Abstract
Aim
To assess if there is deterioration in mental and psychomotor performance during 24-hour voluntary fluid intake deprivation.
Methods
A battery of computer generated psychological tests (Complex Reactionmeter Drenovac) was applied to 10 subjects to test light signal position discrimination, short-term memory, simple visual orientation, simple arithmetic, and complex motor coordination. We measured total test solving time, minimum (best) single task solving time, total ballast time, and total number of errors. Mood self-estimate scales of depression, working energy, anxiety, and self-confidence were used to determine the emotional status of subjects. During the first day of the experiment, subjects had free access to drinks. After a 48-hour interval, subjects voluntarily abstained from fluid intake for 24 hours. During that period, the testing was performed 7 times a day, at 3-hour intervals, except during the night. Z-transformation of the results enabled the comparison of 50 dependent measurements on the same subjects.
Results
During dehydration, there was significant deterioration in total test solving time, minimum single task solving time, and total ballast time. No significant deterioration was found by mood self-estimate scales, except on the scale of energy at 23:00 hours.
Conclusion
Voluntary 24-hour fluid intake deprivation led to deterioration in objective parameters of psychological processing, but not in subjective parameters. The results suggest that the duration of fluid intake deprivation can be a useful indicator of mental and psychomotor deterioration level.
There are many life situations when unfavorable circumstances influence the hydration regime. The problem of dehydration in humans has been extensively studied and the physiology of this phenomenon is generally well understood (1-6). The neurophysiological mechanisms affecting mental and psychomotor performance in dehydration are extremely complex and include structural and functional changes of neuronal and glial cells (7-10).
Mental and psychomotor processing deterioration and the dynamics of its onset during dehydration are of great practical importance but are still not completely understood. Deteriorated mental and psychomotor processing could endanger performance of tasks that require high precision, are performed under forced regime and rhythm, permit no mistakes, or involve sophisticated, expensive, or dangerous equipment. Deteriorated psychological processing was described in experiments in which dehydration was either heat stress induced (11-14) or induced by a combination of heat, stress, and exercise (15-17). Both stressors were necessarily multifactorial and could not represent a valid experimental frame for studying dehydration. In rare experiments in which dehydration was a consequence of voluntary fluid intake deprivation, only deterioration in subjective parameters was found (18-20).
Testing mental and psychomotor performance in possibly dehydrated subjects in a real-life situation and under complex circumstances would be most impractical. In such situations, it would be practical to measure a simple parameter indicating deterioration level of mental and psychomotor abilities, at the same time predicting the level of expected performance efficiency.
The objective of this study was to measure the deterioration in mental and psychomotor performance and dynamics of its onset during voluntary 24-hour fluid intake deprivation by objective and subjective parameters of psychological processing under laboratory conditions.
Subjects and methods
Subjects
The study included 10 male, non-paid volunteers, of an average age of 25 (range 21 to 30). All subjects were healthy, without injuries, and not taking any psychoactive substances 30 days before and during the experiment. The subjects were medical students or young physicians interested in the experiment and motivated by the possibility to use the same equipment in their future research. All subjects signed an informed consent and the study was previously approved by the Ethical Committee of the Split University Medical School, Croatia.
Methods
The study was conducted at the Naval Medical Institute of the Croatian Navy, Split, Croatia, in autumn 2005, in a separate and especially prepared room, which provided the isolation for two subjects at a time. Psychological testing was performed at 7 time points a day during two days of the experiment. Testing started at 08:00 hours and was conducted at 3-hour intervals, except during the night rest from 23:00 to 08:00 of the next day, with a 48-hour interval between the days. During the first day, the subjects were allowed to freely take drinks. During the second day, subjects refrained from any fluid intake. The possibility of cheating was absolutely excluded during the entire experiment because of controlled environment of the test room. During both days of the experiment, all activities of the subjects were reduced to resting and reading.
The daily intake of food during the experiment was previously defined by an experienced nutritionist to 10 460 KJ (2500 KCal), served at usual times (09:00, 13:00, and 19:00 hours). Daily water intake in food mass was 500.0 ± 43.4 g. The menu did not include any food subjects would dislike or reject for any reasons. The room was adequately lighted and furnished to enable comfort. The room temperature was adjusted to 22°C, which was within the temperature comfort frame for light sitting work of lightly dressed persons (21).
Psychological testing of objective parameters
Complex Reactionmeter Drenovac (CRD-series), a battery of computer generated psychological tests, was used to measure objective parameters of psychological performance (22). Prior to the experiment, subjects practiced operating on CRD-series instruments for 3 consecutive days, two hours a day, and reached stable baseline results, without a tendency of improvement.
In several earlier studies (23-34), CRD-series was able to detect even the slightest mental and psychomotor changes. CRD-series is based on chronometric principles (22,34,35). It consists of the software and four instruments. Out of its 34 standard tests, 5 representative tests, given in order from the simplest to the most complex one and covering a broad spectrum of mental and psychomotor processing, were selected for the study. The subjects were instructed to solve the tests as accurately and as quickly as possible. At each single testing, each subject was assigned a new variety of each of the tests, so any possible memorizing of the problem sequences was excluded. CRD-series tests used in this experiment were: light signal position discrimination, short-term memory, simple visual orientation, simple arithmetic, and complex motor coordination.
Four parameters were recorded: total test solving time, minimum (best) single task solving time, total ballast (representing “lost” time expressed as a sum of differences between minimum single task solving time and each of all other single task solving times), and total number of errors committed during any of the tests. Total test solving time and minimum single task solving time are descriptors of speed, reliability (accuracy), and mental endurance; total ballast is a descriptor of stability; and total number of errors of concentration and alertness. Thus, these descriptors could be understood as descriptors of speed (total test solving time, minimum single task solving time) and variability of reactions (total ballast, total number of errors).
CRD-series measures the time needed to perform and solve single tasks in the tests, as well as the complete test. The time of solving psychological tests of different complexity provides information about organizational and functional characteristics of individual psychological processes, and therefore on organization and quality of psychological mechanisms hidden behind cognitive and psychomotor functions. A strong correlation was found between the results on CRD-series tests and cognitive and psychomotor functions (22,34). In each of the 5 tests, consisting of 30 to 60 single tasks, subjects were required to provide the correct answer by pressing the corresponding light-emitting diodes with their dominant hand as quickly as possible. The correct answer would automatically start the next single task. A more detailed description of CRD-series tests could be found elsewhere (22,23).
Psychological testing of subjective parameters
The current emotional status of subjects was measured by printed questionnaires with 10-point mood self-estimate scales of depression, working energy status, anxiety, and self-confidence by Wessman and Ricks (36), modified by Drenovac and Horga (37). On each of the scales, subjects were asked to choose the statement which best describes their actual mood. The questionnaires were filled in anonymously, dropped into a sealed box, and collected after the experiment. Self-estimate testing was performed at the same intervals, but prior to the testing on CRD-series, in order to avoid a possible negative feed-back. Subjects were informed that all results on CRD-series would be good, and that the differences between them would be normal and expected. In one of the earlier studies it was noticed that subjects were highly competitive while solving the CRD-series (23). Besides, it is known that subjects, when tested under experimental conditions, are more engaged than during the practicing period (37). Thus, the values even better than control were also expected. Special attention was paid to a possible drop of motivation. The experimenters were constantly observing subjects’ reactions, such as possible disappointment, anger, and drop of motivation since since these reactions might influence the results.
Statistical analysis
Statistica 7.1 software package (StatSoft, Inc., Tulsa, USA) was used to perform statistical analysis of the data. Z-transformation of the results for total test solving time, minimum single task solving time, total ballast, and total number of errors on all 5 tests enabled the comparison of 50 dependent measurements on the same subjects collected on both days of the experiment. Differences between variances of the data obtained at different points of testing and between the two days of the experiment were estimated by General Linear Models ANOVA for repeated measures and LSD post-hoc test (CRD-series data) and by ANOVA for repeated measures with Bonferroni correction (mood self-estimates). Statistical values were considered significant at P<0.05.
Results
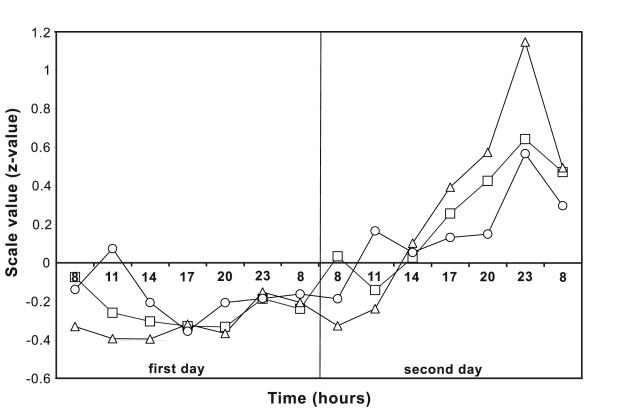
When all 7 time points of measurements were compared between the two days of the experiment, statistically significant deterioration was found on the second day for total test solving time (F = 16.99, df = 13, P<0.001, repeated measures ANOVA), minimum single task solving time (F = 25.97, df = 13, P<0.01), and total ballast (F = 4.77, df = 13, P<0.001, Figure 1). On the first day, no statistically significant difference was found among all points of measurement for total test solving time (F = 2.17, df = 6, P = 0.06), minimum single task solving time (F = 1.36, df = 6, P = 0.231), and total ballast (F = 1.47, df = 6, P = 0.187), but the difference became statistically significant during dehydration (F = 12.17, df = 6, P<0.01; F = 19.22, df = 6, P<0.001; and F = 4.59, df = 6, P<0.001, respectively, Figure 1). A significant increase in mean value (z-scores, first day vs second day) was noticed on the second day of the experiment for total test solving time at 14:00 (-0.3 ± 0.96 vs 0.03 ± 0.98, P = 0.001), 17:00 (-0.33 ± 0.84 vs 0.26 ± 0.89, P<0.001), 20:00 (-0.33 ± 0.88 vs 0.43 ± 1.12, P<0.001), and 23:00 hours (-0.19 ± 0.95 vs 0.64 ± 0.96, P<0.001). For minimum single task solving time, a significant increase was noticed at 11:00 (-0.39 ± 0.89 vs -0.24 ± 0.89, P = 0.01), 14:00 (-0.39 ± 0.81 vs 0.10 ± 0.76, P<0.001), 17:00 (-0.32 ± 0.83 vs 0.39 ± 0.80, P<0.001), 20:00 (-0.37 ± 0.9 vs 0.57 ± 0.99, P<0.001), 23:00 (-0.15 ± 0.94 vs 1.14 ± 1.04, P<0.001), and at 08:00 hours of the next day (-0.20 ± 1.15 vs 0.49 ± 1.02, P<0.001). For total ballast, a significant increase was noticed at 17:00 (-0.35 ± 1.14 vs 0.13 ± 1.07, P = 0.008), 20:00 (-0.21 ± 0.96 vs 0.15 ± 1.02, P = 0.013), 23:00 (-0.18 ± 0.92 vs 0.57 ± 0.89, P<0.001), and 08:00 hours of the next day (-0.16 ± 1.07 vs 0.30 ± 0.99, P = 0.010).
Figure 1.
The results of testing (z-values) of mental and psychomotor performance on CRD-series during the first (free hydration) and the second (voluntary 24-hour fluid intake deprivation) day of the experiment (n = 10). Squares – total test solving time; triangles – minimum single task solving time; circles – total ballast (“lost” time, a sum of differences between minimum single task solving time and each of all other single task solving times).
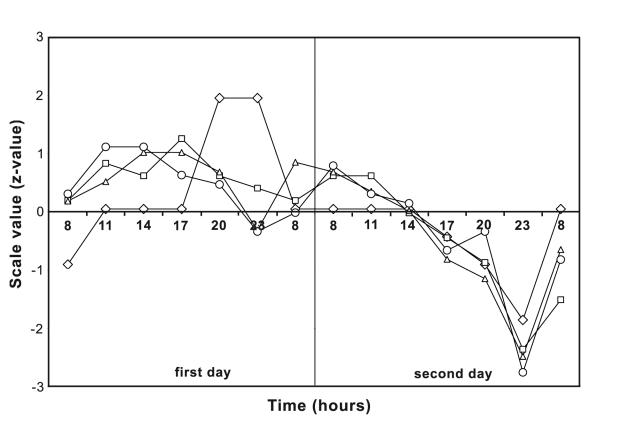
No significant deterioration of mood self-estimate was found on the second day of the experiment (Figure 2), except on the scale of energy at 23:00 hours (P = 0.016).
Figure 2.
The results of mood self-estimate testing (z-values), on the scales of depression, working energy, anxiety, and self-confidence during the first (free hydration) and the second (voluntary 24-hour fluid intake deprivation) day of the experiment (n = 10). Rhombs – depression; squares – energy; triangles – anxiety; circles – self-confidence.
Discussion
Our study showed that mental and psychomotor abilities deteriorated during progressive 24-hour voluntary dehydration. During the second day of the experiment, deterioration in the performance on CRD-series tests was expressed as an increase in total test solving time, minimum single task solving time, and total ballast. All changes became statistically significant 9 hours after the beginning of the second day of the experiment. The descriptors of speed, reliability (accuracy), and mental endurance deteriorated 3 hours (minimum single task solving time) and 6 hours (total test solving time) after the beginning of fluid intake deprivation. The descriptor of stability (total ballast), indicating a change in the problem-solving strategy, deteriorated after 9 hours of dehydration. Statistical analysis of total number of errors did not provide any reliable conclusions. However, deterioration did not occur on all CRD-series tests, in all the subjects, or at every point of testing. The trend of deterioration was clearly marked on the second day of the experiment, especially toward its end. However, no clear dose-response type changes were found with the increase in the time during which the subjects were deprived of fluid intake.
The experiments in which a deteriorated mental and psychomotor performance was a consequence of dehydration induced by exercise, heat stress, or increased ambient temperature (11-17,38) must have been necessarily influenced by fatigue, increased body temperature, and increased ambient temperature as additional stressors. An experiment in which dehydration would be a consequence of voluntary refraining from fluid intake would be free of such stressors and represent a valid study frame. Such an experimental model was used in earlier studies (18-20), but only subjective changes of mental and psychomotor processing were found, possibly because the psychological tests used were not sensitive enough. On the contrary, no changes of mood self-estimates were found in this study, except on the scale of energy at 23:00 hours of the dehydration day. However, the trends of deterioration were clearly marked. Further research with a greater number of subjects could clarify the real significance of such trends. A recent report (38) revealed statistically significant deterioration in cognitive performance in the dehydrated subjects, but this study addressed the beneficial effect of hydration rather than the effects of voluntary dehydration.
Based on the present results, it could be concluded that, in situations when more complex psychological testing is not possible, the duration of fluid intake deprivation could serve as a useful indicator of mental and psychomotor deterioration level. The present study suggests that deteriorated mental and psychomotor processing occurs early after the beginning of inadequate fluid intake, pointing to the importance of a regular and perhaps planned regime of fluid intake (39).
The primary limitation of this study was the small number of subjects, at least when mood self-estimates are concerned. The comparison of performance between the two days of the experiment on each of 5 CRD-series tests would not be reliable, so the parameters (z-scores) obtained on all CRD-series tests (total test solving time, minimum single test solving time, total ballast, and total number of errors) were compared. Another limitation is that only 5 out of 34 CRD-series tests were used and only 4 out of 27 parameters analyzed.
Hormonal and metabolic responses to this type of acute stress are complex and include a variety of changes such as the release of cortisol and catecholamines, activation of glucagon, and growth hormone (40). The research of this kind of responses was beyond the scope of the present study. Besides, we investigated only the effects of dehydration, not of a rehydration regime, so we have no data about the recovery of psychological performance. A similar group of voluntary dehydrated subjects was investigated by Roky et al (41). In their subjects, Ramadan fasting (refraining from drinking and eating from sunrise to sunset) did not dramatically affect the metabolism of lipids, carbohydrates, and proteins, or the daily mean of hormonal serum levels, but affected the circadian distribution of body temperature, cortisol, melatonin, and glycemia.
Further research, with a greater number of subjects and a broader spectrum of CRD-series tests, which would include other dehydration parameters, such as body mass loss, urine osmolality, cortisol in saliva, and hematocrit, might confirm the validity of the present conclusions and reveal other possible indicators of mental and psychomotor deterioration level during dehydration.
References
- 1.Astrand P-O, Rodahl K. Textbook of work physiology. 3rd ed. New York (NY): McGraw-Hill; 1986. [Google Scholar]
- 2.Kokko J, Tannen R, editors. Fluids and electrolytes. 3rd ed. Philadelphia (PA): WB Saunders; 1996. [Google Scholar]
- 3.Jimenez C, Melin B, Koulmann N, Allevard AM, Launay JC, Savourey G. Plasma volume changes during and after acute variations of body hydration level in humans. Eur J Appl Physiol Occup Physiol. 1999;80:1–8. doi: 10.1007/s004210050550. [DOI] [PubMed] [Google Scholar]
- 4.Halperin M, Goldstein M. Fluid, electrolytes and acid-base physiology. 3rd ed. Philadelphia (PA): WB Saunders; 1999. [Google Scholar]
- 5.Rehrer NJ. Fluid and electrolyte balance in ultra-endurance sport. Sports Med. 2001;31:701–15. doi: 10.2165/00007256-200131100-00001. [DOI] [PubMed] [Google Scholar]
- 6.Iversen PO, Nicolaysen G. Water – for life. Tidsskr Nor Laegeforen. 2003;123:3402–5. [In Norwegian]. [PubMed] [Google Scholar]
- 7.Langle SL, Poulain DA, Theodosis DT. Neuronal-glial remodeling: a stuctural basis for neuronal-glial interactions in the adult hypothalamus. J Physiol (Paris) 2002;96:169–75. doi: 10.1016/s0928-4257(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 8.Wilson MM, Morley JE. Impaired cognitive function and mental performance in mild dehydration. Eur J Clin Nutr. 2003;57(Suppl 2):S24–9. doi: 10.1038/sj.ejcn.1601898. [DOI] [PubMed] [Google Scholar]
- 9.Miklos IH, Kovacs KJ. Functional heterogeneity of the responses of histaminergic neuron subpopulations to various stress challenges. Eur J Neurosci. 2003;18:3069–79. doi: 10.1111/j.1460-9568.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- 10.Kadekaro M. Nitric oxide modulation of the hypothalamo-neurohypophyseal system. Braz J Med Biol Res. 2004;37:441–50. doi: 10.1590/s0100-879x2004000400001. [DOI] [PubMed] [Google Scholar]
- 11.Wyon DP, Andersen I, Lundqvist GR. The effects of moderate heat stress on mental performance. Scand J Work Environ Health. 1979;5:352–61. doi: 10.5271/sjweh.2646. [DOI] [PubMed] [Google Scholar]
- 12.Epstein Y, Keren G, Moisseiev J, Gasko O, Yachin S. Psychomotor deterioration during exposure to heat. Aviat Space Environ Med. 1980;51:607–10. [PubMed] [Google Scholar]
- 13.Sharma VM, Sridharan K, Pichan G, Panwar MR. Influence of heat stress-induced dehydration on mental functions. Ergonomics. 1986;29:791–9. doi: 10.1080/00140138608968315. [DOI] [PubMed] [Google Scholar]
- 14.Gopinathan PM, Pichan G, Sharma VM. Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health. 1988;43:15–7. doi: 10.1080/00039896.1988.9934367. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol. 1998;513:895–905. doi: 10.1111/j.1469-7793.1998.895ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cian C, Koulmann N, Barraud PA, Raphel C, Jimenez C, Melin B. Influence of variations in body hydration on cognitive function: effect of hyperhydration, heat stress, and exercise-induced dehydration. Journal of Psychophysiology. 2000;14:29–36. [Google Scholar]
- 17.Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42:243–51. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 18.Neave N, Scholey AB, Emmett JR, Moss M, Kennedy DO, Wesnes KA. Water ingestion improves subjective alertness, but has no effect on cognitive performance in dehydrated healthy young volunteers. Appetite. 2001;37:255–6. doi: 10.1006/appe.2001.0429. [DOI] [PubMed] [Google Scholar]
- 19.Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr. 2004;91:951–8. doi: 10.1079/BJN20041149. [DOI] [PubMed] [Google Scholar]
- 20.Szinnai G, Schachinger H, Arnaud MJ, Linder L, Keller U. Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol. 2005;289:R275–80. doi: 10.1152/ajpregu.00501.2004. [DOI] [PubMed] [Google Scholar]
- 21.Osada Y. Experimental studies on the sexual and seasonal differences of the optimal thermal conditions. J Hum Ergol (Tokyo) 1978;7:145–55. [PubMed] [Google Scholar]
- 22.Drenovac M. CRD-series of psychodiagnostic tests [in Croatian] Zagreb: AKD; 1994. [Google Scholar]
- 23.Petri NM. Change in strategy of solving psychological tests: evidence of nitrogen narcosis in shallow air-diving. Undersea Hyperb Med. 2003;30:293–303. [PubMed] [Google Scholar]
- 24.Bobic J, Pavicevic L, Gomzi M. The difference in complex psychomotor reaction time between patients with and without signs of cerebral circulatory disorders. Coll Antropol. 2002;26:515–20. [PubMed] [Google Scholar]
- 25.Prpic-Majic D, Bobic J, Simic D, House DE, Otto DA, Jurasovic J, et al. Parental education as a confounder in the assessment of low level lead effect on psychological functions in children. Cent Eur J Public Health. 2000;8(Suppl):69. [PubMed] [Google Scholar]
- 26.Bobic J, Pavicevic L. Complex reaction time and EEG characteristics in alcoholics. Arh Hig Rada Toksikol. 1996;47:351–7. [PubMed] [Google Scholar]
- 27.Bobic J, Pavicevic L. Critical values of psychological measuring instruments in evaluation of working ability of elderly alcoholics. Lijec Vjesn. 1987;6:199–202. [in Croatian]. [PubMed] [Google Scholar]
- 28.Bobic J, Pavicevic L, Drenovac M. The difference between alcoholics and their healthy equivalent pairs – psychological approach. Stud Psychol (Bratisl) 1995;37:351–6. [Google Scholar]
- 29.Drenovac M, Zajc M.The efficiency of cognitive functioning across different age groups[in Croatian]Čovjek i promet 1992127–32. [Google Scholar]
- 30.Drenovac S, Drenovac M.Variability of working efficiency of railway dispatchers during day and night 12-hour shift [in Croatian]Čovjek i promet 198915115–27. [Google Scholar]
- 31.Radonjic V, Kafol R, Ercegovac D, Kocijancic R, Neskovic N. Neurophysiologic, psychomotor and psychologic indicators in workers after long-term exposure to cholinesterase-inhibiting insecticides. Arh Hig Rada Toksikol. 1985;36:355–64. [PubMed] [Google Scholar]
- 32.Drenovac M, Drenovac S.Working shift and changes of working efficiency of diesel and electrolocomotives operators [in Croatian]Čovjek i promet 19762223–9. [Google Scholar]
- 33.Bobic J, Gomzi M. Memory and concentration efficiency in workers professionally exposed to xylene. Stud Psychol (Bratisl) 2004;46:65–71. [Google Scholar]
- 34.Kardum G. The application of computerized psychological diagnostic instruments in research of human potential [thesis in Croatian]. Zagreb: University of Zagreb; 2002. [Google Scholar]
- 35.Spoelders-Claes R, Coetsier P. Research note on a new electronic psycho-diagnostic system: The Complex Reactionmeter Drenovac Testbattery. Ghent (Belgium): State University of Ghent; 1976.
- 36.Wessman AE, Ricks DF. Mood and personality. New York (NY): Hodl, Rinehart and Winston; 1966. [Google Scholar]
- 37.Moeller G, Chattin C, Rogers W, Laxar K, Ryack B. Performance effects with repeated exposure to the diving environment. J Appl Psychol. 1981;66:502–10. [Google Scholar]
- 38.Bar-David Y, Urkin J, Kozminsky E. The effect of voluntary dehydration on cognitive functions of elementary school children. Acta Paediatr. 2005;94:1667–73. doi: 10.1080/08035250500254670. [DOI] [PubMed] [Google Scholar]
- 39.Shirreffs SM. The importance of good hydration for work and exercise performance. Nutr Rev. 2005;63:S14–21. doi: 10.1111/j.1753-4887.2005.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 40.Kandel RE. Schwartz HJ, Jessell MT. Principles of neuralscience, 4th ed. New York (NY): McGraw-Hill; 2000. [Google Scholar]
- 41.Roky R, Houti I, Moussamih S, Qotbi S, Aadil N. Physiological and chronobiological changes during Ramadan intermittent fasting. Ann Nutr Metab. 2004;48:296–303. doi: 10.1159/000081076. [DOI] [PubMed] [Google Scholar]