission rate was 8%. With oral capecitabine, pathologically determined complete remission rates, ranged from 4 to 31% mostly in preliminary studies (19-25), whereas in the recent study by Kim et al (26), which was the largest study so far, the pathologically determined complete remission rate was 12%. In our study, the pathologically determined complete remission rate was at somewhat lower rate of 9.1%. We presume that the main reason for this discrepancy was the unfavorable distribution of T- and N-tumor stages in our patients; in 60% of them the preoperative stage of disease was T3-4 and/or N+. Furthermore, contrary to other authors, we did not add a booster dose after the lesser pelvis was irradiated to a tumor dose of 45 Gy (19-24). In spite of a relatively high radiation dose of 55.8 Gy, Dunst et al (20) reported a pathologically determined complete remission rate of only 4% in a population of patients with a rather high proportion (50%) of T4 primaries. On the other hand, Dupois et al (25) observed as much as a 24% pathologically determined complete remission rate after preoperative radiotherapy to a dose of 45 Gy; however, only 2% of the tumors from their study were stage T4.
The other reason for the lower pathologically determined complete remission rate in our series could be the fact that the radiotherapy component of the protocol was prolonged in as many as 45.5% of our patients, with treatment interruptions of 3 days or more introduced in 18.2% of them. In contrast, in the study by Kim et al (26), there were no radiotherapy interruptions of more than 2 days. Furthermore, we found that 10.9% of patients received less than 90% of the planned dose of capecitabine which could also have reduced the likelihood of achieving the desired pathologically determined complete remission rate even though this was not statistically significant. Although the patients were thoroughly instructed before treatment and had diaries to fill in, which were monitored regularly by the radiation oncologist, many of them did not follow the instructions. Compliance in oral therapy in comparison to intravenous treatment could be a drawback of this type of therapy.
In the subgroup of our patients with primary tumors located 5 cm or less from the anal opening, the sphincter preservation rate was 37%, which is only half of 72% reported by Kim et al (26). In the latter study, all but one patient were operated on by a single surgeon, whereas in our study surgery was performed by 24 surgeons from 10 different hospitals. According to our previous experience, the level of expertise in pelvic surgery differs substantially among individual surgeons. Therefore, the probability of sphincter preservation in our series would most probably have been higher if all patients had been operated on by a limited number of highly skilled oncological surgeons.
The second important limitation of our study is the diversity of diagnostic modalities used for staging purposes. We are aware that MRI of the lower abdomen and endoluminal ultrasound of the rectum are the two most adequate and used investigations to determine the pretreatment extent of the disease. However, as the magnetic resonance imaging (MRI) and endorectal ultrasound (EUS) capacities in Slovenia were limited at the time the study was conducted, we did not specify a radiologic procedure for disease evaluation in our entry criteria. Consequently, we compared the preoperative clinical stage of the disease as assessed by different radiological modalities with the postoperative pathological stage for the assessment of treatment efficacy.
In some studies, the financial aspect of chemotherapy with oral capecitabine was also evaluated (27,28). Compared with intravenous administration of 5-FU, the results speak in favor of oral capecitabine. However, the financial benefit of any new regimen should be weighed against the long-term local control rate and patient survival rate and, finally, compared to a standard. As reliable data on these two endpoints for preoperative chemoradiotherapy with oral capecitabine are not yet available, the overall cost-effectiveness analysis of this particular regimen must be postponed into the future.
According to the results of our study and data from the literature, we conclude that in patients with locoregionally advanced rectal cancer, preoperative chemoradiotherapy with oral capecitabine as a radiosensitizer is safe. This treatment has considerable downstaging potential and can significantly increase the possibility of sphincter preservation in low-sited rectal primary tumors. Therefore, it seems that oral capecitabine represents a good alternative to protracted intravenous 5-FU in preoperative chemoradiotherapy protocols from the standpoint of toxicity and downstaging potential. The effectiveness of this new regimen is to be defined through randomized controlled trial(s) comparing all aspects (ie, toxicity, efficacy, and costs) of oral capecitabine therapy and protracted intravenous 5-FU.
References
- 1.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Grem JL. Systemic treatment options in advanced colorectal cancer: perspectives on combination 5-fluorouracil plus leucovorin. Semin Oncol. 1997;24(5 Suppl 18):S18-8-S18-18. [PubMed] [Google Scholar]
- 3.Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45:291–7. doi: 10.1007/s002800050043. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998;55:1091–7. doi: 10.1016/s0006-2952(97)00682-5. [DOI] [PubMed] [Google Scholar]
- 5.Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–81. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 6.Shimma N, Umeda I, Arasaki M, Murasaki C, Masubuchi K, Kohchi Y, et al. The design and synthesis of a new tumor-selective fluoropyrimidine carbamate, capecitabine. Bioorg Med Chem. 2000;8:1697–706. doi: 10.1016/s0968-0896(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–5. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 8.International union against cancer (UICC). Colon and rectum. In: Sobin LH, Wittekind CH, editors. TNM classification of malignant tumours, 6th ed. New York: Wiley; 2002. p.72-6. [Google Scholar]
- 9.World health organization. WHO handbook for reporting results of cancer treatment. Geneva: WHO; 1979. [Google Scholar]
- 10.Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 11.Hyams DM, Mamounas EP, Petrelli N, Rockette H, Jones J, Wieand HS, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: a progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum. 1997;40:131–9. doi: 10.1007/BF02054976. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence TS, Tepper JE, Blackstock AW. Fluoropyrimidine-radiation interactions in cells and tumors. Semin Radiat Oncol. 1997;7:260–6. doi: 10.1053/SRAO00700260. [DOI] [PubMed] [Google Scholar]
- 13.Rich TA. Irradiation Plus 5-Fluorouracil: Cellular mechanisms of action and treatment schedules. Semin Radiat Oncol. 1997;7:267–73. doi: 10.1053/SRAO00700267. [DOI] [PubMed] [Google Scholar]
- 14.Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis group in cancer. J Clin Oncol. 1998;16:301–8. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 15.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002;3:415–24. doi: 10.1016/s1470-2045(02)00788-x. [DOI] [PubMed] [Google Scholar]
- 16.Vaishampayan UN, Ben-Josef E, Philip PA, Vaitkevicius VK, Du W, Levin KJ, et al. A single-institution experience with concurrent capecitabine and radiation therapy in gastrointestinal malignancies. Int J Radiat Oncol Biol Phys. 2002;53:675–9. doi: 10.1016/s0360-3016(02)02772-4. [DOI] [PubMed] [Google Scholar]
- 17.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–7. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 18.Dahlberg M, Glimelius B, Graf W, Pahlman L. Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum. 1998;41:543–9. doi: 10.1007/BF02235256. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Kim JS, Cho MJ, Song KS, Yoon WH. Preoperative chemoradiation using oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54:403–8. doi: 10.1016/s0360-3016(02)02856-0. [DOI] [PubMed] [Google Scholar]
- 20.Kocakova I, Svoboda M, Klocova K, Spelda S, Kocak I, Slampa P, et al. Combined therapy of locally advanced rectal adenocarcinoma with capecitabine and concurrent radiotherapy. 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:3720. [Google Scholar]
- 21.Shi GG, Lin E, Eng C, Delcos M, Crane C, Amos CB, et al. Phase II study of capecitabine and radiotherapy (RT) plus concomitant boost in patients (pts) with locally advanced rectal cancer (LARC). 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:3775. [Google Scholar]
- 22.Dunst J, Reese T, Debus J, Hoelscher T, Budach W, Rudat V, et al. Phase-II-study of preoperative chemoradiation with capecitabine in rectal cancer. 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:3559. [Google Scholar]
- 23.Wong SJ, Sadasiwan C, Erickson B, Ota D, Mulkerin D, Thomas J, et al. A phase II trial of pre-operative capecitabine and concurrent radiation for locally advanced rectal cancer. 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:3771. [Google Scholar]
- 24.De Paoli A, Chiara S, Luppi G, Friso ML, Beretta G, Delprete S, et al. A phase II study of capecitabine (CAP) and pre-operative radiation therapy (RT) in resectable, locally advanced rectal cancer (LARC). 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:3540. [Google Scholar]
- 25.Dupuis O, Vie B, Lledo G, Hennequin C, Noirclerc M, Bennamoun M, et al. Capecitabine (X) chemoradiation (CRT) in the preoperative treatment of patients (pts) with rectal adenocarcinomas: A phase II GERCOR trial. 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:3538. [Google Scholar]
- 26.Kim JC, Kim TW, Kim JH, Yu CS, Kim HC, Chang HM, et al. Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:346–53. doi: 10.1016/j.ijrobp.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 27.Twelves C, Boyer M, Findlay M, Cassidy J, Weitzel C, Barker C, et al. Capecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer. 2001;37:597–604. doi: 10.1016/s0959-8049(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 28.Jansman FG, Postma MJ, van Hartskamp D, Willemse PH, Brouwers JR. Cost-benefit analysis of capecitabine versus 5-fluorouracil/leucovorin in the treatment of colorectal cancer in the Netherlands. Clin Ther. 2004;26:579–89. doi: 10.1016/s0149-2918(04)90060-4. [DOI] [PubMed] [Google Scholar]